Biological Activity of Quaternary Ammonium Salts and Their Derivatives
Abstract
1. Mechanism of Biocidal Action
- Inhibition of cell wall synthesis;
- Inhibition of protein synthesis;
- Inhibition of nucleic acid synthesis;
- Inhibition of metabolic pathways;
- Interference with cell membrane integrity.
2. Biocidal Action of Quaternary Ammonium Salts
2.1. Biocidal Action of Monomeric Quaternary Ammonium Salts
2.1.1. Antibacterial Activity of Quaternary Ammonium Salts
2.1.2. Bacterial Resistance to Quaternary Ammonium Salts
2.1.3. Antifungal Activity of Quaternary Ammonium Salts
2.2. Biocidal Action of Gemini Quaternary Ammonium Salts
2.2.1. Activity of Gemini Quaternary Ammonium Salts Against Bacterial Biofilm Formation
2.2.2. Antifungal Activity of Gemini Quaternary Ammonium Salts
3. Biocidal Action of Zwitterionic Surfactants
4. Current and Future QAS Application Trends
Author Contributions
Funding
Conflicts of Interest
References
- Maillard, J.Y. Bacterial target sites for biocide action. J. Appl. Microbiol. 2002, 92, 16–27. [Google Scholar] [CrossRef]
- White, D.G.; McDermott, P.F. Biocides, drug resistance and microbial evolution. Curr. Opin. Microbiol. 2001, 4, 313–317. [Google Scholar] [CrossRef]
- Wales, A.D.; Davies, R.H. Co-selection of resistance to antibiotics, biocides and heavy metals, and its relevance to foodborne pathogens. Antibiotics 2015, 4, 567–604. [Google Scholar] [CrossRef] [PubMed]
- Diez, B.; Mellado, E.; Rodriguez, M.; Fouces, R.; Barredo, J.L. Recombinant microorganisms for industrial production of antibiotics. Biotechnol. Bioeng. 1997, 55, 216–226. [Google Scholar] [CrossRef]
- Guzmán-Trampe, S.; Ceapa, C.D.; Manzo-Ruiz, M.; Sánchez, S. Synthetic biology era: Improving antibiotic’s world. Biochem. Pharmacol. 2017, 134, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, A.T., Jr. Antiseptic “resistance”: Real or perceived threat? Clin. Infect. Dis. 2005, 40, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.D.; Furr, J.R.; Maillard, J.Y. Microbial susceptibility and resistance to biocides. ASM News-Am. Soc. Microbiol. 1997, 63, 481–487. [Google Scholar]
- Chapman, J.S. Disinfectant resistance mechanisms, cross-resistance, and co-resistance. Int. Biodeterior. Biodegrad. 2003, 51, 271–276. [Google Scholar] [CrossRef]
- Hoque, J.; Akkapeddi, P.; Yarlagadda, V.; Uppu, D.S.S.M.; Kumar, P.; Haldar, J. Cleavable Cationic Antibacterial Amphiphiles: Synthesis, Mechanism of Action, and Cytotoxicities. Langmuir 2012, 28, 12225–12234. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, L.; Kleshcheva, N. Antimicrobial polymers: Mechanism of action, factors of activity, and applications. Appl. Microbiol. Biotechnol. 2010, 89, 475–492. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, S.D.; Avti, P.K.; Hegde, G. Activated carbon nanoparticles from biowaste as new generation antimicrobial agents: A review. Nano-Struct. Nano-Objects 2018, 16, 306–321. [Google Scholar] [CrossRef]
- Denyer, S.P.; Stewart, G.S.A.B. Mechanisms of action of disinfectants. Int. Biodeterior. Biodegrad. 1998, 41, 261–268. [Google Scholar] [CrossRef]
- Denyer, S.P. Mechanisms of action of biocides. Int. Biodeterior. 1990, 26, 89–100. [Google Scholar] [CrossRef]
- Lambert, P.A. Mechanisms of action of biocides. In Principles and Practice of Disinfection, Preservation and Sterilization; Blackwell publishing Ltd.: Oxford, UK, 2008; pp. 139–153. [Google Scholar]
- Barzic, A.I.; Ioan, S. Antibacterial Drugs—From Basic Concepts to Complex Therapeutic Mechanisms of Polymer Systems. In Concepts, Compounds and the Alternatives of Antibacterials; InTech: Rijeka, Croatia, 2015; pp. 3–28. [Google Scholar]
- Ribeiro, M.; Simoes, L.C.; Simões, M. Biocides; Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2018; pp. 478–490. Available online: https://doi.org/10.1016/B978-0-12-809633-8.12118-1 (accessed on 1 June 2020).
- Corrêa, J.A.F.; Evangelista, A.G.; de Melo Nazareth, T.; Luciano, F.B. Fundamentals on the Molecular Mechanism of Action of Antimicrobial Peptides. Materialia 2019, 8, 100494. [Google Scholar] [CrossRef]
- Chakraborty, S.P.; Pramanik, P.; Roy, S. A review on emergence of antibiotic resistant staphylococcus aureus and role of chitosan nanoparticle in drug delivery. Int. J. Life Sci. Pharma Res. 2012, 2, 96–115. [Google Scholar]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef] [PubMed]
- Harley, J.P.; Prescott, M. Laboratory Exercises in Microbiology; The McGraw-Hill Companies: Boston, MA, USA, 2005; pp. 14–46. [Google Scholar]
- Oros, G.; Cserháti, T.; Forgács, E. Separation of the strength and selectivity of the microbiological effect of synthetic dyes by spectral mapping technique. Chemosphere 2003, 52, 185–193. [Google Scholar] [CrossRef]
- Migahed, M.A.; Negm, N.A.; Shaban, M.M.; Ali, T.A.; Fadda, A.A. Synthesis, characterization, surface and biological activity of diquaternary cationic surfactants containing ester linkage. J. Surfactants Deterg. 2016, 19, 119–128. [Google Scholar] [CrossRef]
- Obłąk, E.; Gamian, A. Biologiczna aktywność czwartorzędowych soli amoniowych (CSA) * The biological activity of quaternary ammonium salts (QASs). Postepy Hig. Med. Dosw. (Online) 2010, 64, 201–211. [Google Scholar] [PubMed]
- Gerba, C.P. Quaternary ammonium biocides: Efficacy in application. Appl. Environ. Microbiol. 2015, 81, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Cole, E.C.; Addison, R.M.; Rubino, J.R.; Leese, K.E.; Dulaney, P.D.; Newell, M.S.; Wilkins, J.; Gaber, D.J.; Wineinger, T.; Criger, D.A. Investigation of antibiotic and antibacterial agent cross-resistance in target bacteria from homes of antibacterial product users and nonusers. J. Appl. Microbiol. 2003, 95, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Mbithi, J.N.; Springthorpe, V.S.; Sattar, S.A. Chemical disinfection of hepatitis A virus on environmental surfaces. Appl. Environ. Microbiol. 1990, 56, 3601–3604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ding, S.; Yu, J.; Chen, X.; Lei, Q.; Fang, W. Antibacterial activity, in vitro cytotoxicity, and cell cycle arrest of gemini quaternary ammonium surfactants. Langmuir 2015, 31, 12161–12169. [Google Scholar] [CrossRef] [PubMed]
- Tischer, M.; Pradel, G.; Ohlsen, K.; Holzgrabe, U. Quaternary ammonium salts and their antimicrobial potential: Targets or nonspecific interactions? Chem. Med. Chem. 2012, 7, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.; Moore, L.E. Cationic antiseptics: Diversity of action under a common epithet. J. Appl. Microbiol. 2005, 99, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Buffet-Bataillon, S.; Tattevin, P.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Emergence of resistance to antibacterial agents: The role of quaternary ammonium compounds—A critical review. Int. J. Antimicrob. Agents 2012, 39, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Bragg, R.; Jansen, A.; Coetzee, M.; van der Westhuizen, W.; Boucher, C. Bacterial resistance to quaternary ammonium compounds (QAC) disinfectants. In Infectious Diseases and Nanomedicine II; Adhikari, R., Thapa, S., Eds.; Springer: New Delhi, India, 2014; Volume 808, pp. 1–13. [Google Scholar]
- Russell, A.D. Do biocides select for antibiotic resistance? J. Pharm. Pharmacol. 2000, 52, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.D. Introduction of biocides into clinical practice and the impact on antibiotic-resistant bacteria. J. Appl. Microbiol. 2002, 92, 121S–135S. [Google Scholar] [CrossRef] [PubMed]
- Furi, L.; Ciusa, M.L.; Knight, D.; Di Lorenzo, V.; Tocci, N.; Cirasola, D.; Aragones, L.; Coelho, J.R.; Freitas, A.T.; Marchi, E.; et al. Evaluation of reduced susceptibility to quaternary ammonium compounds and bisbiguanides in clinical isolates and laboratory-generated mutants of Staphylococcus aureus. Antimicrob. Agents Chemother. 2013, 57, 3488–3497. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, I.T.; Firth, N.; Skurray, R.A. Resistance to antimicrobial agents other than β-lactams. In The Staphylococci in Human Disease, 1st ed.; Crossley, K.B., Archer, G.L., Eds.; Churchhill Livingstone: New York, NY, USA, 1997; pp. 175–212. [Google Scholar]
- Paulsen, I.T.; Littlejohn, T.G.; Rådström, P.; Sundström, L.; Sköld, O.; Swedberg, G.; Skurray, R.A. The 3’conserved segment of integrons contains a gene associated with multidrug resistance to antiseptics and disinfectants. Antimicrob. Agents Chemother. 1993, 37, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Kücken, D.; Feucht, H.H.; Kaulfers, P.M. Association of qacE and qacE Δ1 with multiple resistance to antibiotics and antiseptics in clinical isolates of Gram-negative bacteria. FEMS Microbiol. Lett. 2000, 183, 95–98. [Google Scholar] [CrossRef]
- Vieira, D.B.; Carmona-Ribeiro, A.M. Cationic lipids and surfactants as antifungal agents: Mode of action. J. Antimicrob. Chemother. 2006, 58, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Fait, M.E.; Bakas, L.; Garrote, G.L.; Morcelle, S.R.; Saparrat, M.C.N. Cationic surfactants as antifungal agents. Appl. Microbiol. Biotechnol. 2019, 103, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Obłąk, E.; Piecuch, A.; Rewak-Soroczyńska, J.; Paluch, E. Activity of gemini quaternary ammonium salts against microorganisms. Appl. Microbiol. Biotechnol. 2019, 103, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Koziróg, A.; Brycki, B. Monomeric and gemini surfactants as antimicrobial agents-influence on environmental and reference strains. Acta Biochim. Pol. 2015, 62, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Kwaśniewska, D.; Staszak, K.; Wieczorek, D.; Zieliński, R. Synthesis and Interfacial Activity of Novel Heterogemini Sulfobetaines in Aqueous Solution. J. Surfactants Deterg. 2015, 18, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Obłąk, E.; Piecuch, A.; Guz-Regner, K.; Dworniczek, E. Antibacterial activity of gemini quaternary ammonium salts. FEMS Microbiol. Lett. 2014, 350, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Koziróg, A.; Kręgiel, D.; Brycki, B. Action of monomeric/gemini surfactants on free cells and biofilm of Asaia lannensis. Molecules 2017, 22, 2036. [Google Scholar] [CrossRef] [PubMed]
- Sumitomo, T.; Maeda, T.; Nagamune, H.; Kourai, H. Bacterioclastic action of a bis-quaternary ammonium compound against Escherichia coli. Biocontrol Sci. 2004, 9, 1–9. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef] [PubMed]
- Koziróg, A.; Otlewska, A.; Brycki, B. Viability, enzymatic and protein profiles of Pseudomonas aeruginosa biofilm and planktonic cells after monomeric/gemini surfactant treatment. Molecules 2018, 23, 1294. [Google Scholar] [CrossRef] [PubMed]
- Shirai, A.; Sumitomo, T.; Kurimoto, M.; Maseda, H.; Kourai, H. The mode of the antifungal activity of gemini-pyridinium salt against yeast. Biocontrol Sci. 2009, 14, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Obłąk, E.; Piecuch, A.; Krasowska, A.; Łuczyński, J. Antifungal activity of gemini quaternary ammonium salts. Microbiol. Res. 2013, 168, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Lomax, E.G. Amphoteric Surfactant, 2nd ed.; Marcel Dekker, Inc.: New York, NY, USA, 1996; Volume 59, pp. 253–272. [Google Scholar]
- Ward, R.S.; Davies, J.; Hodges, G.; Roberts, D.W. Synthesis of Quaternary Alkylammonium Sulfobetaines. Synthesis 2002, 16, 2431–2439. [Google Scholar] [CrossRef]
- Paulus, W. Relationship between chemical structure and activity or mode of action of microbicides. In Directory of Microbicides for the Protection of Materials; Springer: Dordrecht, The Netherlands, 2004; pp. 9–25. [Google Scholar]
- Cheng, G.; Zhang, Z.; Chen, S.; Bryers, J.D.; Jiang, S. Inhibition of bacterial adhesion and biofilm formation on zwitterionic surfaces. Biomaterials 2007, 28, 4192–4199. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Li, G.; Xue, H.; Chen, S.; Bryers, J.D.; Jiang, S. Zwitterionic carboxybetaine polymer surfaces and their resistance to long-term biofilm formation. Biomaterials 2009, 30, 5234–5240. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, P.; Hall-Stoodley, L.; Costerton, B.; DeMeo, P.; Shirtliff, M.; Gawalt, E.; Kathju, S. Biofilms, Biomaterials, and Device-Related Infections. In Handbook of Polymer Applications in Medicine and Medical Devices; Ebnesajjad, K.M., Ed.; William Andrew Publishing: Oxford, UK, 2013; Volume 5, pp. 77–101. [Google Scholar]
- Wieczorek, D.; Gwiazdowska, D.; Staszak, K.; Chen, Y.L.; Shen, T.L. Surface and antimicrobial activity of sulfobetaines. J. Surfactants Deterg. 2016, 19, 813–822. [Google Scholar] [CrossRef]
- Wieczorek, D.; Dobrowolski, A.; Staszak, K.; Kwaśniewska, D.; Dubyk, P. Synthesis, surface and antimicrobial activity of heterocyclic amine based sulfobetaines. J. Surfactants Deterg. 2017, 20, 151–158. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mrlík, M.; Špírek, M.; Al-Khori, J.; Ahmad, A.A.; Mosnaček, J.; AlMaadeed, M.A.; Kasák, P. Mussel-mimicking sulfobetaine-based copolymer with metal tunable gelation, self-healing and antibacterial capability. Arab. J. Chem. 2020, 13, 193–204. [Google Scholar] [CrossRef]
- Mi, L.; Jiang, S. Integrated antimicrobial and nonfouling zwitterionic polymers. Angew. Chem. Int. Ed. 2014, 53, 1746–1754. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.N.; Wen, T.C.; Chang, Y. Zwitterionic surface grafting of epoxylated sulfobetaine copolymers for the development of stealth biomaterial interfaces. Acta Biomater. 2016, 40, 78–91. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Gao, C.; Li, S.; Chung, C.T.W.; Xin, J.H. Non-leaching and durable antibacterial textiles finished with reactive zwitterionic sulfobetaine. J. Ind. Eng. Chem. 2017, 46, 373–378. [Google Scholar] [CrossRef]
- Lalani, R.; Lingyun, L. Electrospun zwitterionic poly (sulfobetaine methacrylate) for nonadherent, superabsorbent, and antimicrobial wound dressing applications. Biomacromolecules 2012, 13, 1853–1863. [Google Scholar] [CrossRef] [PubMed]
- Zaki, M.E.S.; Bastawy, S.; Montasser, K. Molecular study of resistance of Staphylococcus aureus to antiseptic quaternary ammonium compounds. J. Glob. Antimicrob. Resist. 2019, 17, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhou, Z.C.; Zhu, L.; Wei, Y.Y.; Feng, W.Q.; Xu, L.; Liu, Y.; Lin, Z.-J.; Shuai, X.-Y.; Zhang, Z.-J.; et al. The impact and mechanism of quaternary ammonium compounds on the transmission of antibiotic resistance genes. Environ. Sci. Pollut. Res. 2019, 26, 28352–28360. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Jamaledin, R.; Jabbari, M.; Nikfarjam, N.; Borzacchiello, A. Antibacterial quaternary ammonium compounds in dental materials: A systematic review. Dent. Mater. 2018, 34, 851–867. [Google Scholar] [CrossRef] [PubMed]
- Manouchehri, F.; Sadeghi, B.; Najafi, F.; Mosslemin, M.H.; Niakan, M. Synthesis and characterization of novel polymerizable bis-quaternary ammonium dimethacrylate monomers with antibacterial activity as an efficient adhesive system for dental restoration. Polym. Bull. 2019, 76, 1295–1315. [Google Scholar] [CrossRef]
- Munguía-Moreno, S.; Martínez-Castañón, G.A.; Patiño-Marín, N.; Cabral-Romero, C.; Zavala-Alonso, N.V. Biocompatibility and surface characteristics of resin-modified glass ionomer cements with ammonium quaternary compounds or silver nanoparticles: An in vitro study. J. Nanomater. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Zywicka, A.; Fijałkowski, K.; Junka, A.F.; Grzesiak, J.; El Fray, M. Modification of bacterial cellulose with quaternary ammonium compounds based on fatty acids and amino acids and the effect on antimicrobial activity. Biomacromolecules 2018, 19, 1528–1538. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.L.; Hamood, A.N.; de Souza, A.; Schultz, G.; Liesenfeld, B.; Mehta, D.; Reid, T.W. A study on the ability of quaternary ammonium groups attached to a polyurethane foam wound dressing to inhibit bacterial attachment and biofilm formation. Wound Repair Regen. 2015, 23, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Meghil, M.M.; Rueggeberg, F.; El-Awady, A.; Miles, B.; Tay, F.; Pashley, D.; Cutler, C.W. Novel coating of surgical suture confers antimicrobial activity against Porphyromonas gingivalis and Enterococcus faecalis. J. Periodontol. 2015, 86, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.H.; Kwaśniewska, D.; Wang, S.C.; Shen, T.L.; Wieczorek, D.; Chen, Y.L. Gemini quaternary ammonium compound PMT12-BF4 inhibits Candida albicans via regulating iron homeostasis. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, X.; Liu, N.; Guo, Z.; Singh, P.K.; Fu, S. Effect of surface wettability on the antibacterial activity of nanocellulose-based material with quaternary ammonium groups. Colloids Surf. A Physicochem. Eng. Asp. 2018, 554, 122–128. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Tang, C.Y.; Ma, J.; Liu, M.; Ping, M.; Chen, M.; Wu, Z. Modification of microfiltration membranes by alkoxysilane polycondensation induced quaternary ammonium compounds grafting for biofouling mitigation. J. Membr. Sci. 2018, 549, 165–172. [Google Scholar] [CrossRef]
- Forman, M.E.; Jennings, M.C.; Wuest, W.M.; Minbiole, K.P. Building a better quaternary ammonium compound (QAC): Branched tetracationic antiseptic amphiphiles. Chem. Med. Chem. 2016, 11, 1401–1405. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jin, Y.; Zhou, H.; Wang, H. Synthesis of zwitterionic N-chlorohydantoins for antibacterial applications. Bioorganic Med. Chem. Lett. 2018, 28, 3665–3669. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yu, X.; Zhang, T.; Wang, X. Constructing zwitterionic coatings on thin-film nanofibrous composite membrane substrate for multifunctionality. Appl. Surf. Sci. 2019, 483, 979–990. [Google Scholar] [CrossRef]
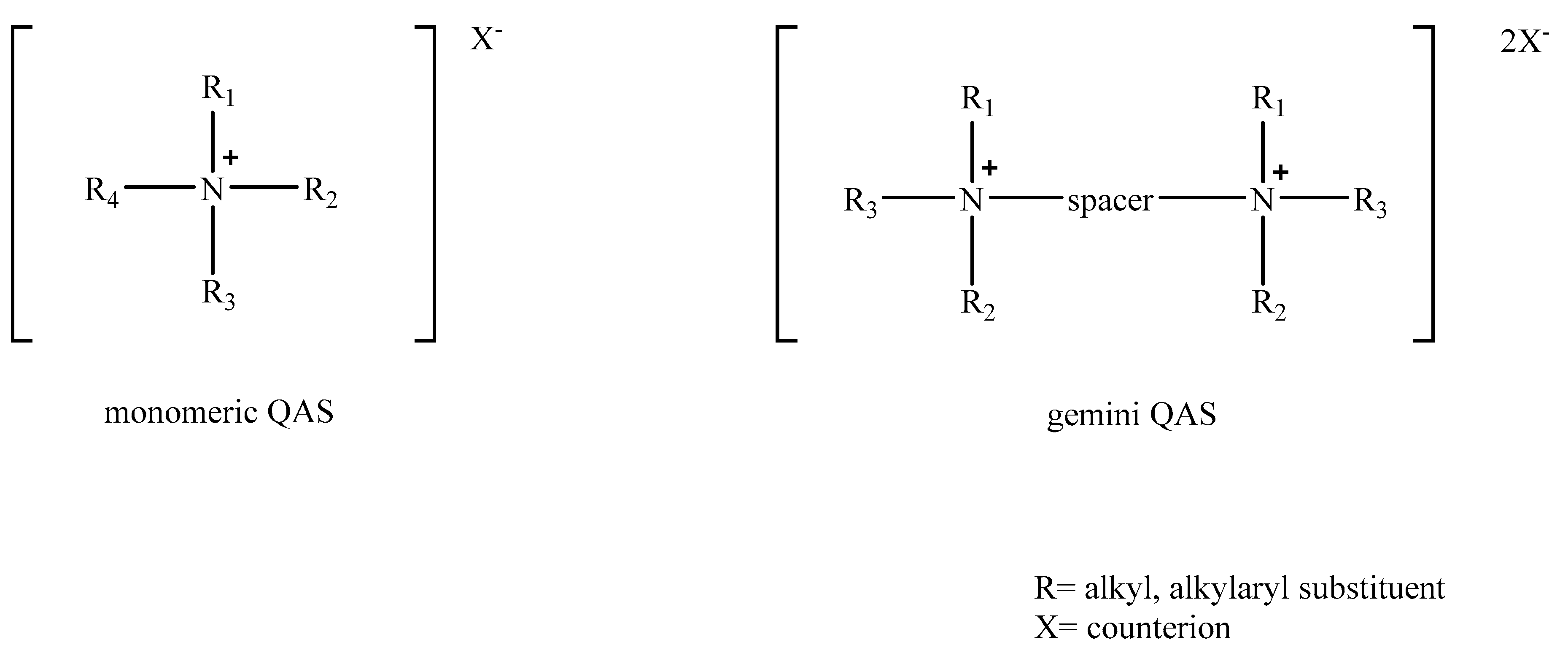
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwaśniewska, D.; Chen, Y.-L.; Wieczorek, D. Biological Activity of Quaternary Ammonium Salts and Their Derivatives. Pathogens 2020, 9, 459. https://doi.org/10.3390/pathogens9060459
Kwaśniewska D, Chen Y-L, Wieczorek D. Biological Activity of Quaternary Ammonium Salts and Their Derivatives. Pathogens. 2020; 9(6):459. https://doi.org/10.3390/pathogens9060459
Chicago/Turabian StyleKwaśniewska, Dobrawa, Ying-Lien Chen, and Daria Wieczorek. 2020. "Biological Activity of Quaternary Ammonium Salts and Their Derivatives" Pathogens 9, no. 6: 459. https://doi.org/10.3390/pathogens9060459
APA StyleKwaśniewska, D., Chen, Y.-L., & Wieczorek, D. (2020). Biological Activity of Quaternary Ammonium Salts and Their Derivatives. Pathogens, 9(6), 459. https://doi.org/10.3390/pathogens9060459





