High Genotypic Diversity, Putative New Types and Intra-Genotype Variants of Bovine Papillomavirus in Northeast Brazil
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chambers, G.; Ellsmore, V.A.; Brien, P.M.O.; Reid, S.W.J.; Love, S.; Campo, M.S.; Nasir, L. Association of bovine papillomavirus with the equine sarcoid. J. Gen. Virol. 2003, 84, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Dyk, E.; Bosman, A.-M.; Wilpe, E.; Williams, J.H.; Bengis, R.G.; Heerden, J.; Venter, E.H. Detection and characterisation of papillomavirus in skin lesions of giraffe and sable antelope in South Africa. Tydskr. S. Afr. Vet. Assoc. 2011, 82, 80–85. [Google Scholar]
- Pangty, K.; Singh, S.; Goswami, R.; Saikumar, G.; Somvanshi, R. Detection of BPV-1 and -2 and Quantification of BPV-1 by Real-Time PCR in Cutaneous Warts in Cattle and Buffaloes. Transbound. Emerg. Dis. 2010, 57, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Campo, M.S. Papillomavirus: Old system, new lessons? In Papillomavirus Research from Natural History to Vaccine and Beyond; Caister Academic Press: Wymondham, UK, 2006; Volume 57, p. 1021. [Google Scholar]
- Mazzuchelli-De-Souza, J.; De Carvalho, R.F.; Módolo, G.G.; Thompson, C.E.; Araldi, R.P.; Stocco, R.C. First detection of bovine papillomavirus type 2 in cutaneous wart lesions from ovines. Transbound. Emerg. Dis. 2018, 65, 939–943. [Google Scholar] [CrossRef]
- Roperto, S.; Russo, V.; Corrado, F.; De Falco, F.; Munday, J.S.; Roperto, F. Oral fibropapillomatosis and epidermal hyperplasia of the lip in newborn lambs associated with bovine Deltapapillomavirus. Sci. Rep. 2018, 8, 13310. [Google Scholar] [CrossRef] [PubMed]
- Savini, F.; Gallina, L.; Prosperi, A.; Puleio, R.; Lavazza, A.; Di Marco, P.; Tumino, S.; Moreno, A.; Lelli, D.; Guercio, A.; et al. Bovine papillomavirus 1 gets out of the flock: Detection in an ovine wart in Sicily. Pathogens 2020, 9, 429. [Google Scholar] [CrossRef]
- PAVE Papillomavirus Episteme. Available online: https://pave.niaid.nih.gov (accessed on 29 March 2020).
- Borzacchiello, G.; Roperto, F. Bovine papillomaviruses, papillomas and cancer in cattle. Vet. Res. 2008, 39, 1–19. [Google Scholar] [CrossRef]
- Yamashita-Kawanishi, N.; Tsuzuki, M.; Kasuya, F.; Chang, H.W.; Haga, T. Genomic characterization of a novel bovine papillomavirus type 28. Virus Genes 2020, 1–6. [Google Scholar] [CrossRef]
- Campo, M.S.; Jarrett, W.F.H.; Barron, R.; Neil, B.W.O.; Smith, K.T. Association of Bovine Papillomavirus Type 2 and Bracken Fern with Bladder Cancer in Cattle. Cancer Res. 1992, 52, 6898–6904. [Google Scholar]
- Martano, M.; Roperto, F.; Stocco, R.C.; Russo, V.; Borzacchiello, G.; Paciello, O.; Iovane, V.; Leonardi, L.; Maiolino, P.; Restucci, B.; et al. Bovine Papillomavirus Type 2 Infection and a Series of Mesenchymal Tumors of the Urinary Bladder in Cattle. Biomed. Res. Int. 2013, 2013, 814635. [Google Scholar] [CrossRef]
- Roperto, S.; Russo, V.; Leonardi, L.; Martano, M.; Corrado, F.; Riccardi, M.G.; Roperto, F. Bovine Papillomavirus Type 13 Expression in the Urothelial Bladder Tumours of Cattle. Transbound. Emerg. Dis. 2015, 63, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Roperto, S.; Munday, J.S.; Corrado, F.; Goria, M.; Roperto, F. Detection of bovine papillomavirus type 14 DNA sequences in urinary bladder tumors in cattle. Vet. Microbiol. 2016, 190, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Munday, J.S.; Thomson, N.; Dunowska, M.; Knight, C.G.; Laurie, R.E.; Hills, S. Genomic characterisation of the feline sarcoid-associated papillomavirus and proposed classification as Bos taurus papillomavirus type 14. Vet. Microbiol. 2015, 177, 289–295. [Google Scholar] [CrossRef] [PubMed]
- De Villiers, E.M.; Fauquet, C.; Broker, T.R.; Bernard, H.U.; Zur Hausen, H. Classification of papillomaviruses. Virology 2004, 324, 17–27. [Google Scholar] [CrossRef]
- Schiffman, M.; Castle, P.E.; Jeronimo, J.; Rodriguez, A.C.; Wacholder, S. Human papillomavirus and cervical cancer. Lancet 2007, 370, 890–907. [Google Scholar] [CrossRef]
- Xi, L.F.; Koutsky, L.A.; Hildesheim, A.; Galloway, D.A.; Wheeler, C.M.; Winer, R.L.; Ho, J.; Kiviat, N.B. Risk for high-grade cervical intraepithelial neoplasia associated with variants of human papillomavirus types 16 and 18. Cancer Epidemiol. Biomark. Prev. 2007, 16, 4–10. [Google Scholar] [CrossRef]
- Xi, L.F.; Schiffman, M.; Koutsky, L.A.; Hughes, J.P.; Winer, R.L.; Mao, C.; Hulbert, A.; Lee, S.K.; Shen, Z.; Kiviat, N.B. Lineages of oncogenic human papillomavirus types other than type 16 and 18 and risk for cervical intraepithelial neoplasia. J. Natl. Cancer Inst. 2014, 106, dju270. [Google Scholar] [CrossRef]
- Chen, A.A.; Gheit, T.; Franceschi, S.; Tommasino, M.; Clifford, G.M. Human Papillomavirus 18 Genetic Variation and Cervical Cancer Risk Worldwide. J. Virol. 2015, 89, 10680–10687. [Google Scholar] [CrossRef]
- Cullen, M.; Boland, J.F.; Schiffman, M.; Zhang, X.; Wentzensen, N.; Yang, Q.; Chen, Z.; Yu, K.; Mitchell, J.; Roberson, D.; et al. Deep sequencing of HPV16 genomes: A new high-throughput tool for exploring the carcinogenicity and natural history of HPV16 infection. Papillomavirus Res. 2015, 1, 3–11. [Google Scholar] [CrossRef]
- Mirabello, L.; Yeager, M.; Cullen, M.; Boland, J.F.; Chen, Z.; Wentzensen, N.; Zhang, X.; Yu, K.; Yang, Q.; Mitchell, J.; et al. HPV16 Sublineage Associations with Histology-Specific Cancer Risk Using HPV Whole-Genome Sequences in 3200 Women. J. Natl. Cancer Inst. 2016, 108, djw100. [Google Scholar] [CrossRef]
- Da Silva, F.R.; Cibulski, S.P.; Daudt, C.; Weber, M.N.; Guimarães, L.L.; Streck, A.F.; Mayer, F.Q.; Roehe, P.M.; Canal, C.W. Novel Bovine Papillomavirus Type Discovered by Rolling-Circle Amplification Coupled with Next-Generation Sequencing. PLoS ONE 2016, 11, e0162345. [Google Scholar] [CrossRef]
- Lunardi, M.; Alfieri, A.A.; Otonel, R.A.A.; Alcântara, B.K.; Rodrigues, W.B.; Miranda, A.B.; Alfieri, A.F. Genetic characterization of a novel bovine papillomavirus member of the Deltapapillomavirus genus. Vet. Microbiol. 2013, 162, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Literak, I.; Ogawa, T.; Jin, Z.; Shirasawa, H. Complete genomes and phylogenetic positions of bovine papillomavirus type 8 and a variant type from a European bison. Virus Genes 2007, 35, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Tomita, Y.; Okada, M.; Shirasawa, H. Complete genome and phylogenetic position of bovine papillomavirus type 7. J. Gen. Virol. 2007, 88, 1934–1938. [Google Scholar] [CrossRef] [PubMed]
- Savini, F.; Gallina, L.; Alberti, A.; Müller, M.; Scagliarini, A. Bovine papillomavirus type 7 in Italy: Complete genomes and sequence variants. Virus Genes 2016, 52, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Claus, M.P.; Lunardi, M.; Alfieri, A.A.; Otonel, R.A.A.; Sartori, D.; Fungaro, M.H.P. Multiple Bovine Papillomavirus Infections Associated with Cutaneous Papillomatosis in Brazilian Cattle Herds. Braz. Arch. Biol. Technol. 2009, 52, 93–98. [Google Scholar] [CrossRef]
- Tozato, C.C.; Lunardi, M.; Alfieri, A.F.; Otonel, R.A.A.; Di Santis, G.W.; De Alcântara, B.K.; Headley, S.A.; Alfieri, A.A. Teat papillomatosis associated with bovine papillomavirus types 6, 7, 9, and 10 in dairy cattle from Brazil. Braz. J. Microbiol. 2013, 44, 905–909. [Google Scholar] [CrossRef]
- Lunardi, M.; de Tozato, C.C.; Alfieri1, A.F.; de Alcântara, B.K.; Vilas-Boas, L.A.; Otonel, R.A.A.; Headley, S.A.; Alfieri, A.A. Genetic diversity of bovine papillomavirus types, including two putative new types, in teat warts from dairy cattle herds. Arch. Virol. 2016, 161, 1569–1577. [Google Scholar] [CrossRef]
- Da Silva, F.R.C.; Daudt, C.; Streeck, A.F.; Weber, M.N.; Filho, R.V.O.; Driemeier, D.; Canal, C.W. Genetic characterization of Amazonian bovine papillomavirus reveals the existence of four new putative types. Virus Genes 2015, 51, 77–84. [Google Scholar] [CrossRef]
- Roth, S.D.; Sapp, M.; Streeck, R.E.; Selinka, H.-C. Characterization of neutralizing epitopes within the major capsid protein of human papillomavirus type 33. Virol. J. 2006, 3, 1–11. [Google Scholar] [CrossRef]
- Shen-Gunther, J.; Cai, H.; Zhang, H.; Wang, Y. Abundance of HPV L1 Intra-Genotype Variants with Capsid Epitopic Modifications Found Within Low- and High-Grade Pap Smears with Potential Implications for Vaccinology. Front. Genet. 2019, 10, 489. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.C.R.; Batista, M.V.A.; Silva, M.A.R.; Balbino, V.Q.; Freitas, A.C. Detection of bovine papillomavirus types, co-infection and a putative new BPV11 subtype in cattle. Transbound. Emerg. Dis. 2012, 59, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, C.J.; Almeida, M.E.; Vicari, C.F.; Carvalho, C.; Yaguiu, A.; Freitas, A.C.; Beçak, W.; Stocco, R.C. Bovine papillomavirus DNA in milk, blood, urine, semen, and spermatozoa of bovine papillomavirus-infected animals. Genet. Mol. Res. 2009, 8, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.; Ignacchiti, M.; Giuriato, P.; Nunes, L.; Pereira-Júnior, O. Detecçao do papilomavírus bovino tipo 2 em bexigas de bovinos com hematúria enzoótica pela técnica de reaçao em cadeia de polimerase no Sul do Espirito Santo, Brasil. Rev. Bras. Med. Vet. 2012, 34, 146–151. [Google Scholar]
- Batista, M.V.A.; Silva, M.A.R.; Pontes, N.E.; Reis, M.C.; Corteggio, A.; Castro, R.S.; Borzacchiello, G.; Balbino, V.Q.; Freitas, A.C. Molecular epidemiology of bovine papillomatosis and the identification of a putative new virus type in Brazilian cattle. Vet. J. 2013, 197, 368–373. [Google Scholar] [CrossRef]
- Daudt, C.; Da Silva, F.R.C.; Lunardi, M.; Alves, C.B.D.T.; Weber, M.N.; Cibulski, S.P.; Alfieri, A.F.; Alfieri, A.A.; Canal, C.W. Papillomaviruses in ruminants: An update. Transbound. Emerg. Dis. 2018, 65, 1381–1395. [Google Scholar] [CrossRef]
- Campo, M.S.; Moar, M.H.; Jarrett, W.F.H.; Laird, H.M. A New Papillomavirus Assoiated with Alimentary Cancer in Cattle. Nature 1980, 286, 180–182. [Google Scholar] [CrossRef]
- Claus, M.P.; Lunardi, M.; Alfieri, A.F.; Ferracin, L.M.; Fungaro, M.H.P.; Alfieri, A.A. Identification of unreported putative new bovine papillomavirus types in Brazilian cattle herds. Vet. Microbiol. 2008, 132, 396–401. [Google Scholar] [CrossRef]
- Bianchi, R.M.; Alves, C.D.B.T.; Schwertz, C.I.; Panziera, W.; De Lorenzo, C.; Da Silva, F.S.; De Cecco, B.S.; Daudt, C.; Chaves, F.R.; Canal, C.W.; et al. Molecular and pathological characterization of teat papillomatosis in dairy cows in southern Brazil. Braz. J. Microbiol. 2020, 51, 369–375. [Google Scholar] [CrossRef]
- Silva, M.A.R.; Pontes, N.E.; Da Silva, K.M.G.; Guerra, M.M.P.; Freitas, A.C. Detection of bovine papillomavirus type 2 DNA in commercial frozen semen of bulls (Bos taurus). Anim. Reprod. Sci. 2011, 129, 146–151. [Google Scholar] [CrossRef]
- Da Agricultura, B.M.; Abastecimento, P.E. Instrução Normativa no 13, de 3 de março de 2020. Diário. União 2020, 44, 6–8. [Google Scholar]
- Da Agricultura, B.M.; Abastecimento, P.E. Instrução Normativa SDA no 48, de 17 de junho de 2003. Diário. União 2003, 1, 6–7. [Google Scholar]
- De Falco, F.; Gentile, I.; Cerino, P.; Cutarelli, A.; Catoi, C.; Roperto, S. Prohibitin 2 is Involved in Parkin-Mediated Mitophagy in Urothelial Cells of Cattle Infected with Bovine Papillomavirus. Pathogens 2020, 9, 621. [Google Scholar] [CrossRef] [PubMed]
- Forslund, O.; Antonsson, A.; Nordin, P.; Stenquist, B.; Hansson, B.G. A broad range of human papillomavirus types detected with a general PCR method suitable for analysis of cutaneous tumours and normal skin. J. Gen. Virol. 1999, 80, 2437–2443. [Google Scholar] [CrossRef]
- Staden, R. The Staden Sequence Analysis Package. Mol. Biotechnol. 1996, 5, 233–241. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and high-performance computing. Nat. Methods 2015, 9, 772. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Sali, A.; Blundell, T.L. Comparative Protein Modelling by Satisfaction of Spatial Restraints. J. Mol. Biol. 1993, 234, 779–815. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhang, Y. Improving the Physical Realism and Structural Accuracy of Protein Models by a Two-Step Atomic-Level Energy Minimization. Biophys. J. 2011, 101, 2525–2534. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, D.; Nowotny, J.; Cao, R.; Cheng, J. 3Drefine: An interactive web server for efficient protein structure refinement. Nucleic Acids Res. 2016, 44, 406–409. [Google Scholar] [CrossRef]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Pandurangan, A.P.; Ochoa-Montaño, B.; Ascher, D.B.; Blundell, T.L. SDM: A server for predicting effects of mutations on protein stability. Nucleic Acids Res. 2017, 45, 229–235. [Google Scholar] [CrossRef] [PubMed]
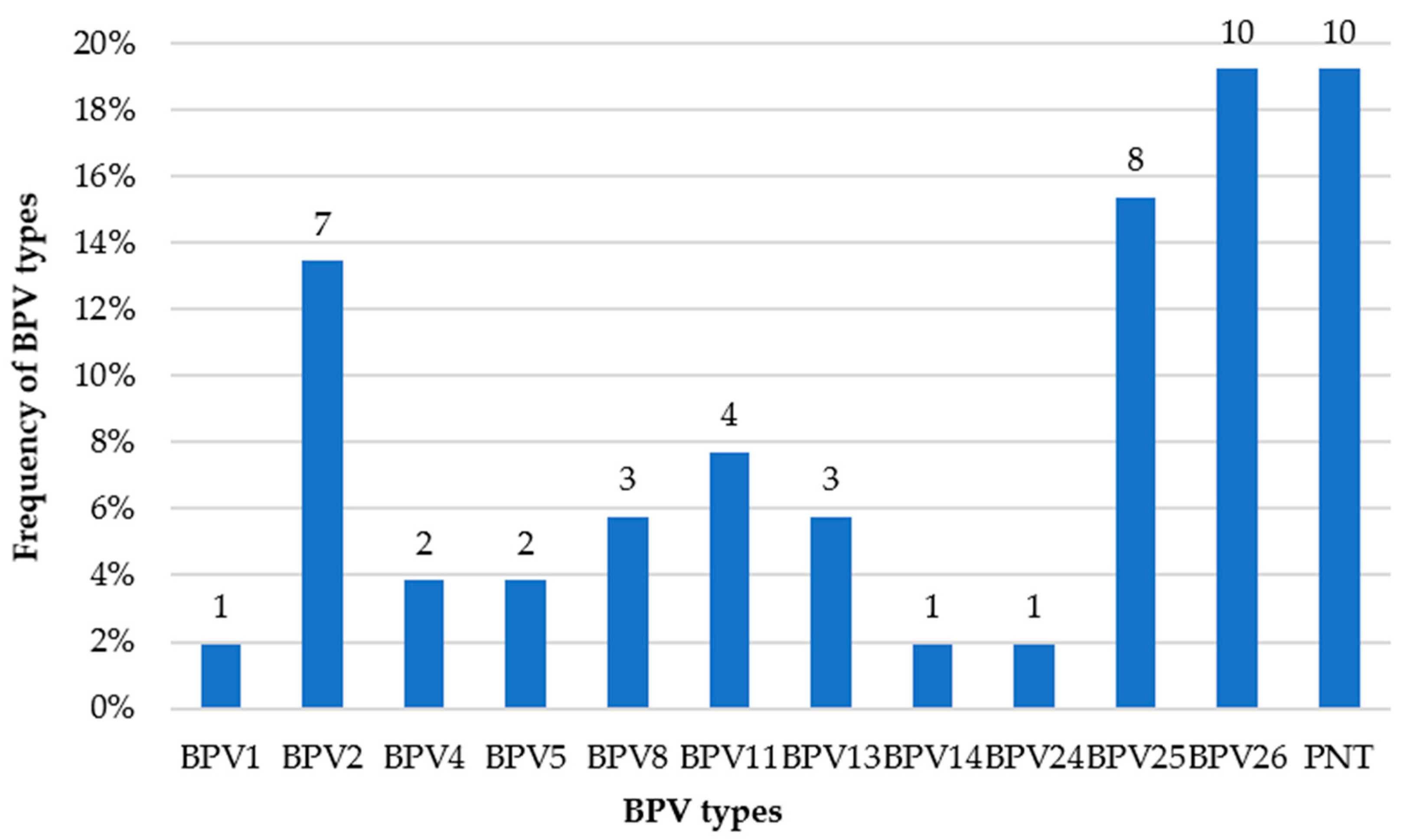
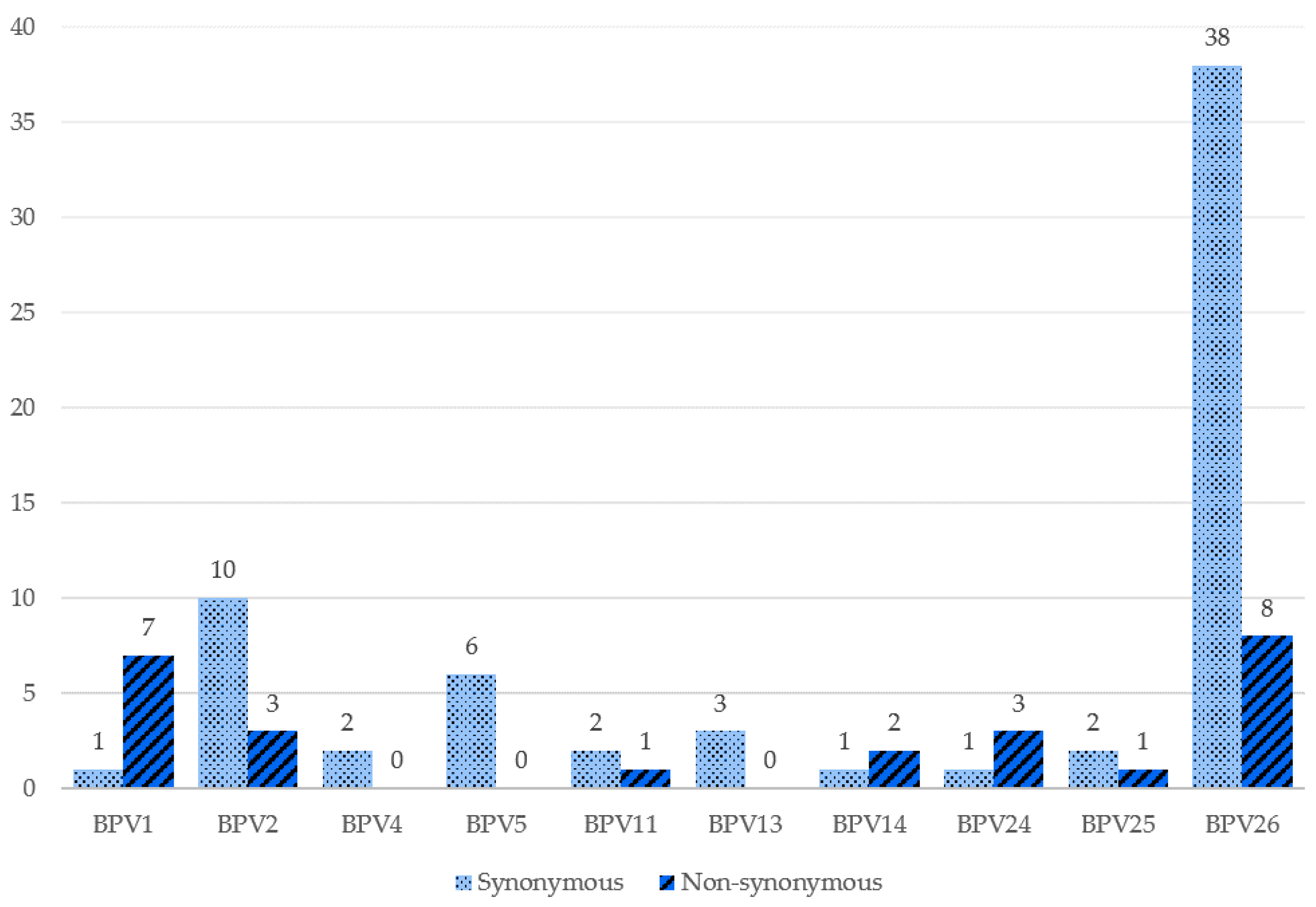

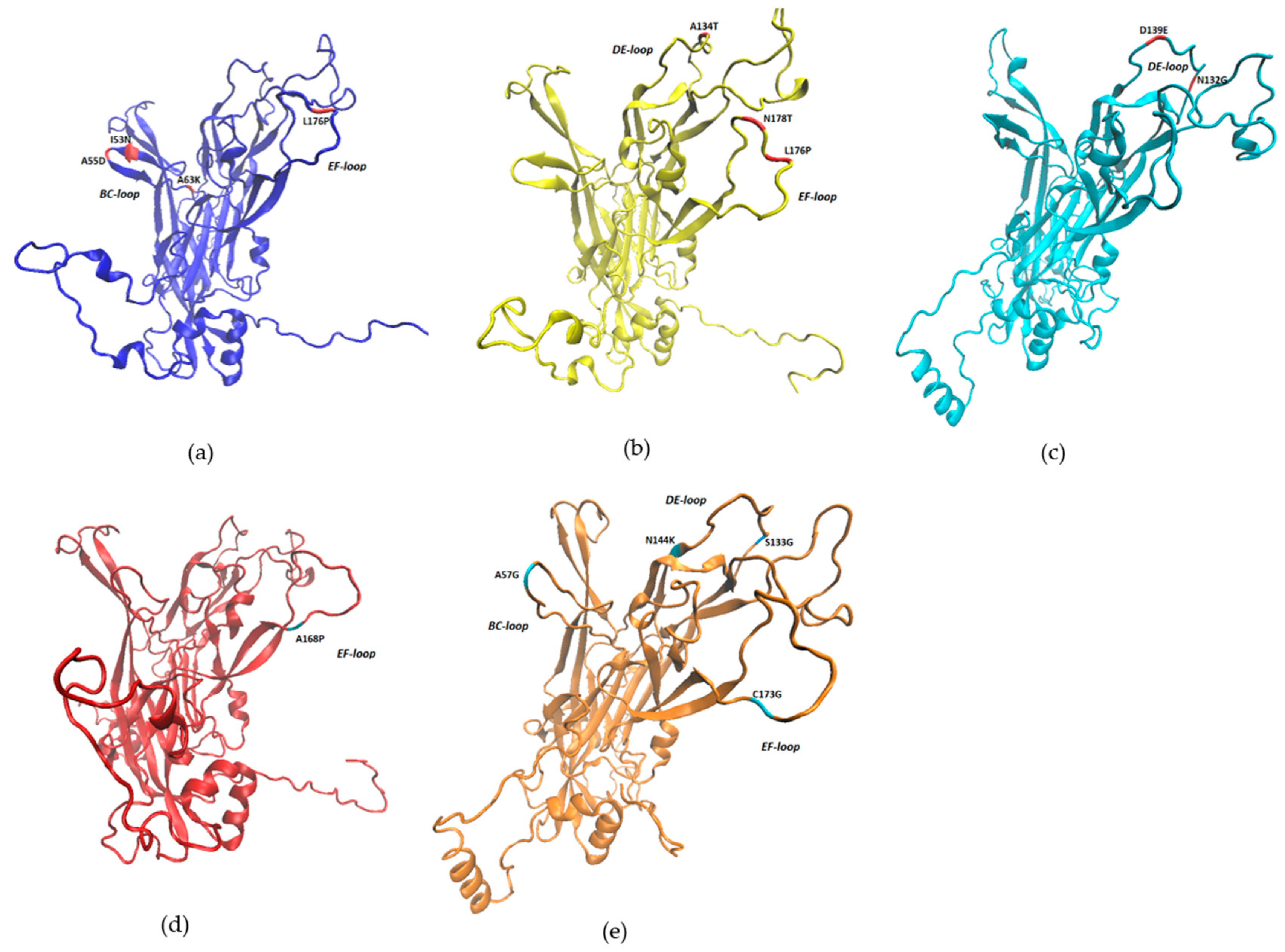
| Sample | Identity | Reference Type | Isolate with the Highest Identity | GenBank Accession Number |
|---|---|---|---|---|
| BPVUFSBR-19 | 87.8% | BPV26 | BPV26: 87.8% | MG281846 |
| BPVUFSBR-20 | 86.06% | BPV26 | BR-UEL2: 86.62% | EU293538 |
| BPVUFSBR-28 | 76.39% | BPV3 | BR-UEL6: 95.92% | KP892554 |
| BPVUFSBR-30 | 78.36% | BPV26 | BR-UEL2: 85.67% | GQ471901 |
| BPVUFSBR-31 | 77.25% | BPV3 | BR-UEL6: 98.42% | KP892554 |
| BPVUFSBR-34 | 77.81% | BPV12 | 06AC14: 93.88% | KP701433 |
| BPVUFSBR-36 | 79.57% | BPV24 | BR-UEL3: 99.78% | EU293539 |
| BPVUFSBR-44 | 78.38% | BPV3 | SW03: 79.21% | KF751802 |
| BPVUFSBR-45 | 77.63% | BPV12 | 06AC14: 93.88% | EU293539 |
| BPVUFSBR-49 | 74.13% | BPV3 | BR-UEL6: 98.43% | KX924620 |
| BPV Type | Mutation | ΔΔG | Structure Stability |
|---|---|---|---|
| BPV1 | I53N | −1.65 | Reduced |
| A55D | −0.02 | Reduced | |
| A63K | −2.79 | Reduced | |
| R105I | 0.76 | Increased | |
| L176P | 0.58 | Increased | |
| BPV2 | A134T | −0.93 | Reduced |
| L176P | −1.46 | Reduced | |
| N178T | 0.05 | Increased | |
| BPV11 | K189R | −0.16 | Reduced |
| BPV14 | H220P | −1.98 | Reduced |
| I223K | −2.6 | Reduced | |
| BPV24 | N132G | 0.9 | Increased |
| D139E | 0.53 | Increased | |
| BPV25 | A168P | −1.37 | Reduced |
| BPV26 | A57G | 0.57 | Increased |
| P85Q | −0.08 | Reduced | |
| Y90N | −2.24 | Reduced | |
| P92H | 0.76 | Increased | |
| S133G | 0.59 | Increased | |
| N144K | 0.82 | Increased | |
| C173G | −0.29 | Reduced | |
| N190I | 1.7 | Increased |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueirêdo, R.P.; Santos, G.F.; Oliveira, L.B.; Santos, L.A.B.O.; Barreto, D.M.; Cândido, A.L.; Campos, A.C.; Azevedo, E.O.; Batista, M.V.A. High Genotypic Diversity, Putative New Types and Intra-Genotype Variants of Bovine Papillomavirus in Northeast Brazil. Pathogens 2020, 9, 748. https://doi.org/10.3390/pathogens9090748
Figueirêdo RP, Santos GF, Oliveira LB, Santos LABO, Barreto DM, Cândido AL, Campos AC, Azevedo EO, Batista MVA. High Genotypic Diversity, Putative New Types and Intra-Genotype Variants of Bovine Papillomavirus in Northeast Brazil. Pathogens. 2020; 9(9):748. https://doi.org/10.3390/pathogens9090748
Chicago/Turabian StyleFigueirêdo, Rebeca P., Gabriela F. Santos, Luana B. Oliveira, Lucas A. B. O. Santos, Débora M. Barreto, Alexandre L. Cândido, Ana C. Campos, Edisio O. Azevedo, and Marcus V. A. Batista. 2020. "High Genotypic Diversity, Putative New Types and Intra-Genotype Variants of Bovine Papillomavirus in Northeast Brazil" Pathogens 9, no. 9: 748. https://doi.org/10.3390/pathogens9090748
APA StyleFigueirêdo, R. P., Santos, G. F., Oliveira, L. B., Santos, L. A. B. O., Barreto, D. M., Cândido, A. L., Campos, A. C., Azevedo, E. O., & Batista, M. V. A. (2020). High Genotypic Diversity, Putative New Types and Intra-Genotype Variants of Bovine Papillomavirus in Northeast Brazil. Pathogens, 9(9), 748. https://doi.org/10.3390/pathogens9090748






