Should We Screen HIV-Positive Migrants for Strongyloidiasis?
Abstract
1. Introduction
2. Results
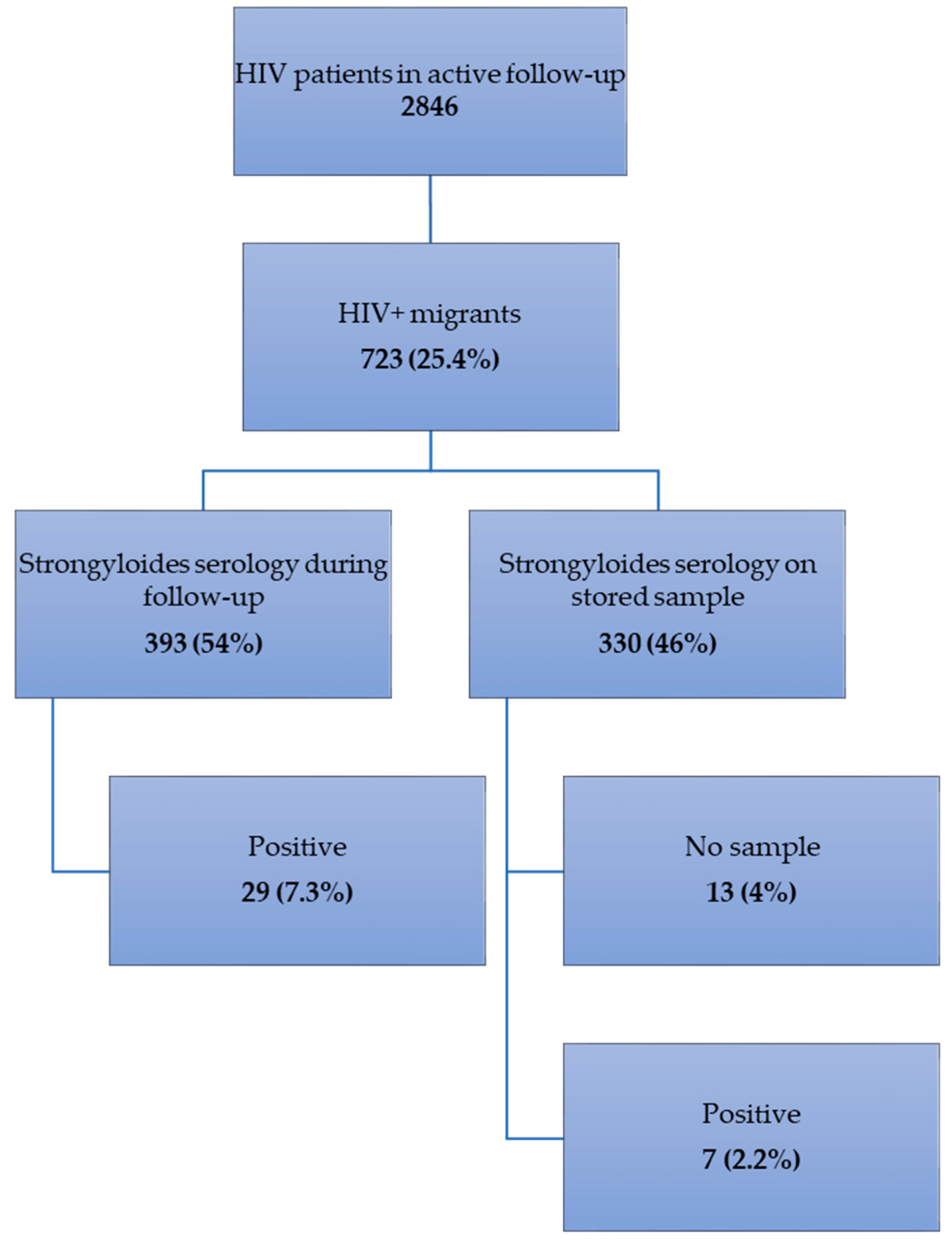
2.1. Frequency of Strongyloidiasis (Figure 1)
2.2. Clinical Presentation
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Serology
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bisoffi, Z.; Buonfrate, D.; Montresor, A.; Requena-Méndez, A.; Muñoz, J.; Krolewiecki, A.J.; Gotuzzo, E.; Mena, M.A.; Chiodini, P.L.; Anselmi, M.; et al. Strongyloides stercoralis: A plea for action. PLoS Negl. Trop. Dis. 2013, 7, e2214. [Google Scholar] [CrossRef] [PubMed]
- Genta, R.M. Global prevalence of strongyloidiasis: Critical review with epidemiologic insights into the prevention of disseminated disease. Rev. Infect. Dis. 1989, 11, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Geri, G.; Rabbat, A.; Mayaux, J.; Zafrani, L.; Chalumeau-Lemoine, L.; Guidet, B.; Azoulay, E.; Pène, F. Strongyloides stercoralis hyperinfection syndrome: A case series and a review of the literature. Infection 2015, 43, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.C.; Pacheco, F.T.; Souza, J.N.; Silva, M.L.; Inês, E.J.; Soares, N.M. Strongyloides stercoralis Infection in Alcoholic Patients. Biomed. Res. Int. 2016, 2016, 4872473. [Google Scholar] [CrossRef]
- Sudarshi, S.; Stümpfle, R.; Armstrong, M.; Ellman, T.; Parton, S.; Krishnan, P.; Chiodini, P.L.; Whitty, C.J. Clinical presentation and diagnostic sensitivity of laboratory tests for Strongyloides stercoralis in travellers compared with immigrants in a non-endemic country. Trop. Med. Int. Health 2003, 8, 728–732. [Google Scholar] [CrossRef]
- Luvira, V.; Trakulhun, K.; Mungthin, M.; Naaglor, T.; Chantawat, N.; Pakdee, W.; Phiboonbanakit, D.; Dekumyoy, P. Comparative Diagnosis of Strongyloidiasis in Immunocompromised Patients. Am. J. Trop. Med. Hyg. 2016, 95, 401–404. [Google Scholar] [CrossRef]
- Bisoffi, Z.; Buonfrate, D.; Sequi, M.; Mejia, R.; Cimino, R.O.; Krolewiecki, A.J.; Albonico, M.; Gobbo, M.; Bonafini, S.; Angheben, A.; et al. Diagnostic accuracy of five serologic tests for Strongyloides stercoralis infection. PLoS Negl. Trop. Dis. 2014, 8, e2640. [Google Scholar] [CrossRef]
- Buonfrate, D.; Salas-Coronas, J.; Muñoz, J.; Maruri, B.T.; Rodari, P.; Castelli, F.; Zammarchi, L.; Bianchi, L.; Gobbi, F.; Cabezas-Fernández, T.; et al. Multiple-dose versus single-dose ivermectin for Strongyloides stercoralis infection (Strong Treat 1 to 4): A multicenter, open-label, phase 3, randomized controlled superiority trial. Lancet Infect. Dis. 2019, 19, 1181–1190. [Google Scholar] [CrossRef]
- Parasites-Strongyloides. Available online: https://www.cdc.gov/parasites/strongyloides/health_professionals/index.html (accessed on 17 April 2020).
- Public Health Guidance on Screening and Vaccination for Infectious Diseases in Newly Arrived Migrants within the EU/EEA. Available online: https://www.ecdc.europa.eu/en/publications-data/public-health-guidance-screening-and-vaccination-infectious-diseases-newly (accessed on 17 April 2020).
- Agbata, E.N.; Morton, R.L.; Bisoffi, Z.; Bottieau, E.; Greenaway, C.; Biggs, B.A.; Montero, N.; Tran, A.; Rowbotham, N.; Arevalo-Rodriguez, I.; et al. Effectiveness of Screening and Treatment Approaches for Schistosomiasis and Strongyloidiasis in Newly Arrived Migrants from Endemic Countries in the EU/EEA: A Systematic Review. Int. J. Environ. Res. Public Health 2018, 16, 11. [Google Scholar] [CrossRef]
- Petithory, J.C.; Derouin, F. AIDS and strongyloidiasis in Africa. Lancet 1987, 1, 921. [Google Scholar] [CrossRef]
- Hochberg, N.S.; Moro, R.N.; Sheth, A.N.; Montgomery, S.P.; Steurer, F.; McAuliffe, I.T.; Wang, Y.F.; Armstrong, W.; Rivera, H.N.; Lennox, J.L.; et al. High prevalence of persistent parasitic infections in foreign-born, HIV-infected persons in the United States. PLoS Negl. Trop. Dis. 2011, 5, e1034. [Google Scholar] [CrossRef] [PubMed]
- Schär, F.; Trostdorf, U.; Giardina, F.; Khieu, V.; Muth, S.; Marti, H.; Vounatsou, P.; Odermatt, P. Strongyloides stercoralis: Global Distribution and Risk Factors. PLoS Negl Trop Dis 2013, 7, e2288. [Google Scholar] [CrossRef] [PubMed]
- Asundi, A.; Beliavsky, A.; Liu, X.J.; Akaberi, A.; Schwarzer, G.; Bisoffi, Z.; Requena-Méndez, A.; Shrier, I.; Greenaway, C. Prevalence of strongyloidiasis and schistosomiasis among migrants: A systematic review and meta-analysis. Lancet Glob. Health 2019, 7, e236–e248, Erratum in: Lancet Glob. Health 2019, 7, e419. [Google Scholar] [CrossRef]
- Nabha, L.; Krishnan, S.; Ramanathan, R.; Mejia, R.; Roby, G.; Sheikh, V.; McAuliffe, I.; Nutman, T.; Sereti, I. Prevalence of Strongyloides stercoralis in an urban US AIDS cohort. Pathog Glob. Health 2012, 106, 238–244. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mascarello, M.; Gobbi, F.; Angheben, A.; Gobbo, M.; Gaiera, G.; Pegoraro, M.; Lanzafame, M.; Buonfrate, D.; Concia, E.; Bisoffi, Z. Prevalence of Strongyloides stercoralis infection among HIV-positive immigrants attending two Italian hospitals, from 2000 to 2009. Ann. Trop. Med. Parasitol. 2011, 105, 617–623. [Google Scholar] [CrossRef]
- Costiniuk, C.T.; Cooper, C.L.; Doucette, S.; Kovacs, C.M. Parasitic disease screening among HIV patients from endemic countries in a Toronto clinic. Can. J. Infect Dis. Med. Microbiol. 2012, 23, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Requena-Mendez, A.; Chiodini, P.; Bisoffi, Z.; Buonfrate, D.; Gotuzzo, E.; Muñoz, J. The laboratory diagnosis and follow up of strongyloidiasis: A systematic review. PLoS Negl. Trop. Dis. 2013, 7, e2002. [Google Scholar] [CrossRef]
- Puthiyakunnon, S.; Boddu, S.; Li, Y.; Zhou, X.; Wang, C.; Li, J.; Chen, X. Strongyloidiasis—An Insight into Its Global Prevalence and Management. Simon, G.L., Ed. PLoS Negl. Trop. Dis. 2014, 8, e3018. [Google Scholar] [CrossRef]
- Pinlaor, S.; Mootsikapun, P.; Pinlaor, P.; Pipitgool, V.; Tuangnadee, R. Detection of opportunistic and non-opportunistic intestinal parasites and liver flukes in HIV-positive and HIV-negative subjects. Southeast Asian J. Trop. Med. Public Health 2005, 36, 841–845. [Google Scholar]
- McCarthy, A.E.; Weld, L.H.; Barnett, E.D.; So, H.; Coyle, C.; Greenaway, C.; Stauffer, W.; Leder, K.; Lopez-Velez, R.; Gautret, P.; et al. Spectrum of illness in international migrants seen at GeoSentinel clinics in 1997–2009, part 2: Migrants resettled internationally and evaluated for specific health concerns. Clin. Infect. Dis. 2013, 56, 925–933. [Google Scholar] [CrossRef]
- Assefa, S.; Erko, B.; Medhin, G.; Assefa, Z.; Shimelis, T. Intestinal parasitic infections in relation to HIV/AIDS status, diarrhea and CD4 T-cell count. BMC Infect. Dis. 2009, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Tamarozzi, F.; Martello, E.; Giorli, G.; Fittipaldo, A.; Staffolani, S.; Montresor, A.; Bisoffi, Z.; Buonfrate, D. Morbidity Associated with Chronic Strongyloides stercoralis Infection: A Systematic Review and Meta-Analysis. Am. J. Trop. Med. Hyg. 2019, 100, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Deeks, J.J.; Altman, D.G. Diagnostic tests 4: Likelihood ratios. BMJ 2004, 329, 168–169. [Google Scholar] [CrossRef] [PubMed]
- Vaiyavatjamai, P.; Boitano, J.J.; Techasintana, P.; Tungtrongchitr, A. Immunocompromised group differences in the presentation of intestinal strongyloidiasis. Jpn. J. Infect. Dis. 2008, 61, 5–8. [Google Scholar]
- Lanzafame, M.; Faggian, F.; Lattuada, E.; Antolini, D.; Vento, S. Strongyloidiasis in an HIV-1-infected patient after highly active antiretroviral therapy-induced immune restoration. J. Infect. Dis. 2005, 191, 1027. [Google Scholar] [CrossRef]
- Keiser, P.B.; Nutman, T.B. Strongyloides stercoralis in the Immunocompromised Population. Clin. Microbiol. Rev. 2004, 17, 208–217. [Google Scholar] [CrossRef]
- Fincham, J.E.; Markus, M.B.; Adams, V.J. Could control of soil-transmitted helminthic infection influence the HIV/AIDS pandemic. Acta Trop. 2003, 86, 315–333. [Google Scholar] [CrossRef]
| Parameters | With Strongyloidiasis | Without Strongyloidiasis | p-Value |
|---|---|---|---|
| (n = 36) | (n = 674) | ||
| Serologic testing on doctor’s request (n, %) | 29 (81) | 364 (54) | <0.001 |
| Age (y) (median, IQR) | 35.3 (30.3–44.4) | 34.6 (29.4–40.9) | 0.39 |
| Male gender (n,%) | 19 (53) | 300 (45) | 0.42 |
| Origin (n, %) | 0.71 | ||
| Sub-Saharan Africa | 28 (78) | 480 (71) | |
| Asia | 5 (14) | 81 (12) | |
| South & Central America | 3 (8) | 90 (14) | |
| North Africa | 0 (0) | 22 (3) | |
| Time in Belgium(y) (median, IQR) | 3.5 (0.8–5.7) | 2.1 (0.9–6.4) | 1.00 |
| Mode of transmission (n, %) | 0.42 | ||
| heterosexual | 26 (72) | 458 (68) | |
| Homo- and bisexual | 9 (25) | 142 (21) | |
| other | 1 (3) | 28 (4) | |
| unknown | 0 (0) | 46 (7) | |
| CD4 (median, IQR) | 386 (299–518) | 374 (234–531) | 0.53 |
| CD4 < 100 (n, %) | 2 (6) | 57 (8) | 0.76 |
| Undetectable VL (n, %) | 5 (14) | 185 (27) | 0.08 |
| Symptoms (n, %) | |||
| Any | 17 (47) | 214 (31) | 0.07 |
| Abdominal | 10 (28) | 96 (16) | 0.1 |
| pain | 7 (19) | 39 (6.5) | |
| diarrhoea | 0 | 25 (4) | |
| anorexia/weight loss | 3 (8) | 17 (2.5) | |
| Cutaneous | 3 (8) | 66 (11) | 0.79 |
| rash | 1 (3) | 45 (7.5) | |
| itching | 3 (8) | 29 (5) | |
| Respiratory | 7 (19) | 55 (9) | 0.07 |
| chest pain | 1 (3) | 14 (2) | |
| cough | 6 (17) | 38 (6) | |
| dyspnoea | 0 | 3 (0.5) | |
| Eosinophils/µL (median, IQR) | 555 (162.5–1167.5) | 120 (50–230) | <0.001 |
| Eosinophilia > 450 (N, %) | 19 (53) | 66 (10) | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theunissen, C.; Bottieau, E.; Van Esbroeck, M.; Tsoumanis, A.; Florence, E. Should We Screen HIV-Positive Migrants for Strongyloidiasis? Pathogens 2020, 9, 388. https://doi.org/10.3390/pathogens9050388
Theunissen C, Bottieau E, Van Esbroeck M, Tsoumanis A, Florence E. Should We Screen HIV-Positive Migrants for Strongyloidiasis? Pathogens. 2020; 9(5):388. https://doi.org/10.3390/pathogens9050388
Chicago/Turabian StyleTheunissen, Caroline, Emmanuel Bottieau, Marjan Van Esbroeck, Achilleas Tsoumanis, and Eric Florence. 2020. "Should We Screen HIV-Positive Migrants for Strongyloidiasis?" Pathogens 9, no. 5: 388. https://doi.org/10.3390/pathogens9050388
APA StyleTheunissen, C., Bottieau, E., Van Esbroeck, M., Tsoumanis, A., & Florence, E. (2020). Should We Screen HIV-Positive Migrants for Strongyloidiasis? Pathogens, 9(5), 388. https://doi.org/10.3390/pathogens9050388





