Patterns of Ecological Adaptation of Aedes aegypti and Aedes albopictus and Stegomyia Indices Highlight the Potential Risk of Arbovirus Transmission in Yaoundé, the Capital City of Cameroon
Abstract
1. Introduction
2. Results
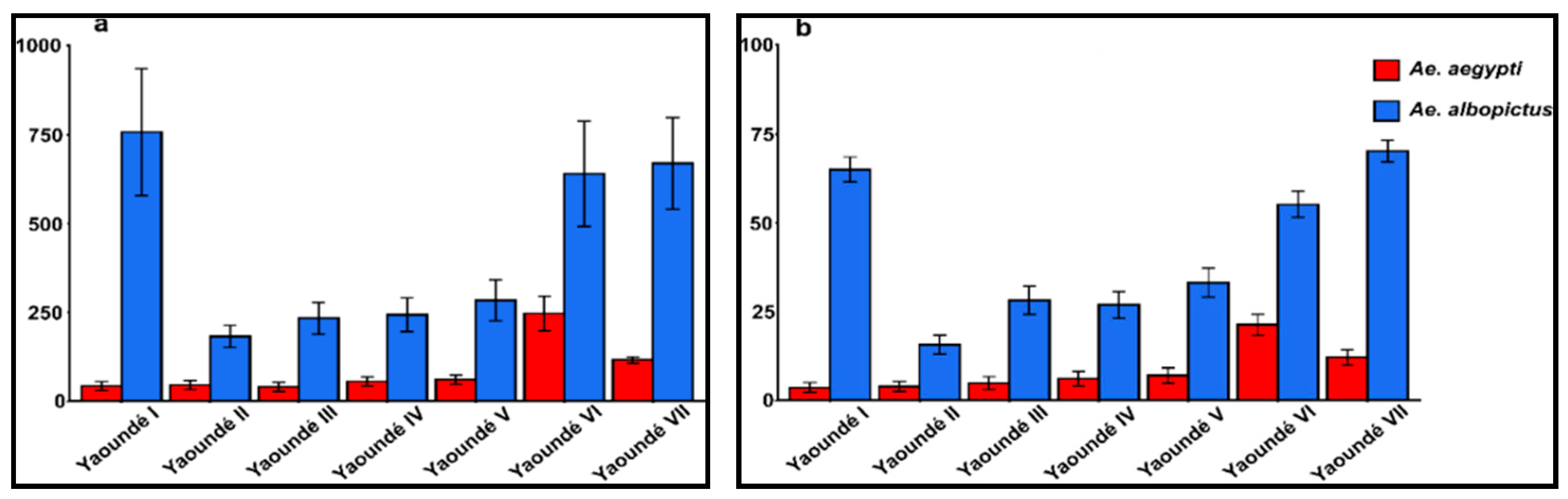
2.1. Pre-Imaginal Infestation
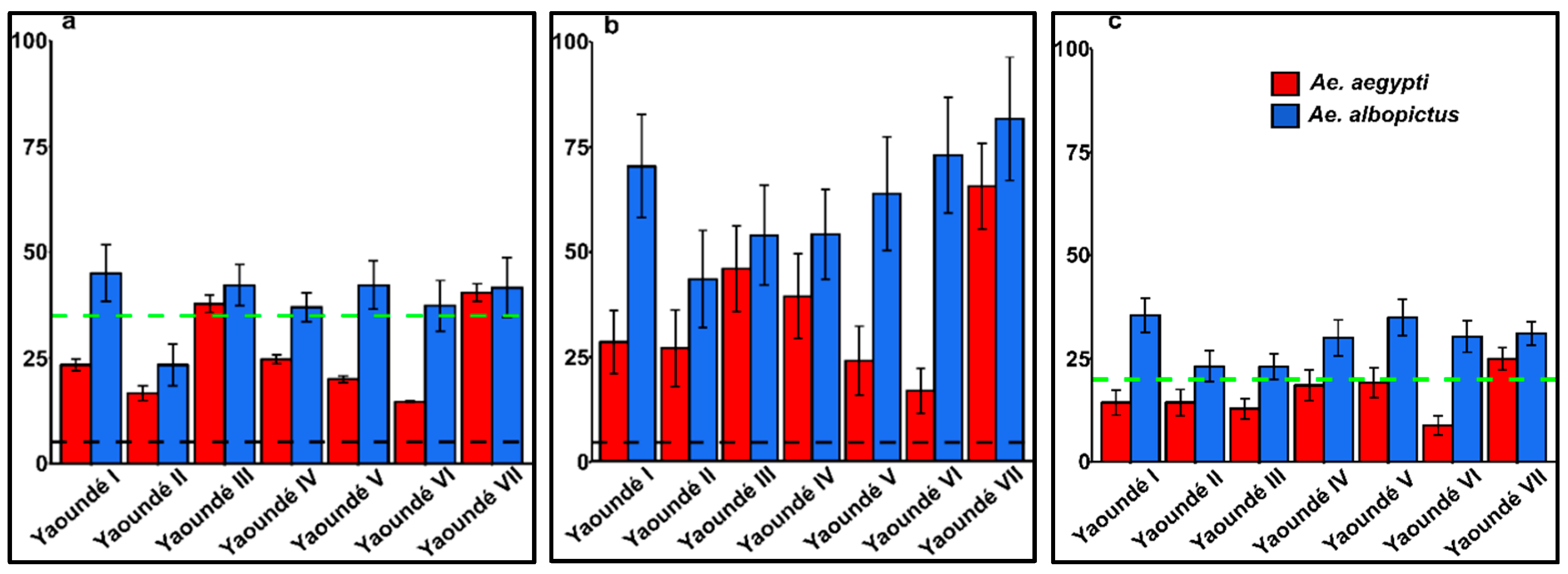
2.2. Larval/Pupal Indices and Risk of Dengue and Yellow Fever Transmission
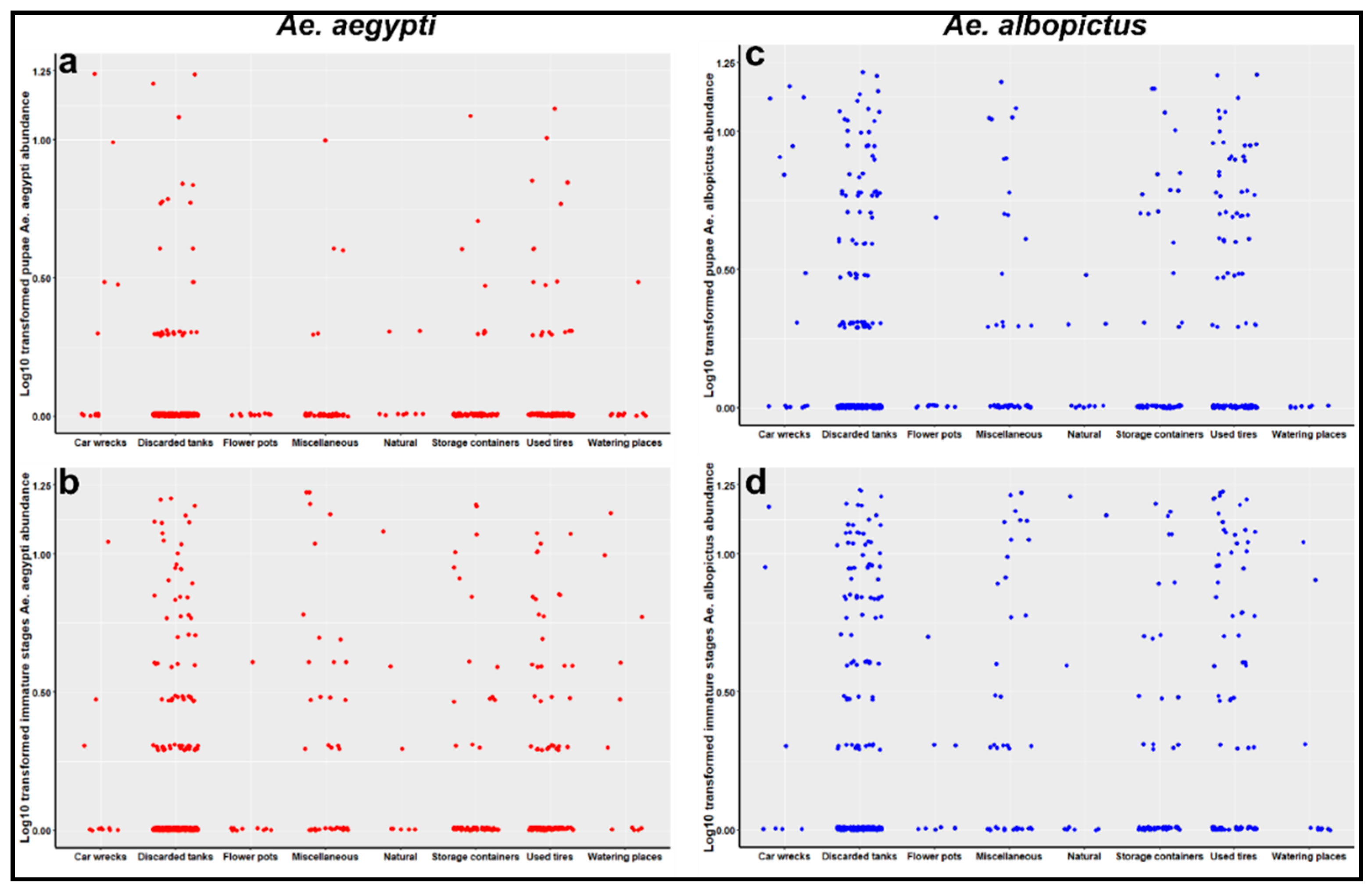
2.3. Container Prevalence and Preferences of Ae. albopictus and Ae. aegypti
2.4. Environmental Characteristics Influencing the Presence of Aedes Species
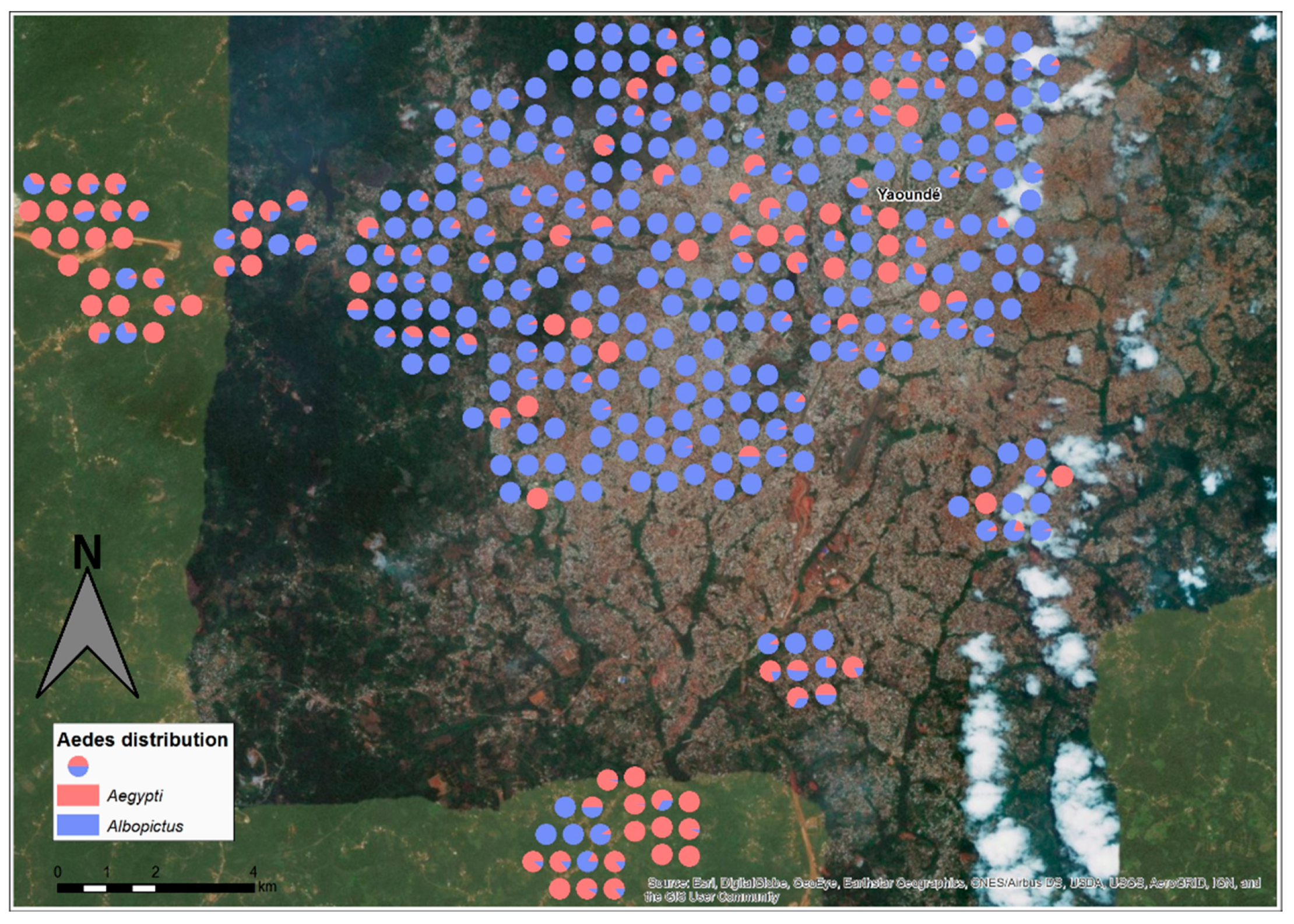
2.5. Spatial Distribution of Immature Stages of Ae. albopictus and Ae. aegypti
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
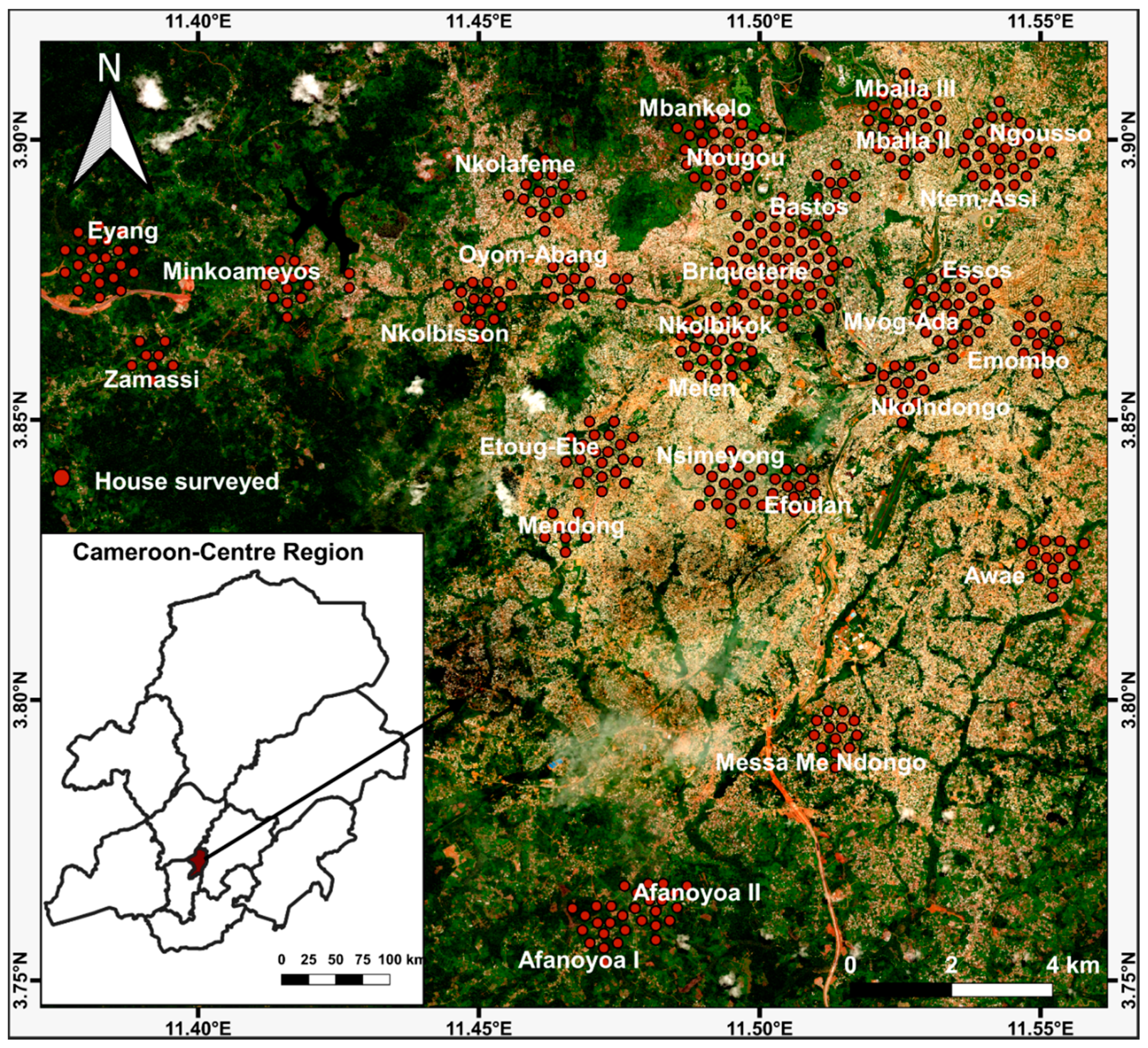
4.2. Study Sites
4.3. Study Design
4.4. Entomological Indices
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Global Vector Control Response 2017–2030; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Leta, S.; Beyene, T.J.; De Clercq, E.M.; Amenu, K.; Kraemer, M.U.G.; Revie, C.W. Global risk mapping for major diseases transmitted by Aedes aegypti and Aedes albopictus. Int. J. Infect. Dis. 2018, 67, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.; Sylla, M.; Goss, L.; Burugu, M.W.; Sang, R.; Kamau, L.W.; Kenya, E.U.; Bosio, C.; Munoz Mde, L.; Sharakova, M.; et al. Dual African origins of global Aedes aegypti s.l. populations revealed by mitochondrial DNA. PLoS Negl. Trop. Dis. 2013, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mattingly, P.F. Genetical Aspects of the Aedes aegypti Problem. Ann. Trop. Med. Parasit. 1957, 51, 392–408. [Google Scholar] [CrossRef] [PubMed]
- Gratz, N.G. Critical review of the vector status of Aedes albopictus. Med. Vet. Entomol. 2004, 18, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Enserink, M. A mosquito goes global. Science 2008, 320, 864–866. [Google Scholar] [CrossRef] [PubMed]
- Juliano, S.A.; Lounibos, L.P. Ecology of invasive mosquitoes: Effects on resident species and human health. Ecol. Lett. 2005, 8, 558–574. [Google Scholar] [CrossRef]
- Kraemer, M.U.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife 2015, 4, e08347. [Google Scholar] [CrossRef]
- Reiter, P. Aedes albopictus and the world of trade in used tires, 1988–1995: The shape of things to come. J. Am. Mosquito. Contr. 1998, 14, 83–94. [Google Scholar]
- Paupy, C.; Delatte, H.; Bagny, L.; Corbel, V.; Fontenille, D. Aedes albopictus, an arbovirus vector: From the darkness to the light. Microbes Infect. 2009, 11, 1177–1185. [Google Scholar] [CrossRef]
- Fontenille, D.; Toto, J.C. Aedes (Stegomyia) albopictus (Skuse), a Potential New Dengue Vector in Southern Cameroon. Emerg. Infect. Dis. 2001, 7, 1066–1067. [Google Scholar] [CrossRef]
- Ngoagouni, C.; Kamgang, B.; Nakouné, E.; Paupy, C.; Kazanji, M. Invasion of Aedes albopictus (Diptera: Culicidae) into central Africa: What consequences for emerging diseases? Parasit. Vectors 2015, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kamgang, B.; Yougang, A.P.; Tchoupo, M.; Riveron, J.M.; Wondji, C. Temporal distribution and insecticide resistance profile of two major arbovirus vectors Aedes aegypti and Aedes albopictus in Yaounde, the capital city of Cameroon. Parasit. Vectors 2017, 10, 469. [Google Scholar] [CrossRef] [PubMed]
- Braks, M.A.H.; Lourenco-De-Oliveira, R.; Juliano, S.A.; Honorio, N.A.; Lounibos, L.P. Convergent habitat segregation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in Southeastern Brazil and Florida. Fac. Publ. Biol. Sci. 2003, 40, 13. [Google Scholar] [CrossRef] [PubMed]
- Sivan, A.; Shriram, A.N.; Sunish, I.P.; Vidhya, P.T. Host-feeding pattern of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in heterogeneous landscapes of South Andaman, Andaman and Nicobar Islands, India. Parasitol. Res. 2015, 114, 3539–3546. [Google Scholar] [CrossRef] [PubMed]
- Ponlawat, A.; Harrington, L.C. Blood Feeding Patterns of Aedes aegypti and Aedes albopictus in Thailand. J. Med. Entomol. 2005, 42, 844–849. [Google Scholar] [CrossRef]
- Lounibos, L.P.; Bargielowski, I.; Carrasquilla, M.C.; Nishimura, N. Coexistence of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in Peninsular Florida Two Decades After Competitive Displacements. J. Med. Entomol. 2016, 53, 1385–1390. [Google Scholar] [CrossRef]
- Kamgang, B.; Happi, J.Y.; Boisier, P.; Njiokou, F.; Herve, J.P.; Simard, F.; Paupy, C. Geographic and ecological distribution of the dengue and chikungunya virus vectors Aedes aegypti and Aedes albopictus in three major Cameroonian towns. Med. Vet. Entomol. 2010, 24, 10. [Google Scholar] [CrossRef]
- Kamgang, B.; Ngoagouni, C.; Manirakiza, A.; Nakouné, E.; Paupy, C.; Kazanj, M. Temporal Patterns of Abundance of Aedes aegypti and Aedes albopictus (Diptera Culicidae) and Mitochondrial DNA analysis of Ae. albopictus in Central African Republic. PLoS Negl. Trop. Dis. 2013, 7, 12. [Google Scholar] [CrossRef]
- Weetman, D.; Kamgang, B.; Badolo, A.; Moyes, C.L.; Shearer, F.M.; Coulibaly, M.; Pinto, J.; Lambrechts, L.; McCall, P.J. Aedes Mosquitoes and Aedes-borne arboviruses in Africa: Current and future threats. Int. J. Environ. Res. Public. Health 2018, 15, 220. [Google Scholar] [CrossRef]
- O’Meara, G.F.; Evans, L.F., Jr.; Gettman Alan, D.; Cuda, J.P. Spread of Aedes albopictus and decline of Aedes aegypti in Florida. J. Med. Entomol. 1995, 32, 9. [Google Scholar]
- Bracks, M.A.H.; Honorio, N.A.; Lounibos, L.P.; Lourenço-De-Oliveira, R.; Juliano, S.A. Interspecific competition between two invasive species of container mosquitoes, Aedes aegypti and Aedes albopictus (Diptera: Culicidae), in Brazil. Ann. Entomol. Soc. Am. 2004, 97, 130–139. [Google Scholar] [CrossRef]
- Honório, N.A.; Da Costa Silva, W.; José Leite, P.; Gonçalves, J.M.; Lounibos, L.P.; Lourenço-de-Oliveira, R. Dispersal of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in an Urban Endemic Dengue Area in the State of Rio de Janeiro, Brazil. Mem. Inst. Oswaldo Cruz 2003, 98, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Rey, J.R.; Nishimura, N.; Wagner, B.; Braks, M.A.H.; O’Connell, S.M.; Lounibos, L.P. Habitat segregation of mosquito Arbovirus in South Florida. J. Med. Entomol. 2006, 43, 7. [Google Scholar] [CrossRef]
- Juliano, S.A.; Lounibos, L.P.; O’Meara, G.F. A field test for competitive effects of Aedes albopictus on A. aegypti in South Florida: Differences between sites of coexistence and exclusion. Oecologia 2004, 139, 19. [Google Scholar] [CrossRef]
- Muzari, M.; Davis, J.; Bellwood, R.; Crunkhorn, B.; Gunn, E.; Sabatino, U.; Gair, R. Dominance of the tiger: The displacement of Aedes aegypti by Aedes albopictus in parts of the Torres Strait, Australia. Commun. Dis. Intell. 2019, 43. [Google Scholar] [CrossRef]
- Bagny, L.; Delatte, H.; Quilici, S.; Fontenille, D. Progressive decrease in Aedes aegypti distribution in Reunion Island since the 1900s. J. Med. Entomol. 2009, 46, 1541–1545. [Google Scholar] [CrossRef]
- Salvan, M.; Mouchet, J. Aedes albopictus et Aedes aegypti à l’île de la Reunion. Ann. Soc. Belg. Med. Trop. 1994, 74, 323–326. [Google Scholar]
- Bagny Beilhe, L.; Arnoux, S.; Delatte, H.; Lajoie, G.; Fontenille, D. Spread of invasive Aedes albopictus and decline of resident Aedes aegypti in urban areas of Mayotte 2007–2010. Biol. Invasions 2012, 14, 1623–1633. [Google Scholar] [CrossRef]
- Kamgang, B.; Wilson-Bahun, T.A.; Irving, H.; Kusimo, M.O.; Lenga, A.; Wondji, C.S. Geographical distribution of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) and genetic diversity of invading population of Aedes albopictus in the Republic of the Congo. Wellcome Open Res. 2018, 3, 1–17. [Google Scholar] [CrossRef]
- Seng, M.C.; Jute, N. Breeding of Aedes aegypti and Aedes albopictus in urban housing of Sibu town, Sarawak. Southeast Asian J. Trop. Med. Public Health 1994, 25, 543–548. [Google Scholar]
- Chan, Y.C.; Chan, K.L.; Ho, B.C. Aedes aegypti and Aedes albopictus in Singapore city: Distribution and density. Bull. World Health Org. 1971, 44, 617–627. [Google Scholar] [PubMed]
- Tedjou, A.N.; Kamgang, B.; Yougang, A.P.; Njiokou, F.; Wondji, C.S. Update on the geographical distribution and prevalence of Aedes aegypti and Aedes albopictus (Diptera: Culicidae), two major arbovirus vectors in Cameroon. PLoS Negl. Trop. Dis. 2019, 13, e0007137. [Google Scholar] [CrossRef] [PubMed]
- Simard, F.; Nchoutpouen, E.; Toto, J.C.; Fontenille, D. Geographic Distribution and Breeding Site Preference of Aedes albopictus and Aedes aegypti (Diptera: Culicidae) in Cameroon, Central Africa. J. Med. Entomol. 2005, 42, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Tchuandom, S.B.; Tchadji, J.C.; Tchouangueu, T.F.; Biloa, M.Z.; Atabonkeng, E.P.; Fumba, M.I.M.; Massom, E.S.; Nchinda, G.; Kuiate, J.R. A cross-sectional study of acute dengue infection in paediatric clinics in Cameroon. BMC Public Health 2019, 19, 958. [Google Scholar] [CrossRef] [PubMed]
- Nemg Simo, F.B.; Sado Yousseu, F.B.; Evouna Mbarga, A.; Bigna, J.J.; Melong, A.; Ntoude, A.; Kamgang, B.; Bouyne, R.; Moundipa Fewou, P.; Demanou, M. Investigation of an Outbreak of Dengue Virus Serotype 1 in a Rural Area of Kribi, South Cameroon: A Cross-Sectional Study. Intervirology 2018, 61, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Yousseu, S.F.B.; Nemg, S.F.B.; Ngouanet, A.S.; Mekanda, O.F.M.; Demanou, M. Detection and serotyping of dengue viruses in febrile patients consulting at the new-bell district hospital in Douala, Cameroon. PLoS ONE 2018, 13, e0204143. [Google Scholar] [CrossRef]
- Tchuandom, S.B.; Tchouangueu, T.F.; Antonio-Nkondjio, C.; Lissom, A.; Ngono Djang, J.O.; Atabonkeng, E.P.; Kechia, A.; Nchinda, G.; Kuiate, J.-R. Seroprevalence of dengue virus among children presenting with febrile illness in some public health facilities in Cameroon. Pan Afr. Med. J. 2018, 31, 31–38. [Google Scholar] [CrossRef]
- Monamele, G.C.; Demanou, M. First documented evidence of dengue and malaria co-infection in children attending two health centers in Yaounde, Cameroon. Pan Afr. Med. J. 2018, 29, 227. [Google Scholar] [CrossRef]
- Kamgang, B.; Vazeille, M.; Yougang, A.P.; Tedjou, A.N.; Wilson-Bahun, T.A.; Mousson, L.; Wondji, C.S.; Failloux, A.B. Potential of Aedes albopictus and Aedes aegypti (Diptera: Culicidae) to transmit yellow fever virus in urban areas in Central Africa. Emerg. Microbes Infect. 2019, 8, 1636–1641. [Google Scholar] [CrossRef]
- Kamgang, B.; Vazeille, M.; Tedjou, A.N.; Wilson-Bahun, T.A.; Yougang, A.P.; Mousson, L.; Wondji, C.S.; Failloux, A.B. Risk of dengue in Central Africa: Vector competence studies with Aedes aegypti and Aedes albopictus (Diptera: Culicidae) populations and dengue 2 virus. PLoS Negl. Trop. Dis. 2020, 13, e0007985. [Google Scholar] [CrossRef]
- Kamgang, B.; Vazeille, M.; Tedjou, A.; Yougang, A.P.; Wilson-Bahun, T.A.; Mousson, L.; Wondji, C.S.; Failloux, A.-B. Different populations of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) from Central Africa are susceptible to Zika virus infection. PLoS Negl. Trop. Dis. 2020, 14, e0008163. [Google Scholar] [CrossRef] [PubMed]
- PAHO. Dengue and Dengue Hemorrhagic Fever in the Americas: Guidelines for Prevention and Control; Pan American Health Organisation: Washington, DC, USA, 1994. [Google Scholar]
- WHO. Technical Guide for a System of Yellow Fever Surveillance; World Health Organisation: Geneva, Switzerland, 1971. [Google Scholar]
- Camara, D.C.; Codeco, C.T.; Juliano, S.A.; Lounibos, L.P.; Riback, T.I.; Pereira, G.R.; Honorio, N.A. Seasonal Differences in Density But Similar Competitive Impact of Aedes albopictus (Skuse) on Aedes aegypti (L.) in Rio de Janeiro, Brazil. PLoS ONE 2016, 11, e0157120. [Google Scholar] [CrossRef] [PubMed]
- Juliano, S.A.; O’Meara, G.F.; Morrill, J.R.; Cutwa, M.M. Desiccation and thermal tolerance of eggs and the coexistence of competing mosquitoes. Oecologia 2002, 130, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Tripet, F.; Lounibos, L.P.; Robbins, D.; Moran, J.; Nishimura, N.; Blosser, E.M. Competitive reduction by satyrization? Evidence for interspecific mating in nature and asymmetric reproductive competition between invasive mosquito vectors. Am. J. Trop. Med. Hyg. 2011, 85, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Bargielowski, I.E.; Lounibos, L.P.; Carrasquilla, M.C. Evolution of resistance to satyrization through reproductive character displacement in populations of invasive dengue vectors. Proc. Natl. Acad. Sci. USA 2013, 110, 2888–2892. [Google Scholar] [CrossRef]
- Bargielowski, I.E.; Lounibos, L.P. Satyrization and satyrization-resistance in competitive displacements of invasive mosquito species. Insect Sci. 2016, 23, 162–174. [Google Scholar] [CrossRef]
- Reiskind, M.H.; Lounibos, L.P. Spatial and temporal patterns of abundance of Aedes aegypti L. (Stegomyia aegypti) and Aedes albopictus (Skuse) [Stegomyia albopictus (Skuse)] in southern Florida. Med. Vet. Entomol. 2013, 27, 421–429. [Google Scholar] [CrossRef]
- Lounibos, L.P. Invasions by insect vectors of Human disease. Annu. Rev. Entomol. 2002, 47, 233–266. [Google Scholar] [CrossRef]
- Hammond, S.N.; Gordon, A.L.; Lugo, E.D.C.; Moreno, G.; Kuan, G.M.; López, M.M.; López, J.D.; Delgado, M.A.; Valle, S.I.; Espinoza, P.M. Characterization of Aedes aegypti (Diptera: Culcidae) production sites in urban Nicaragua. J. Med. Entomol. 2007, 44, 851–860. [Google Scholar] [CrossRef]
- Dutta, P.; Khan, S.A.; Khan, A.M.; Sharma, C.K.; Doloi, P.K.; Mahanta, J. Solid waste pollution and breeding potential of dengue vectors in urban and industrial environmentof Assam. J. Environ. Biol. 1999, 20, 343–345. [Google Scholar]
- Duccombe, J.; Espino, F.; Marollano, K.; Velazco, A.; Ritchie, S.A.; Wenbiao, H.; Weinstein, P.; Clements, A.C.A. Characterising the spatial dynamics of sympatric Aedes aegypti and Aedes albopictus populations in the Philippines. Geospat. Health 2013, 8, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Barrera, R.; Amador, M.; Clark, G.G. Ecological factors influencing Aedes aegypti (Diptera: Culicidae) productivity in artificial containers in Salinas, Puerto Rico. J. Med. Entomol. 2006, 43, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Grillet, M.E.; Ramos, O.M.; Amador, M.; Barrera, R. Habitat segregation of dengue vectors along an urban environmental gradient. Am. J. Trop. Med. Hyg. 2007, 76, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Alto, B.W.; Lounibos, L.P.; Mores, C.N.; Reiskind, M.H. Larval competition alters susceptibility of adult Aedes mosquitoes to dengue infection. Proc. Biol. Sci. 2008, 275, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Lutomiah, J.; Barrera, R.; Makio, A.; Mutisya, J.; Koka, H.; Owaka, S.; Koskei, E.; Nyunja, A.; Eyase, F.; Coldren, R.; et al. Dengue outbreak in Mombasa city, Kenya, 2013–2014: Entomologic investigations. PLoS Negl. Trop. Dis. 2016, 10, e0004981. [Google Scholar] [CrossRef] [PubMed]
- Getachew, D.; Tekie, H.; Gebre-Michael, T.; Balkew, M.; Mesfin, A. Breeding Sites of Aedes aegypti: Potential Dengue Vectors in Dire Dawa, East Ethiopia. Interdiscip. Perspect. Infect. Dis. 2015, 2015, 706276. [Google Scholar] [CrossRef]
- Mboera, L.E.; Mweya, C.N.; Rumisha, S.F.; Tungu, P.K.; Stanley, G.; Makange, M.R.; Misinzo, G.; De Nardo, P.; Vairo, F.; Oriyo, N.M. The risk of dengue virus transmission in Dar es Salaam, Tanzania during an epidemic period of 2014. PLoS Negl. Trop. Dis. 2016, 10, e0004313. [Google Scholar] [CrossRef]
- BUCREP. Projections Démographiques, 2010th ed.; BUCREP: Yaoundé, Cameroun, 2010; Volume 3, p. 91. [Google Scholar]
- Letouzey, R. Notice de la Carte Phytogéographique du Cameroun au 1:500 000 (1985); Institut de la Carte Internationale de la Végétation: Toulouse, France, 1985; p. 50. [Google Scholar]
- Anguh Nkwemoh, C.; Tchindjang, M.; Ngwatung Afungang, R. The impact of urbanization on the vegetation of Yaoundé, (Cameroon). Int. J. Innov. Res. Dev. 2017, 6, 6–18. [Google Scholar] [CrossRef]
- Jupp, P.G. Mosquitoes of Southern Africa. Culicinae and Toxorhynchitinae; Ekogilde: Hartebeespoort, South Africa, 1996; p. 164. [Google Scholar]
- Edwards, J. Mosquitoes of the Ethiopian Region; Oxford University Press: Oxford, UK, 1941; p. 513. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- QGIS Development Team. QGIS 3.8.1 Geographic Information System. Open Source Geospatial Foundation Project. 2019. Available online: https://www.npackd.org/p/qgis/3.8.1 (accessed on 2 May 2012).
| Type of Containers | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Domestic | Peri-Domestic | Natural ** | Total | |||||||
| Boroughs | Watering Place | Flowerpots | Storage Containers | Used Tyres | Discarded Tanks | Car Wrecks | Miscellaneous * | |||
| Yaoundé I | N | 3 | 4 | 15 | 23 | 52 | 2 | 12 | 1 | 112 |
| % Positive | 33.3 | 25 | 53.3 | 47.8 | 38.5 | 50 | 58.3 | 0 | 4.4 | |
| % Ae. albopictus only | 0 | 100 | 37.5 | 72.7 | 55 | 100 | 85.7 | 0 | 61.2 | |
| % Ae. aegypti only | 0 | 0 | 0 | 9.1 | 5 | 0 | 0 | 0 | 4.1 | |
| % Mixed | 100 | 0 | 62.5 | 18.2 | 40 | 0 | 14.3 | 0 | 34.7 | |
| Yaoundé II | N | 0 | 9 | 24 | 21 | 34 | 3 | 6 | 97 | |
| % Positive | 0 | 0 | 12.5 | 66.7 | 23.5 | 33.3 | 66.7 | 3.1 | ||
| % Ae. albopictus only | 0 | 0 | 0 | 42.9 | 62.5 | 0 | 25 | 0 | 40 | |
| % Ae. aegypti only | 0 | 0 | 0 | 7.1 | 0 | 0 | 0 | 0 | 3.3 | |
| % Mixed | 0 | 0 | 100 | 50 | 37.5 | 100 | 75 | 0 | 56.7 | |
| Yaoundé III | N | 1 | 1 | 25 | 13 | 119 | 2 | 9 | 7 | 177 |
| % Positive | 0 | 100 | 28 | 30.8 | 25.2 | 100 | 44.4 | 28.6 | 2.8 | |
| % Ae. albopictus only | 0 | 100 | 57.1 | 75 | 50 | 50 | 25 | 50 | 52 | |
| % Ae. aegypti only | 0 | 0 | 14.3 | 25 | 10 | 50 | 25 | 0 | 14 | |
| % Mixed | 0 | 0 | 28.6 | 0 | 40 | 0 | 50 | 50 | 34 | |
| Yaoundé IV | N | 0 | 4 | 6 | 14 | 59 | 1 | 6 | 0 | 90 |
| % Positive | 0 | 50 | 33.3 | 57.1 | 32.2 | 0 | 83.3 | 0 | 4 | |
| % Ae. albopictus only | 0 | 100 | 50 | 25 | 42.1 | 0 | 40 | 0 | 41.7 | |
| % Ae. aegypti only | 0 | 0 | 0 | 0 | 10.5 | 0 | 0 | 0 | 5.6 | |
| % Mixed | 0 | 0 | 50 | 75 | 47.4 | 0 | 60 | 0 | 52.8 | |
| Yaoundé V | N | 5 | 1 | 19 | 11 | 45 | 9 | 7 | 3 | 100 |
| % Positive | 60 | 100 | 47.4 | 54.6 | 44.4 | 44.4 | 42.9 | 66.7 | 4.8 | |
| % Ae. albopictus only | 0 | 100 | 66.7 | 50 | 60 | 50 | 0 | 50 | 52.1 | |
| % Ae. aegypti only | 66.7 | 0 | 0 | 0 | 15 | 0 | 0 | 50 | 12.5 | |
| % Mixed | 33.3 | 0 | 33.3 | 50 | 25 | 50 | 100 | 0 | 35.4 | |
| Yaoundé VI | N | 0 | 2 | 3 | 27 | 77 | 3 | 20 | 3 | 135 |
| % Positive | 0 | 0 | 0 | 55.6 | 31.2 | 0 | 60 | 0 | 3.8 | |
| % Ae. albopictus only | 0 | 0 | 0 | 86.7 | 70.8 | 0 | 66.7 | 0 | 74.5 | |
| % Ae. aegypti only | 0 | 0 | 0 | 6.7 | 12.5 | 0 | 16.7 | 0 | 11.8 | |
| % Mixed | 0 | 0 | 0 | 6.7 | 16.7 | 0 | 16.7 | 0 | 13.7 | |
| Yaoundé VII | N | 8 | 5 | 30 | 62 | 119 | 3 | 15 | 2 | 244 |
| % Positive | 25 | 40 | 10 | 48.4 | 37 | 100 | 66.7 | 100 | 3.9 | |
| % Ae. albopictus only | 0 | 50 | 33.3 | 56.7 | 15.9 | 33.33 | 30 | 50 | 32.3 | |
| % Ae. aegypti only | 0 | 0 | 0 | 0 | 25 | 0 | 30 | 50 | 15.6 | |
| % Mixed | 100 | 50 | 66.7 | 43.3 | 59.1 | 66.67 | 40 | 0 | 52.1 | |
| Total | N | 17 | 26 | 122 | 171 | 505 | 23 | 75 | 16 | 955 |
| % Positive | 35.3 | 26.9 | 26.2 | 51.5 | 32.7 | 47.83 | 60 | 37.5 | 37.7 | |
| % Ae. albopictus only | 0 | 85.7 | 46.9 | 59.1 | 45.5 | 45.45 | 46.7 | 50 | 49.2 | |
| % Ae. aegypti only | 33.3 | 0 | 3.1 | 4.6 | 13.9 | 9.1 | 13.33 | 33.3 | 10.8 | |
| % Mixed | 66.7 | 14.3 | 50 | 36.4 | 40.6 | 45.45 | 40 | 16.67 | 40 | |
| Type of Containers | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Domestic | Peri-Domestic | Natural ** | Total n (%) | ||||||
| Species | Watering Places n (%) | Flowerpots n (%) | Storage Containers n (%) | Used Tyres n (%) | Discarded Tanks n (%) | Car Wrecks n (%) | Miscellaneous * n (%) | Natural n (%) | |
| Ae. albopictus | 42 (0.4) | 176 (1.6) | 654 (6.1) | 2763 (25.6) | 4008 (37.1) | 482 (4.5) | 982 (9.1) | 68 (0.6) | 9175 (85) |
| Ae. aegypti | 33 (0.3) | 3 (0.03) | 105 (1) | 214 (2) | 930 (8.6) | 105 (1) | 221 (2.1) | 15 (0.2) | 1626 (15) |
| Total | 75 (0.7) | 179 (1.7) | 759 (7) | 2977 (27.6) | 4938 (45.7) | 587(5.4) | 1203 (11.1) | 83 (0.8) | 10,801 (100) |
| Pupae | Larvae and Pupae | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ae. aegypti | Ae. albopictus | Ae. aegypti | Ae. albopictus | ||||||||
| Categories | Number | % | OR (CI 95%) | % | OR (CI 95%) | Number | % | OR (CI 95%) | % | OR (CI 95%) | |
| Distance to the nearest building (m) | medium [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20] | 70 | 27.8 | Ref | 29.9 | Ref | 133 | 33.3 | Ref | 31 | Ref |
| low (<5) | 163 | 66.7 | 1 (0.5–2.3) | 69.2 | 1 (0.7–1.4) | 272 | 60.6 | 0.8 (0.5–1.1) | 66.9 | 0.9 (0.7–1.2) | |
| high (>20) | 4 | 5.6 | 2.2 (0.3–8.8) | 1 | 0.3 (0.1–1) | 14 | 6.1 | 2.3 (0.9–5.3) | 2.1 | 0.6 (0.2–1.5) | |
| Distance to the nearest plant (m) | medium [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20] | 24 | 13.9 | Ref | 9.5 | Ref | 36 | 6.8 | Ref | 9.4 | Ref |
| low (<5) | 211 | 86.1 | 0.7 (0.3–2.1) | 89.6 | 1.1 (0.7–1.9) | 381 | 93.2 | 1.6 (0.8–3.6) | 89.9 | 1.1 (0.7–1.8) | |
| high (>20) | 2 | 0.00 (NA–1.6 × 1017) | 1 | 2.7 (0.3–17.4) | 2 | 0 (NA–2.6 × 1023) | 0.7 | 1.7 (0.2–10.8) | |||
| Distance to the ground (m) | medium [1,2,3] | 21 | 5.6 | Ref | 9.5 | Ref | 42 | 10.6 | Ref | 9.8 | Ref |
| high [3,4,5] | 2 | 2.8 | 21.5 (0.8–363.1) * | 0.5 | 1.8 (0.1–20) | 4 | 1.5 | 10.6 (1–236.8) | 0.7 | 4.3 (0.4–94.5) | |
| low (<1) | 214 | 91.7 | 1.7 (0.5–10.6) | 90 | 1 (0.6–1.7) | 373 | 87.9 | 0.8 (0.5–1.6) | 89.5 | 0.9 (0.6–1.5) | |
| Sun exposure | partially shaded | 104 | 33.3 | Ref | 45.8 | Ref | 186 | 40.9 | Ref | 46 | Ref |
| exposed | 94 | 47.2 | 2 (0.9–4.3) | 38.3 | 1.2 (0.8–1.7) | 170 | 44.7 | 1.6 (1.1–2.4) * | 38.7 | 1.20 (0.9–1.6) | |
| shaded | 39 | 19.4 | 1.8 (0.7–4.7) | 15.9 | 1.1 (0.7–1.7) | 63 | 14.4 | 1.1 (0.6–1.9) | 15.3 | 1.04 (0.7–1.6) | |
| Material | miscellaneous | 12 | 2.8 | Ref | 5.5 | Ref | 25 | 5.3 | Ref | 6.3 | Ref |
| rubber | 68 | 27.8 | 3.8 (0.7–69.6) | 28.9 | 2.6 (1.2–5.1) * | 101 | 16.7 | 1.2 (0.5–3) | 27.5 | 2.1 (1.1–4) * | |
| metal | 28 | 8.3 | 1.5 (0.2–29.9) | 12.4 | 1.13 (0.5–2.6) | 49 | 12.9 | 1.2 (0.5–3.3) | 11.1 | 0.8 (0.4–1.6) | |
| natural | 2 | 0 (0–3.2 × 1011) | 1 | 1.3 (0.2–6.3) | 2 | 0 | 0.7 | 0.7 (0.1–3.2) | |||
| plastic | 127 | 61.1 | 2.3 (0.5–42.1) | 52.2 | 1 (0.51–2.05) | 242 | 65.2 | 1.3 (0.6–3.3) | 54.4 | 0.9 (0.5–1.6) | |
| Capacity of the container (L) | high [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50] | 70 | 30.6 | Ref | 29.4 | Ref | 113 | 22 | Ref | 29.3 | Ref |
| medium [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20] | 82 | 38.9 | 0.86 (0.4–2) | 33.8 | 0.7 (0.5–1.1) | 138 | 29.5 | 0.9 (0.5–1.5) | 34.5 | 0.7 (0.5–1) | |
| low <5 | 72 | 25 | 0.5 (0.2–1.3) | 31.3 | 0.7 (0.4–1) * | 144 | 42.4 | 1.4 (0.8–2.2) | 30.7 | 0.6 (0.4–0.9) * | |
| very high > 50 | 13 | 5.6 | 0.6 (0.1–2.5) | 5.5 | 0.6 (0.3–1.2) | 24 | 6.1 | 1 (0.4–2.2) | 5.6 | 0.6 (0.3–1.1) | |
| Volume of water (L) | high [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50] | 5 | Ref | 2.5 | Ref | 9 | 1.5 | Ref | 2.4 | Ref | |
| medium [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20] | 42 | 16.7 | 4 × 106 (0–3.4 × 10168) | 17.9 | 1.3 (0.5–4.2) | 72 | 17.4 | 2.2 (0.6–14) | 17.1 | 1.3 (0.6–3.5) | |
| low <5 | 188 | 83.3 | 4.9 × 106 (0–4.1 × 10166) | 78.9 | 1.5 (0.6–4.3) | 335 | 80.3 | 2.5 (0.7–15.5) | 79.8 | 1.6 (0.7–4) | |
| very high > 50 | 2 | 1 (0–8.7 × 1015) | 1 | 0.5 (0.1–2.5) | 3 | 0.8 | 0.7 (0–7.3) | 0.7 | 0.3 (0.1–1.5) | ||
| Origin of water | well | 1 | Ref | 0.5 | Ref | 2 | 0 | Ref | 0.7 | Ref | |
| rain | 227 | 97.2 | 6.1 × 105 (0–NA) | 95.5 | 1.1 (0.2–20.6) | 407 | 98.5 | 942714.93 (0–NA) | 96.5 | 0.6 (0.1–4.9) | |
| tap | 9 | 2.8 | 6.5 × 105 (0–9.5 × 10164) | 4 | 1.9 (0.2–40) | 10 | 1.5 | 5 × 105 (486–9.1 × 1116) | 2.8 | 0.7 (0.1–6.2) | |
| urine | 0 | 1 (0–2.2 × 1024) | 0 (NA–4.6 × 1034) | 0 | 1 (0–1.7 × 107) | 0 (NA–3 × 1041) | |||||
| Quality of water | clear | 132 | 55.6 | Ref | 55.7 | Ref | 243 | 59.1 | Ref | 57.5 | Ref |
| polluted | 6 | 2.8 | 0.3 (0.02–1.56) | 2.5 | 0.3 (0.1–0.6) * | 10 | 1.5 | 0.2 (0–0.5) * | 2.8 | 0.3 (0.1–0.5) * | |
| turbid | 99 | 41.7 | 2 (1–4) * | 41.8 | 2.4 (1.8–3.4) * | 166 | 39.4 | 1.9 (1.30–2.8) * | 39.7 | 2.5 (1.8–3.4) * | |
| Presence of plant debris | no | 88 | 38.9 | Ref | 36.8 | Ref | 167 | 39.4 | Ref | 40.1 | Ref |
| yes | 149 | 61.1 | 1.9 (1–3.9) | 63.2 | 2.5 (1.8–3.5) * | 252 | 60.6 | 2.1 (1.41–3) * | 59.9 | 2.3 (1.8–3.1) * | |
| Type of container | watering places | 1 | 2.8 | Ref | Ref | 8 | 3.8 | Ref | 1 | Ref | |
| miscellaneous | 25 | 8.3 | 0.7 (0.1–14) | 10.9 | NA | 51 | 14.4 | 0.8 (0.3–2.8) | 11.1 | 3.5 (1–16) | |
| car wrecks | 14 | 11.1 | 3.4 (0.4–69.5) | 5 | NA | 14 | 3.8 | 0.7 (0.2–2.9) | 3.1 | 3 (0.7–15.7) | |
| natural | 3 | 0 (0–3.9 × 1027) | 1.5 | NA | 6 | 1.5 | 0.3 (0–1.9) | 1.4 | 1.6 (0.3–9.3) | ||
| used tyres | 69 | 27.8 | 1 (0.2–18.8) | 29.4 | NA | 103 | 17.4 | 0.4 (0.1–1.3) | 27.9 | 4.1 (1.3–18.3) * | |
| flowerpots | 2 | 0 (0–1.4 × 1020) | 1 | NA | 6 | 0.8 | 0.1 (0–0.7) * | 1.7 | 1.1 (0.2–6.1) | ||
| discarded tanks | 101 | 38.9 | 0.46 (0.08–8.51) | 43.3 | NA | 191 | 47.7 | 0.34 (0.12–1.10) | 44.6 | 1.6 (0.5–7) | |
| storage containers | 22 | 11.1 | 0.5 (0.1–11) | 9 | NA | 40 | 10.6 | 0.3 (0.1–1.1) | 9.1 | 1.3 (0.4–5.8) | |
| Colour | mixed | 31 | 2.8 | Ref | 14.9 | Ref | 69 | 19.7 | Ref | 15 | Ref |
| single | 184 | 83.3 | 7.3 (1.6–131) | 76.6 | 1.3 (0.8–2) | 304 | 66.7 | 0.8 (0.5–1.3) | 75.3 | 1.3 (0.9–1.9) | |
| transparent | 22 | 13.9 | 6.6 (1.1–127.3) | 8.5 | 0.7 (0.4–1.3) | 46 | 13.6 | 0.9 (0.4–1.7) | 9.8 | 0.8 (0.5–1.4) | |
| Mobility of the container | fixed | 6 | Ref | 3 | Ref | 8 | 1.5 | Ref | 2.1 | Ref | |
| lightweight | 222 | 94.4 | 1.6 × 106 (0–NA) | 93.5 | 0.6 (0.2–2.2) | 395 | 94.7 | 0.9 (0.2–5.8) | 94.1 | 0.5 (0.2–1.6) | |
| heavyweight | 9 | 5.6 | 2.9 × 106 (0–5.9 × 10164) | 3.5 | 0.7 (0.2–3) | 14 | 3.8 | 1.1 (0.2–8.2) | 3.1 | 0.5 (0.1–1.9) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tedjou, A.N.; Kamgang, B.; Yougang, A.P.; Wilson-Bahun, T.A.; Njiokou, F.; Wondji, C.S. Patterns of Ecological Adaptation of Aedes aegypti and Aedes albopictus and Stegomyia Indices Highlight the Potential Risk of Arbovirus Transmission in Yaoundé, the Capital City of Cameroon. Pathogens 2020, 9, 491. https://doi.org/10.3390/pathogens9060491
Tedjou AN, Kamgang B, Yougang AP, Wilson-Bahun TA, Njiokou F, Wondji CS. Patterns of Ecological Adaptation of Aedes aegypti and Aedes albopictus and Stegomyia Indices Highlight the Potential Risk of Arbovirus Transmission in Yaoundé, the Capital City of Cameroon. Pathogens. 2020; 9(6):491. https://doi.org/10.3390/pathogens9060491
Chicago/Turabian StyleTedjou, Armel N., Basile Kamgang, Aurélie P. Yougang, Theodel A. Wilson-Bahun, Flobert Njiokou, and Charles S. Wondji. 2020. "Patterns of Ecological Adaptation of Aedes aegypti and Aedes albopictus and Stegomyia Indices Highlight the Potential Risk of Arbovirus Transmission in Yaoundé, the Capital City of Cameroon" Pathogens 9, no. 6: 491. https://doi.org/10.3390/pathogens9060491
APA StyleTedjou, A. N., Kamgang, B., Yougang, A. P., Wilson-Bahun, T. A., Njiokou, F., & Wondji, C. S. (2020). Patterns of Ecological Adaptation of Aedes aegypti and Aedes albopictus and Stegomyia Indices Highlight the Potential Risk of Arbovirus Transmission in Yaoundé, the Capital City of Cameroon. Pathogens, 9(6), 491. https://doi.org/10.3390/pathogens9060491






