Insecticidal Activity of Bacteria from Larvae Breeding Site with Natural Larvae Mortality: Screening of Separated Supernatant and Pellet Fractions
Abstract
:1. Introduction
- We explored 20 larval breeding sites that have never been treated with insecticides, looking for possible mosquito larval mortality. Only one had a natural larva mortality;
- We isolated bacterial strains from this larva breeding site that presented a natural mortality of mosquito larvae;
- We tested the bacteria-free supernatant and disrupted bacterial pellet separately, or the mixture of both of the different strains of bacteria.
2. Results
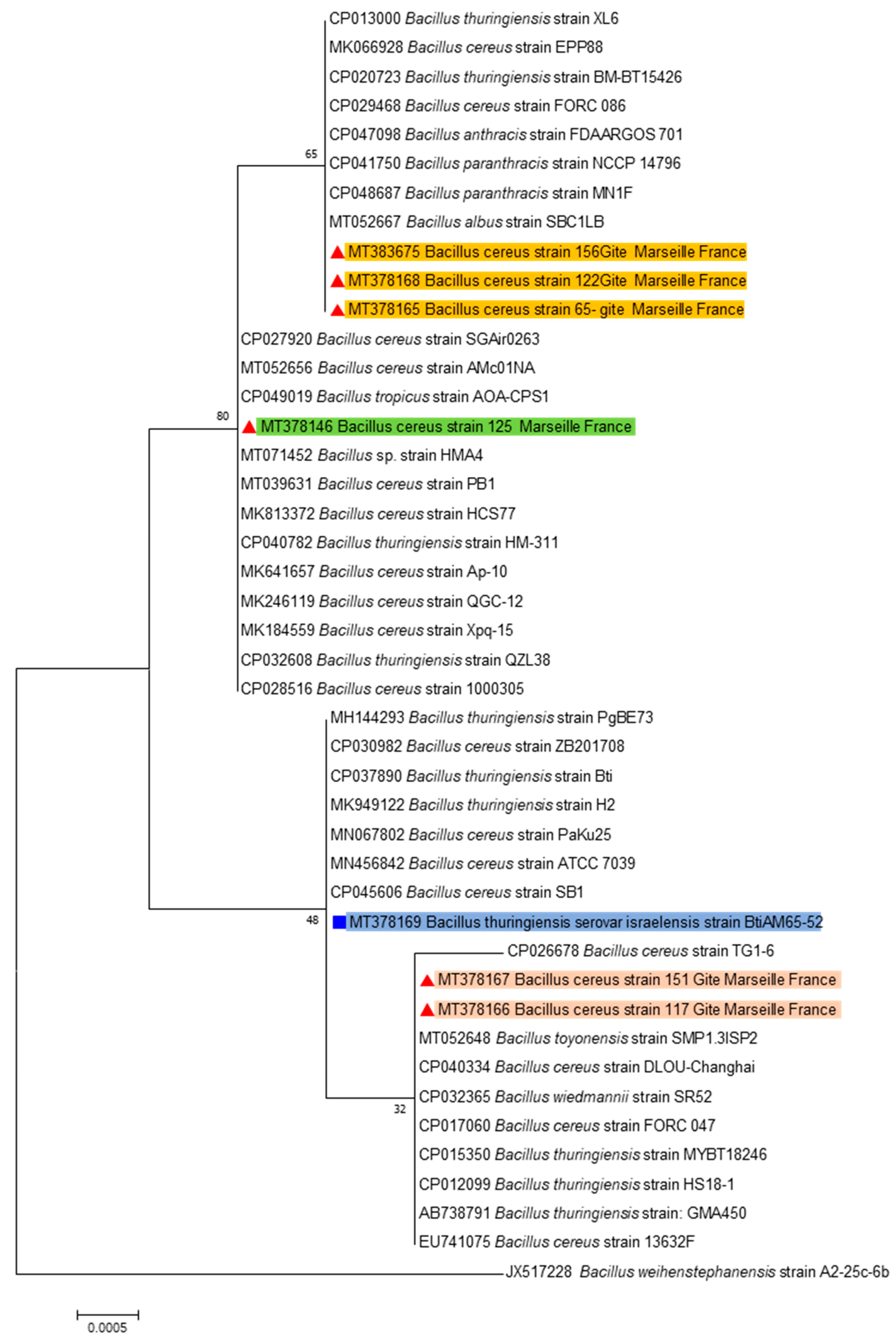
2.1. Isolated Strains
2.2. Insecticidal Activity Prediction
2.3. Screening for Insecticidal Activity
3. Discussion
4. Materials and Methods
4.1. Bacteria Isolation
4.2. Strains Identification
4.3. Fractions Preparation
4.3.1. Bacteria Culture
4.3.2. Releasing Inclusions and Main Cell Components
4.3.3. Fractions Used in Larvae Assays
4.4. Plasmid DNA Isolation and Investigation of Bacterial Genes Encoding Toxins
4.5. Screening for Insecticidal Activity
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dhanasekaran, D.; Thangaraj, R. Microbial secondary metabolites are an alternative approaches against insect vector to prevent zoonotic diseases. Asian Pac. J. Trop. Dis. 2014, 4, 253–261. [Google Scholar] [CrossRef]
- Trevors, J.T.; Barkay, T.; Bourquin, A.W. Gene transfer among bacteria in soil and aquatic environments: A review. Can. J. Microbiol. 1987, 33, 191–198. [Google Scholar] [CrossRef]
- Mishra, S.K.; Keller, J.E.; Miller, J.R.; Heisey, R.M.; Nair, M.G.; Putnam, A.R. Insecticidal and nematicidal properties of microbial metabolites. J. Ind. Microbiol. 1987, 2, 267–276. [Google Scholar] [CrossRef]
- Kuta, F. Antifungal effect of Calotropis procera stem bark on Epidermophyton flocosum and Trichophyton gypseum. African J. Biotechnol. 2008, 7, 2116–2118. [Google Scholar]
- Polanczyk, R.A.; van Frankenhuyzen, K.; Pauli, G. The american Bacillus thuringiensis based biopesticides market. In Bacillus Thuringiensis and Lysinibacillus Sphaericus: Characterization and Use in the Field of Biocontrol; Springer International Publishing: Cham, Switzerland, 2017; pp. 173–184. ISBN 9783319566788. [Google Scholar]
- Dahmana, H.; Mediannikov, O. Mosquito-Borne Diseases Emergence/Resurgence and How to Effectively Control It Biologically. Pathogens 2020, 9, 310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Hua, G.; Adang, M.J. Effects and mechanisms of Bacillus thuringiensis crystal toxins for mosquito larvae. Insect Sci. 2017, 24, 714–729. [Google Scholar] [CrossRef]
- Palma, L.; Muñoz, D.; Berry, C.; Murillo, J.; Caballero, P. Bacillus thuringiensis toxins: An overview of their biocidal activity. Toxins 2014, 6, 3296–3325. [Google Scholar] [CrossRef] [Green Version]
- Tilquin, M.; Paris, M.; Reynaud, S.; Despres, L.; Ravanel, P.; Geremia, R.A.; Gury, J. Long lasting persistence of Bacillus thuringiensis Subsp. israelensis (Bti) in mosquito natural habitats. PLoS ONE 2008, 3, e3432. [Google Scholar]
- Bravo, A.; Sarabia, S.; Lopez, L.; Ontiveros, H.; Abarca, C.; Ortiz, A.; Ortiz, M.; Lina, L.; Villalobos, F.J.; Peña, G.; et al. Characterization of cry genes in a Mexican Bacillus thuringiensis strain collection. Appl. Environ. Microbiol. 1998, 64, 4965–4972. [Google Scholar] [CrossRef] [Green Version]
- Par, P.; Paris, M.; Chevillon, C.; David, J.-P. Evolution de la résistance au bactério-insecticide Bti chez les moustiques. Ph.D. Thesis, Université de Grenoble, Grenoble, France, 2010. [Google Scholar]
- Su, T. Resistance and Its Management to Microbial and Insect Growth Regulator Larvicides in Mosquitoes; IntechOpen: London, UK, 2016. [Google Scholar]
- Amin, M.; Rakhisi, Z.; Zarei, A.A. Isolation and Identification of Bacillus Species From Soil and Evaluation of Their Antibacterial Properties. Avicenna J. Clin. Microb. Infect. 2015, 2, 23233. [Google Scholar] [CrossRef]
- Tariq, A.L.; Sudha, S.; Reyaz, A.L. Isolation and Screening of Bacillus Species from Sediments and Application in Bioremediation. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 916–924. [Google Scholar] [CrossRef] [Green Version]
- Galarza-Seeber, R.; Latorre, J.D.; Hernandez-Velasco, X.; Wolfenden, A.D.; Bielke, L.R.; Menconi, A.; Hargis, B.M.; Tellez, G. Isolation, screening and identification of Bacillus spp. as direct-fed microbial candidates for aflatoxin B1 biodegradation. Asian Pac. J. Trop. Biomed. 2015, 5, 702–706. [Google Scholar] [CrossRef] [Green Version]
- Nair, K.; Al-Thani, R.; Al-Thani, D.; Al-Yafei, F.; Ahmed, T.; Jaoua, S. Diversity of Bacillus thuringiensis Strains From Qatar as Shown by Crystal Morphology, δ-Endotoxins and Cry Gene Content. Front. Microbiol. 2018, 9, 708. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, L.K.J.; de Oliveira, M.R.; Marinotti, O.; Rocha, E.M.; Håkansson, S.; Tadei, W.P.; de Souza, A.Q.L.; Terenius, O. Characterization of Bacterial Communities in Breeding Waters of Anopheles darlingi in Manaus in the Amazon Basin Malaria-Endemic Area. Microb. Ecol. 2019, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ullah, I.; Latif Khan, A.; Ali, L.; Khan, A.R.; Waqas, M.; Lee, I.-J.; Shin, J.-H. An Insecticidal Compound Produced by an Insect-Pathogenic Bacterium Suppresses Host Defenses through Phenoloxidase Inhibition. Molecules 2014, 19, 20913–20928. [Google Scholar] [CrossRef] [Green Version]
- Foldes, T.; Banhegyi, I.; Herpai, Z.; Varga, L.; Szigeti, J. Isolation of Bacillus strains from the rhizosphere of cereals and in vitro screening for antagonism against phytopathogenic, food-borne pathogenic and spoilage micro-organisms. J. Appl. Microbiol. 2000, 89, 840–846. [Google Scholar] [CrossRef] [Green Version]
- Darriet, F.; Hougard, J.-M. An isolate of Bacillus circulans toxic to mosquito larvae. J. Am. Mosq. Control. Assoc. 2002, 18, 65–67. [Google Scholar]
- Favret, M.E.; Yousten, A.A. Insecticidal activity of Bacillus laterosporus. J. Invertebr. Pathol. 1985, 45, 195–203. [Google Scholar] [CrossRef]
- Orlova, M.V.; Smirnova, T.A.; Ganushkina, L.A.; Yacubovich, V.Y.; Azizbekyan, R.R. Insecticidal activity of Bacillus laterosporus. Appl. Environ. Microbiol. 1998, 64, 2723–2725. [Google Scholar] [CrossRef] [Green Version]
- Aronson, A. Sporulation and delta-endotoxin synthesis by Bacillus thuringiensis. Cell. Mol. Life Sci. 2002, 59, 417–425. [Google Scholar] [CrossRef]
- Ahmed, I.; Yokota, A.; Yamazoe, A.; Fujiwara, T. Proposal of Lysinibacillus boronitolerans gen. nov. sp. nov., and transfer of Bacillus fusiformis to Lysinibacillus fusiformis comb. nov. and Bacillus sphaericus to Lysinibacillus sphaericus comb. nov. Int. J. Syst. Evol. Microbiol. 2007, 57, 1117–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulaiman, I.M.; Hsieh, Y.-H.; Jacobs, E.; Miranda, N.; Simpson, S.; Kerdahi, K. Identification of Lysinibacillus fusiformis Isolated from Cosmetic Samples Using MALDI-TOF MS and 16S rRNA Sequencing Methods. J. AOAC Int. 2018, 101, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Jung, Y.-T.; Park, S.; Oh, T.-K.; Yoon, J.-H. Lysinibacillus xylanilyticus sp. nov., a xylan-degrading bacterium isolated from forest humus. Int. J. Syst. Evol. Microbiol. 2010, 60, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Berry, C. The bacterium, Lysinibacillus sphaericus, as an insect pathogen. J. Invertebr. Pathol. 2012, 109, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Abideen, S.; Babuselvam, M. Antagonistic activity of Lysinibacillus fusiformis n 139 strain isolated from marine fish Triacanthus strigilifer and genome sequence. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 1066–1072. [Google Scholar]
- Gallegos-Monterrosa, R.; Kankel, S.; Götze, S.; Barnett, R.; Stallforth, P.; Kovács, Á.T. Lysinibacillus fusiformis M5 Induces Increased Complexity in Bacillus subtilis 168 Colony Biofilms via Hypoxanthine. J. Bacteriol. 2017, 199, e00204-17. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.-P.; Liu, B.; Liu, G.-H.; Chen, D.-J.; Zhu, Y.-J.; Chen, Z.; Che, J.-M. Genome Sequence of Virgibacillus pantothenticus DSM 26T (ATCC 14576), a Mesophilic and Halotolerant Bacterium Isolated from Soil. Genome Announc. 2015, 3, e01064-15. [Google Scholar]
- Duc, L.H.; Hong, H.A.; Barbosa, T.M.; Henriques, A.O.; Cutting, S.M. Characterization of Bacillus probiotics available for human use. Appl. Environ. Microbiol. 2004, 70, 2161–2171. [Google Scholar] [CrossRef] [Green Version]
- Ianiro, G.; Rizzatti, G.; Plomer, M.; Lopetuso, L.; Scaldaferri, F.; Franceschi, F.; Cammarota, G.; Gasbarrini, A. Bacillus clausii for the Treatment of Acute Diarrhea in Children: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2018, 10, 1074. [Google Scholar] [CrossRef] [Green Version]
- Urdaci, M.C.; Bressollier, P.; Pinchuk, I. Bacillus clausii probiotic strains: Antimicrobial and immunomodulatory activities. J. Clin. Gastroenterol. 2004, 38, S86–S90. [Google Scholar] [CrossRef] [Green Version]
- Guo, D.-L.; Wan, B.; Xiao, S.-J.; Allen, S.; Gu, Y.-C.; Ding, L.-S.; Zhou, Y. Cyclic Lipopeptides with Herbicidal and Insecticidal Activities Produced by Bacillus clausii DTM1. Nat. Prod. Commun. 2015, 10, 2151–2153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazra, C.; Kundu, D.; Chaudhari, A. Lipopeptide biosurfactant from Bacillus clausii BS02 using sunflower oil soapstock: Evaluation of high throughput screening methods, production, purification, characterization and its insecticidal activity. RSC Adv. 2015, 5, 2974–2982. [Google Scholar] [CrossRef]
- de Boer, A.S.; Priest, F.; Diderichsen, B. On the industrial use of Bacillus licheniformis: A review. Appl. Microbiol. Biotechnol. 1994, 40, 595–598. [Google Scholar] [CrossRef]
- Zhaolin, J.; Huiwen, H.; Huijuan, Z.; Feng, H.; Yunhui, T.; Zhengwen, Y.; Jingyou, X. The Biocontrol Effects of the Bacillus licheniformis W10 Strain and Its Antifungal Protein Against Brown Rot in Peach. Hortic. Plant. J. 2015, 1, 131–138. [Google Scholar]
- Radhakrishnan, R.; Hashem, A.; Abd Allah, E.F. Bacillus: A Biological Tool for Crop Improvement through Bio-Molecular Changes in Adverse Environments. Front. Physiol. 2017, 8, 667. [Google Scholar] [CrossRef]
- Khan, S.; Zaffar, H.; Irshad, U.; Ahmad, R.; Khan, A.; Shah, M.; Bilal, M.; Iqbal, M.; Naqvi, T. Biodegradation of malathion by Bacillus licheniformis strain ML-1. Arch. Biol. Sci. 2016, 68, 51–59. [Google Scholar] [CrossRef]
- Theoduloz, C.; Vega, A.; Salazar, M.; Gonzalez, E.; Meza-Basso, L. Expression of a Bacillus thuringiensis delta-endotoxin cry1Ab gene in Bacillus subtilis and Bacillus licheniformis strains that naturally colonize the phylloplane of tomato plants (Lycopersicon esculentum, Mills). J. Appl. Microbiol. 2003, 94, 375–381. [Google Scholar] [CrossRef] [Green Version]
- Yan, M.; Roehrl, M.H.; Wang, J.Y. Discovery of crystalline inclusions in Bacillus licheniformis that resemble parasporal crystals of Bacillus thuringiensis. Can. J. Microbiol. 2007, 53, 1111–1115. [Google Scholar] [CrossRef]
- Jumas-Bilak, E.; Jean-Pierre, H.; Carlier, J.-P.; Teyssier, C.; Bernard, K.; Gay, B.; Campos, J.; Morio, F.; Marchandin, H. Dialister micraerophilus sp. nov. and Dialister propionicifaciens sp. nov., isolated from human clinical samples. Int. J. Syst. Evol. Microbiol. 2005, 55, 2471–2478. [Google Scholar] [CrossRef] [Green Version]
- Shida, O.; Takagi, H.; Kadowaki, K.; Komagata, K. Proposal for Two New Genera, Brevibacillus gen. nov. and Aneurinibacillus gen. nov. Int. J. Syst. Bacteriol. 1996, 46, 939–946. [Google Scholar] [CrossRef] [Green Version]
- Berditsch, M.; Afonin, S.; Ulrich, A.S. The ability of Aneurinibacillus migulanus (Bacillus brevis) to produce the antibiotic gramicidin S is correlated with phenotype variation. Appl. Environ. Microbiol. 2007, 73, 6620–6628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alenezi, F.N.; Rekik, I.; Bełka, M.; Ibrahim, A.F.; Luptakova, L.; Jaspars, M.; Woodward, S.; Belbahri, L. Strain-level diversity of secondary metabolism in the biocontrol species Aneurinibacillus migulanus. Microbiol. Res. 2016, 182, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Alenezi, F.N.; Rekik, I.; Chenari Bouket, A.; Luptakova, L.; Weitz, H.J.; Rateb, M.E.; Jaspars, M.; Woodward, S.; Belbahri, L. Increased Biological Activity of Aneurinibacillus migulanus Strains Correlates with the Production of New Gramicidin Secondary Metabolites. Front. Microbiol. 2017, 8, 517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palanimanickam, A.; Sepperumal, U. Profenofos Degradation Potential of Bacillus cereus and Aneurinibacillus migulanus Isolated from Paddy Crop Field Soil. J. Pure Appl. Microbiol. 2017, 11, 221–227. [Google Scholar] [CrossRef]
- Palmisano, M.M.; Nakamura, L.K.; Duncan, K.E.; Istock, C.A.; Cohan, F.M. Bacillus sonorensis sp. nov., a close relative of Bacillus licheniformis, isolated from soil in the Sonoran Desert, Arizona. Int. J. Syst. Evol. Microbiol. 2001, 51, 1671–1679. [Google Scholar] [CrossRef]
- Nerurkar, M.; Joshi, M.; Pariti, S.; Adivarekar, R. Application of Lipase from Marine Bacteria Bacillus sonorensis as an Additive in Detergent Formulation. J. Surfactants Deterg. 2013, 16, 435–443. [Google Scholar] [CrossRef]
- Arya, R.; Mishra, N.K.; Sharma, A.K. Brevibacillus borstelensis and Streptomyces albogriseolus have roles to play in degradation of herbicide, sulfosulfuron. 3 Biotech 2016, 6, 246. [Google Scholar] [CrossRef] [Green Version]
- Hadad, D.; Geresh, S.; Sivan, A. Biodegradation of polyethylene by the thermophilic bacterium Brevibacillus borstelensis. J. Appl. Microbiol. 2005, 98, 1093–1100. [Google Scholar] [CrossRef]
- Khalil, A.B.; Sivakumar, N.; Arslan, M.; Saleem, H.; Qarawi, S. Insights into Brevibacillus borstelensis AK1 through Whole Genome Sequencing: A Thermophilic Bacterium Isolated from a Hot Spring in Saudi Arabia. Biomed. Res. Int. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, J.; Pei, Z.; Lutwick, L.; Dalal, S.; Yang, L.; Cassai, N.; Sandhu, K.; Hanna, B.; Wieczorek, R.L.; Bluth, M.; et al. Case report: Paenibacillus thiaminolyticus: A new cause of human infection, inducing bacteremia in a patient on hemodialysis. Ann. Clin. Lab. Sci. 2008, 38, 393–400. [Google Scholar]
- Grady, E.N.; MacDonald, J.; Liu, L.; Richman, A.; Yuan, Z.-C. Current knowledge and perspectives of Paenibacillus: A review. Microb. Cell Fact. 2016, 15, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.M.; Park, M.H.; Jung, T.S.; Moon, K.H.; Kim, K.M.; Kang, J.S. Characterization of Bacillus mojavensis KJS-3 for industrial applications. Arch. Pharm. Res. 2011, 34, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Liu, J.; Go, Y.S.; Kang, J.S. Characterization of Bacillus mojavensis KJS-3 for the Promotion of Plant Growth. J. Life Sci. 2015, 25, 910–916. [Google Scholar] [CrossRef] [Green Version]
- Jasim, B.; Sreelakshmi, S.; Mathew, J.; Radhakrishnan, E.K. Identification of endophytic Bacillus mojavensis with highly specialized broad spectrum antibacterial activity. 3 Biotech 2016, 6, 187. [Google Scholar] [CrossRef] [Green Version]
- La Scola, B.; Raoult, D. Direct identification of bacteria in positive blood culture bottles by matrix-assisted laser desorption ionisation time-of-flight mass spectrometry. PLoS ONE 2009, 4, e8041. [Google Scholar] [CrossRef] [Green Version]
- Seng, P.; Drancourt, M.; Gouriet, F.; La Scola, B.; Fournier, P.; Rolain, J.M.; Raoult, D. Ongoing Revolution in Bacteriology: Routine Identification of Bacteria by Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry. Clin. Infect. Dis. 2009, 49, 543–551. [Google Scholar] [CrossRef]
- Dahmana, H.; Granjon, L.; Diagne, C.; Davoust, B.; Fenollar, F.; Mediannikov, O. Rodents as Hosts of Pathogens and Related Zoonotic Disease Risk. Pathogens 2020, 9, 202. [Google Scholar] [CrossRef] [Green Version]
- La Scola, B.; Liang, Z.; Zeaiter, Z.; Houpikian, P.; Grimont, P.A.D.; Raoult, D. Genotypic characteristics of two serotypes of Bartonella henselae. J. Clin. Microbiol. 2002, 40, 2002–2008. [Google Scholar] [CrossRef] [Green Version]
- Dahmana, H.; Amanzougaghene, N.; Davoust, B.; Normand, T.; Carette, O.; Demoncheaux, J.-P.; Mulot, B.; Fabrizy, B.; Scandola, P.; Chik, M.; et al. Great diversity of Piroplasmida in Equidae in Africa and Europe, including potential new species. Vet. Parasitol. Reg. Stud. Rep. 2019, 18, 100332. [Google Scholar] [CrossRef]
- Carozzi, N.B.; Kramer, V.C.; Warren, G.W.; Evola, S.; Koziel, M.G. Prediction of insecticidal activity of Bacillus thuringiensis strains by polymerase chain reaction product profiles. Appl. Environ. Microbiol. 1991, 57, 3057–3061. [Google Scholar] [CrossRef] [Green Version]
- Porcar, M.; Iriarte, J.; Dumanoir, V.C.; Ferrandis, M.D.; Lecadet, M.M.; Ferré, J.; Caballero, P. Identification and characterization of the new Bacillus thuringiensis serovars pirenaica (serotype H57) and iberica (serotype h59). J. Appl. Microbiol. 1999, 87, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Zghal, R.Z.; Trigui, H.; Ali, M.B.; Jaoua, S. Evidence of the importance of the Met115 for Bacillus thuringiensis subsp. israelensis Cyt1Aa protein cytolytic activity in Escherichia coli. Mol. Biotechnol. 2008, 38, 121–127. [Google Scholar] [PubMed]
- Guerchicoff, A.; Ugalde, R.A.; Rubinstein, C.P. Identification and characterization of a previously undescribed cyt gene in Bacillus thuringiensis subsp. israelensis. Appl. Environ. Microbiol. 1997, 63, 2716–2721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Guidelines for Laboratory and Field Testing of Mosquito Larvicides; WHO: Geneva, Switzerland, 2005. [Google Scholar]
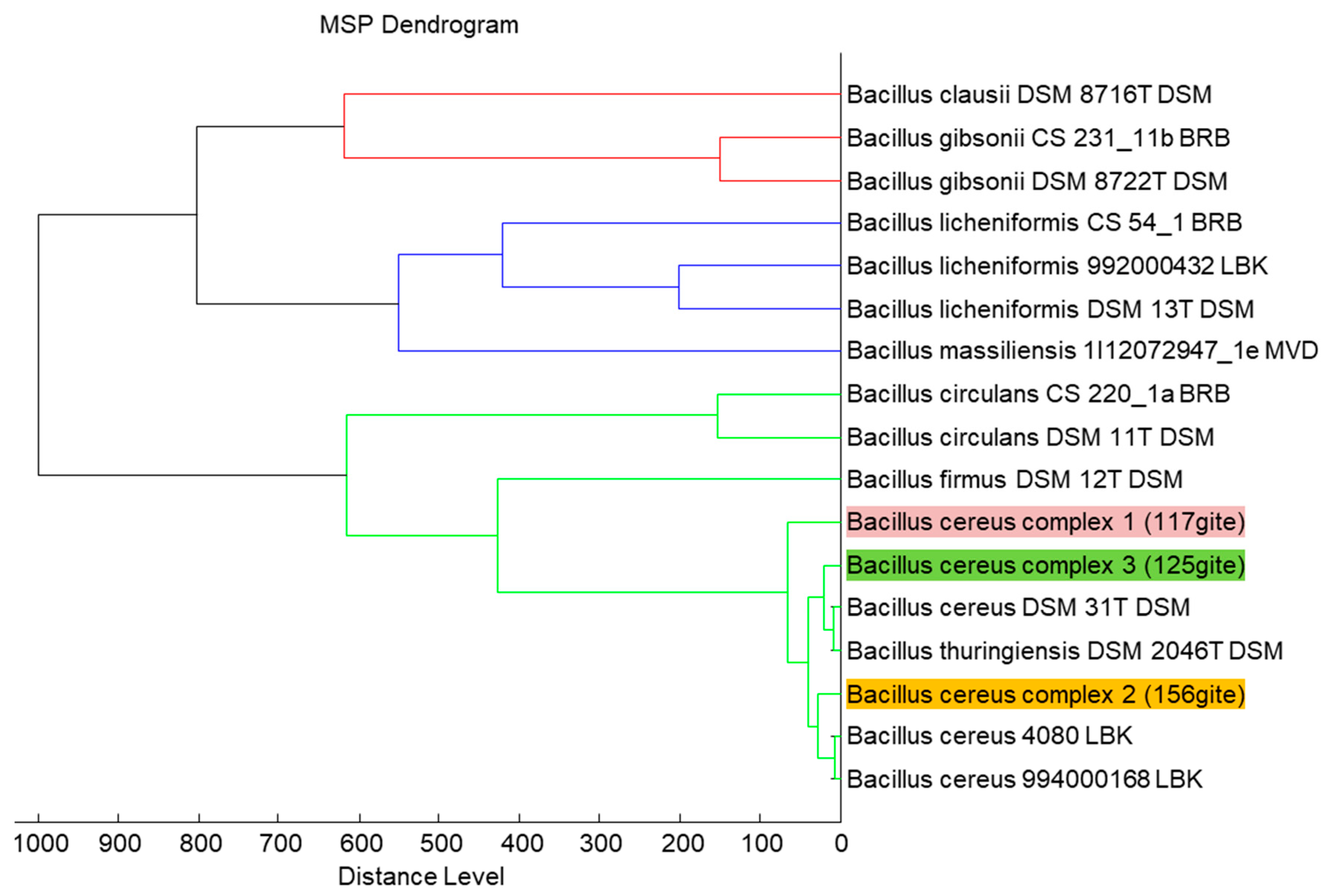
| Phylum | Order | Genus-Species | Strain Name | CSUR Collection Number | MALDI-TOF Score Value |
|---|---|---|---|---|---|
| Firmicutes | Bacillale | Bacillus clausii | 33 gite | Q3263 | 2.224 |
| Bacillus licheniformis | 45 gite | Q3264 | 2.19 | ||
| 139 gite | Q3262 | 2.483 | |||
| Bacillus sonorensis | 46 gite | Q3261 | 2.310 | ||
| Bacillus mojavensis | 133 gite | Q3258 | 2.402 | ||
| Bacillus cereus complex | 117 gite | Q3259 | 2.095 | ||
| Bacillus cereus complex | 156 gite | Q3265 | 2.135 | ||
| Bacillus cereus complex | 125 gite | Q3260 | 2.063 | ||
| Brevibacillusborstelensis | 12 gite | Q3257 | 2.493 | ||
| Lysinibacillus fusiformis | 99 gite | Q3255 | 2.345 | ||
| Virgibacillus pantothenticus | 91 gite | Q3256 | 2.129 | ||
| Aneurinibacillus migulanus | 126 gite | Q3254 | 2.474 | ||
| Paenibacillus thiaminolyticus | 136 gite | Q3253 | 2.035 | ||
| Selenomonadales | Dialister propionicifaciens | 172 gite | Q3287 | 2.432 |
| Strain | Code | Pellet | Supernatant | Supernatant + Pellet (6 mg/L) | |
|---|---|---|---|---|---|
| (6 mg/L) * | 2 mg/L | 6 mg/L | |||
| B. cereus Complex 3 | 125 gite | 0% | 88% | 94% | 84% |
| L. fusiformis | 99 gite | 0% | 47% | 85% | 76% |
| B. cereusComplex 1 | 117 gite | 0% | 65% | 80% | 88% |
| V. pantothenticus | 91 gite | 0% | 61% | 79% | 84% |
| B. clausii | 33 gite | 0% | 48% | 75% | 84% |
| B. licheniformis | 45 gite | 0% | 60% | 71% | 92% |
| D. propionicifaciens | 172 gite | 0% | 52% | 66% | 64% |
| B. cereus Complex 2 | 156 gite | 0% | 15% | 56% | 64% |
| A. migulanus | 126 gite | 56% | 0% | 5% | 52% |
| B. sonorensis | 46 gite | 0% | 24% | 51% | 56% |
| B. licheniformis | 139 gite | 0% | 16% | 37% | 40% |
| B. borstelensis | 12 gite | 0% | 15% | 19% | 16% |
| P. thiaminolyticus | 136 gite | 0% | 0% | 01% | 00% |
| B. mojavensis | 133 gite | 0% | 0% | 0% | 0% |
| Bti | AM65-52 | 0% | 15% | 33% | 34% |
| Strain | Code | Mortality Rate 6 mg/L | STDEV | Mean Rank | p-Value | Decision |
|---|---|---|---|---|---|---|
| Bti | AM65-52 | 33% | Ref | Ref | Ref | Ref |
| B. cereus Complex 3 | 125 gite | 94% | 0.239 | −457.5 | ≤0.0001 | Pos. S * |
| L. fusiformis | 99 gite | 85% | 0.359 | −390 | ≤0.0001 | Pos. S * |
| B. cereus Complex 1 | 117 gite | 80% | 0.402 | −352.5 | ≤0.0001 | Pos. S * |
| V. pantothenticus | 91 gite | 79% | 0.409 | −345 | ≤0.0001 | Pos. S * |
| B. clausii | 33 gite | 75% | 0.435 | −315 | ≤0.0001 | Pos. S * |
| B. licheniformis | 45 gite | 71% | 0.456 | −285 | ≤0.0001 | Pos. S * |
| D. propionicifaciens | 172 gite | 66% | 0.476 | −247.5 | ≤0.0001 | Pos. S * |
| B. cereus Complex 2 | 156 gite | 56% | 0.499 | −172.5 | 0.001 | Pos. S * |
| A. migulanus | 126 gite | 56% | 0.499 | −172.5 | 0.001 | Pos. S * |
| B. sonorensis | 46 gite | 51% | 0.502 | −135 | 0.011 | Pos. S * |
| B. licheniformis | 139 gite | 37% | 0.485 | −30 | 0.572 | NS *** |
| B. borstelensis | 12 gite | 19% | 0.394 | 105 | 0.048 | Neg. S ** |
| P. thiaminolyticus | 136 gite | 01% | 0.1 | 240 | ≤0.0001 | Neg. S ** |
| B. mojavensis | 133 gite | 0% | 0 | 247.5 | ≤0.0001 | Neg. S ** |
| Gene | Primers | Sequences | Amplicon Size | References |
|---|---|---|---|---|
| cry4a/4b | dip2a | GGTGCTTCCTATTCTTTGGC | 1290 bp | [63] |
| dip1b | ATGGCTTGTTTCGCTACATC | |||
| cry10 | cry10-1 | ATATGAAATATTCAATGCTC | 614 bp | [64] |
| cry10-2 | ATAAATTCAAGTGCCAAGTA | |||
| cry11 | cry11-1 | TTAGAAGATACGCCAGATCAAGC | 304 bp | [10] |
| cry11-2 | CATTTGTACTTGAAGTTGTAATCCC | |||
| cyt1a | cyt1A1 | GTTGTAAGCTTATGGAAAAT | 701 bp | [65] |
| cyt1A2 | TTAGAAGCTTCCATTAATA | |||
| cyt1c | cyt1C1 | CAAAATCTACGGGAGCAAGG | 1320 bp | [16] |
| cyt1C2 | GGAAGGATCCCTTTGACTTTT | |||
| cyt2a | cyt2A1 | AATACATTTCAAGGAGCTA | 471 bp | [66] |
| cyt2A2 | TTTCATTTTAACTTCATATC | |||
| p19 | p19-1 | GCAGGAGGAACATCACCATT | 291 bp | [16] |
| p19-2 | GGATTTGCTGAGCAGGTCAT | |||
| p20 | p20-1 | TGACGAGGAAACAGAGTATACGA | 704 bp | [16] |
| p20-2 | TGAAAGGTTAAACGTTCCGATT |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahmana, H.; Raoult, D.; Fenollar, F.; Mediannikov, O. Insecticidal Activity of Bacteria from Larvae Breeding Site with Natural Larvae Mortality: Screening of Separated Supernatant and Pellet Fractions. Pathogens 2020, 9, 486. https://doi.org/10.3390/pathogens9060486
Dahmana H, Raoult D, Fenollar F, Mediannikov O. Insecticidal Activity of Bacteria from Larvae Breeding Site with Natural Larvae Mortality: Screening of Separated Supernatant and Pellet Fractions. Pathogens. 2020; 9(6):486. https://doi.org/10.3390/pathogens9060486
Chicago/Turabian StyleDahmana, Handi, Didier Raoult, Florence Fenollar, and Oleg Mediannikov. 2020. "Insecticidal Activity of Bacteria from Larvae Breeding Site with Natural Larvae Mortality: Screening of Separated Supernatant and Pellet Fractions" Pathogens 9, no. 6: 486. https://doi.org/10.3390/pathogens9060486
APA StyleDahmana, H., Raoult, D., Fenollar, F., & Mediannikov, O. (2020). Insecticidal Activity of Bacteria from Larvae Breeding Site with Natural Larvae Mortality: Screening of Separated Supernatant and Pellet Fractions. Pathogens, 9(6), 486. https://doi.org/10.3390/pathogens9060486






