Toll-Like Receptor-4 Dependent Intestinal and Systemic Sequelae Following Peroral Campylobacter coli Infection of IL10 Deficient Mice Harboring a Human Gut Microbiota
Abstract
1. Introduction
2. Results
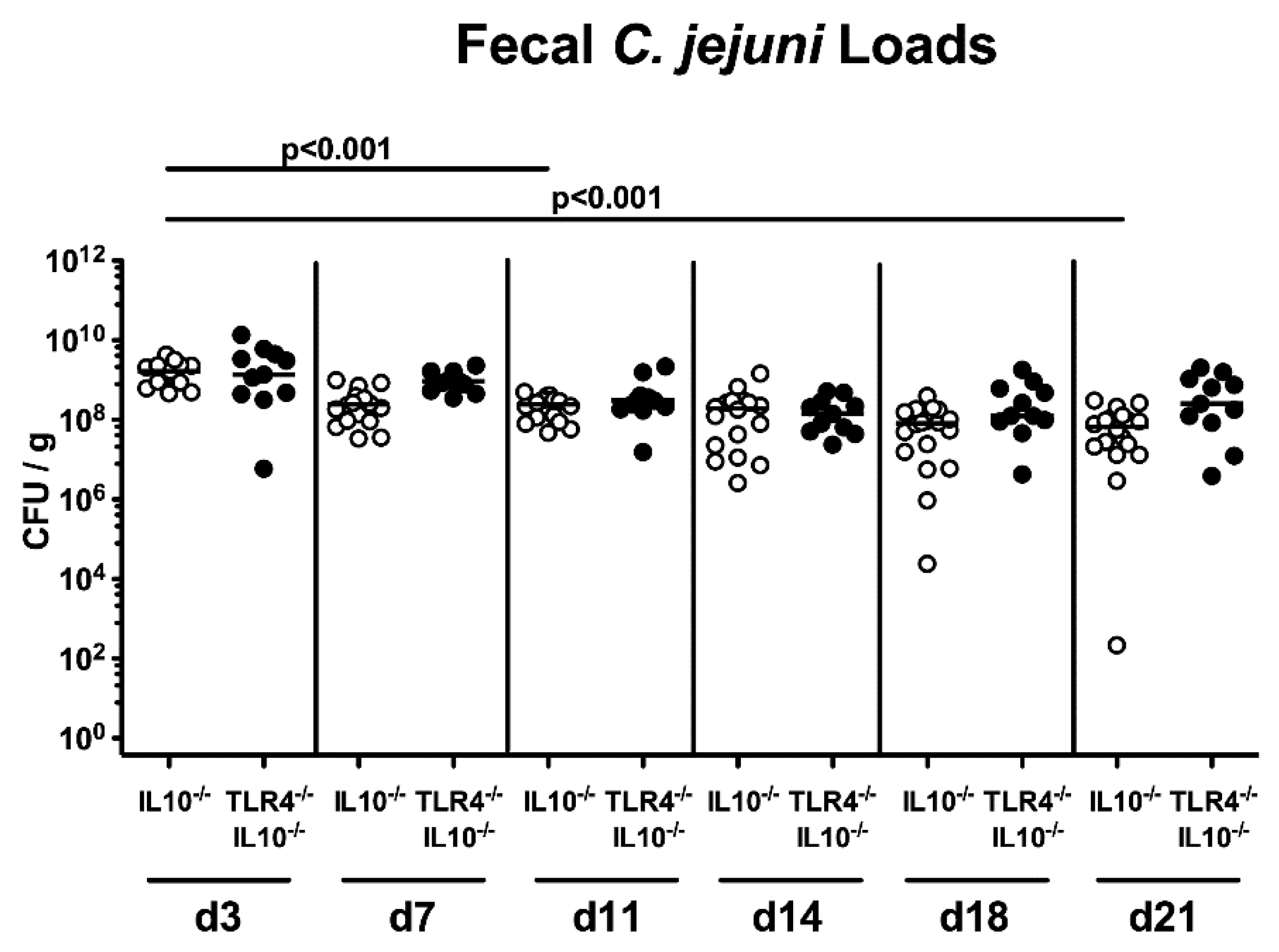
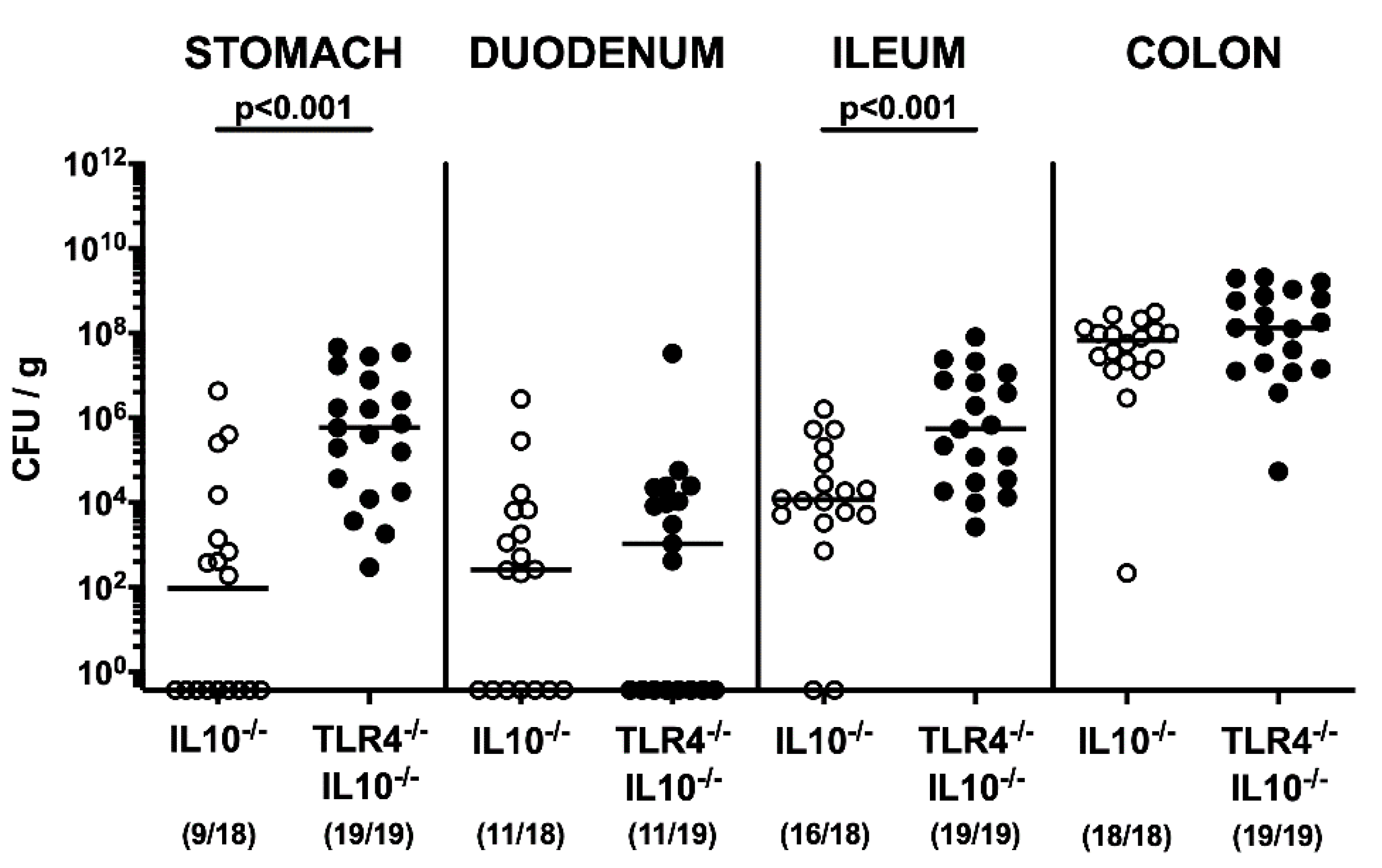
2.1. Gastrointestinal Pathogen Loads Following Peroral C. coli Infection of Human Microbiota-Associated TLR4-Deficient IL10-/- Mice
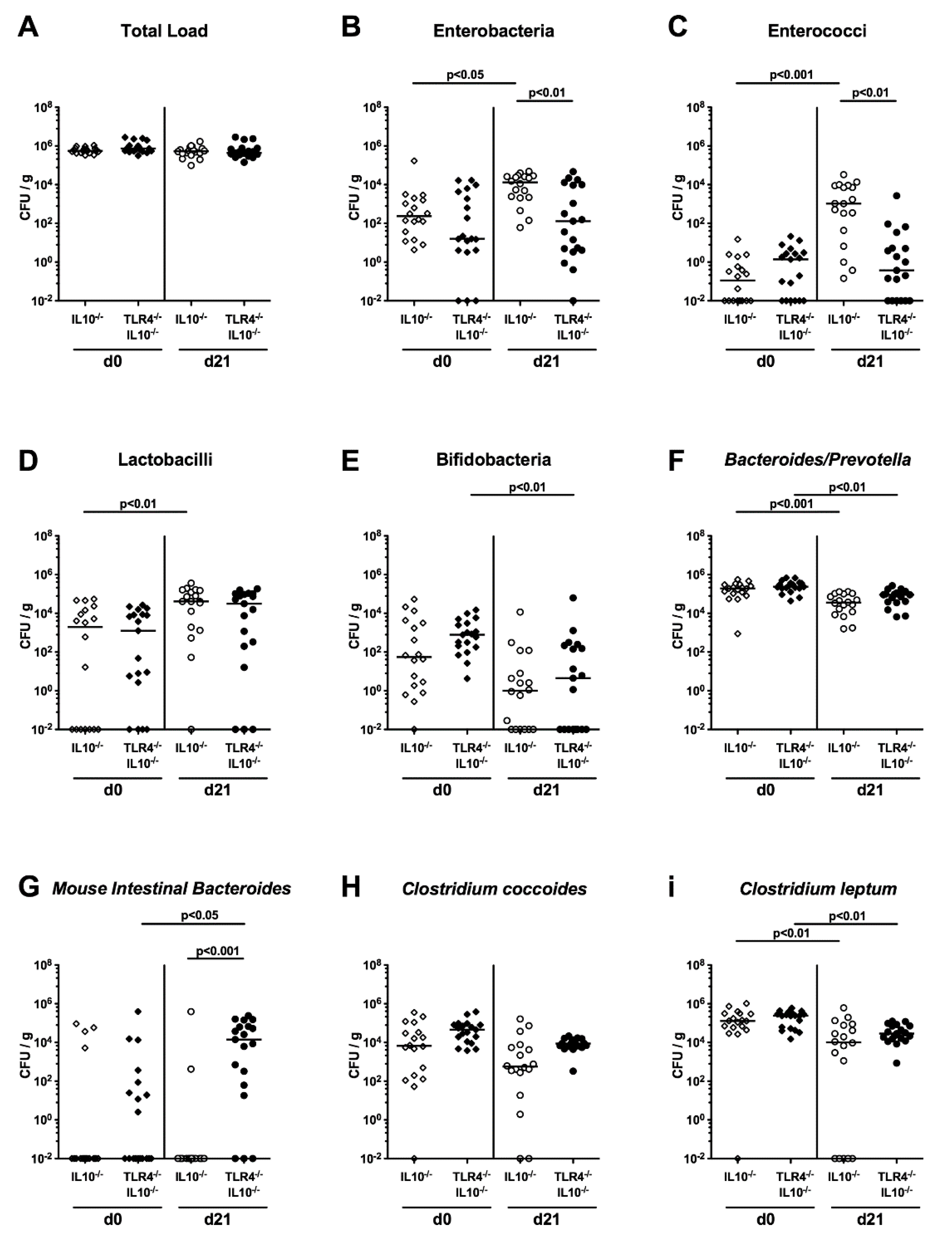
2.2. Commensal Gut Microbiota Changes Following C. coli Infection of Human Microbiota-Associated TLR4-Deficient IL10-/- Mice
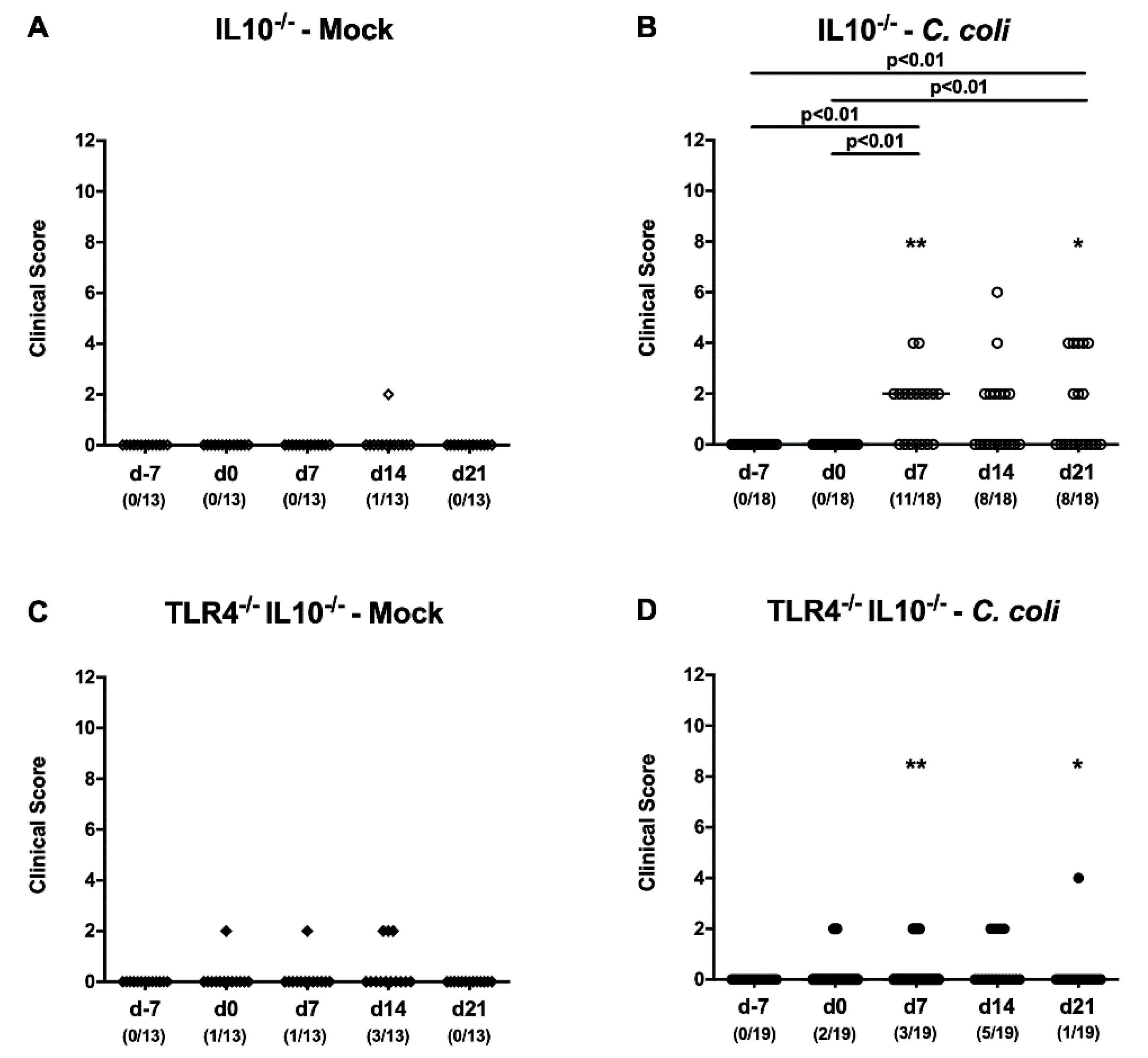
2.3. Kinetic Survey of Clinical Signs Exerted by C. coli-Infected Human Microbiota-Associated TLR4-Deficient IL10-/- Mice
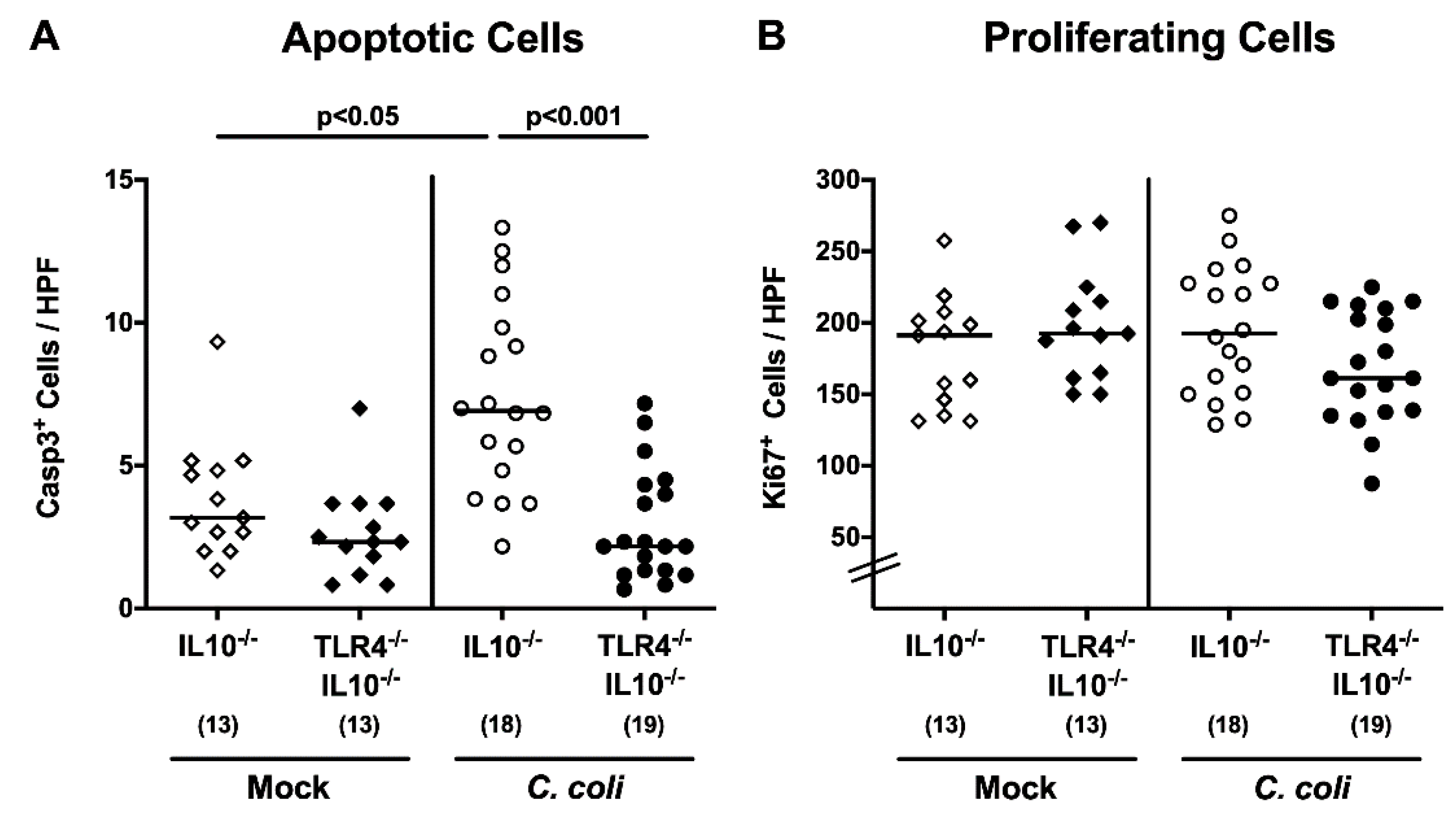
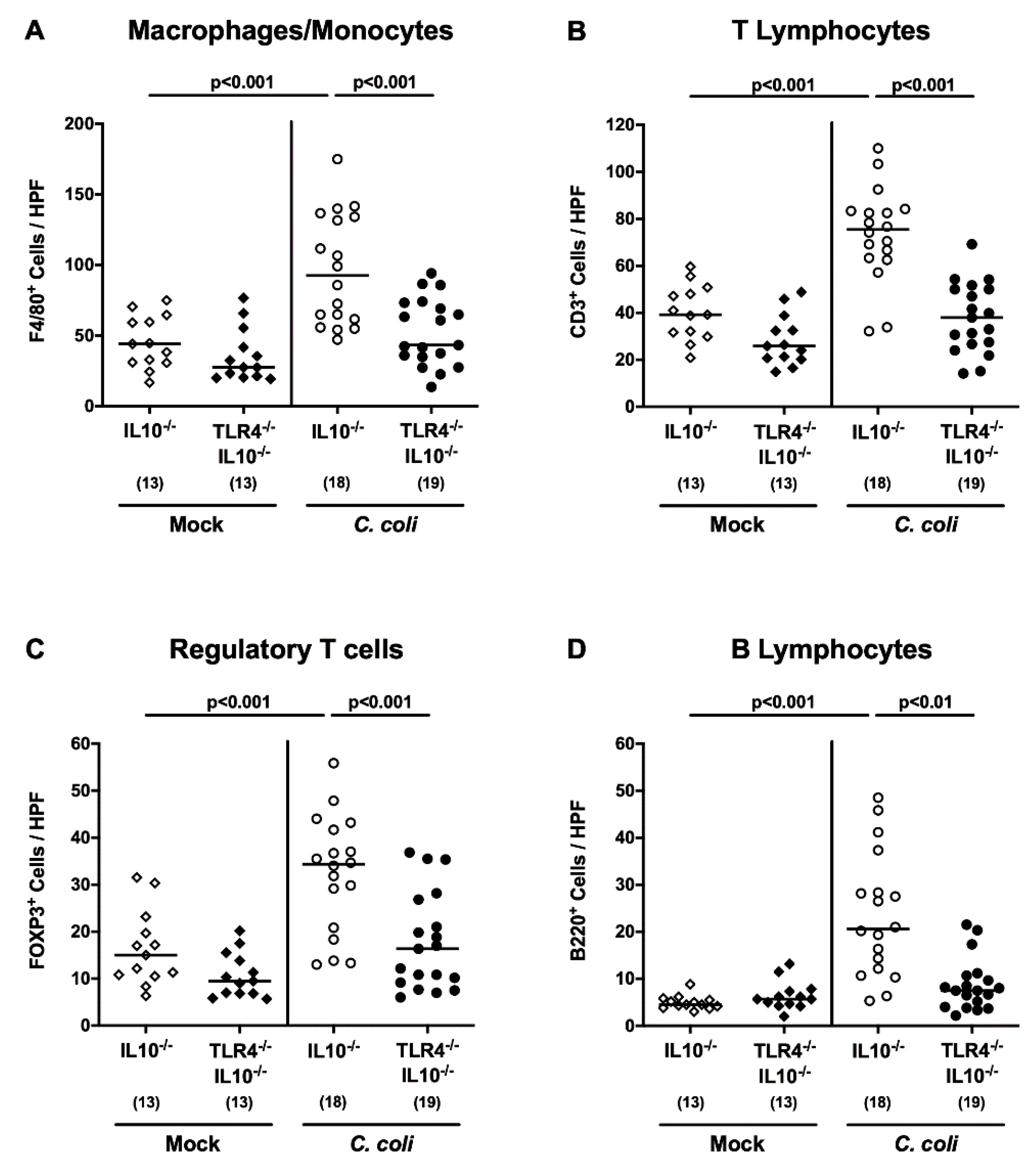
2.4. Colonic Apoptotic and Immune Cell Responses Following C. coli Infection of Human Microbiota-Associated TLR4-Deficient IL10-/- Mice
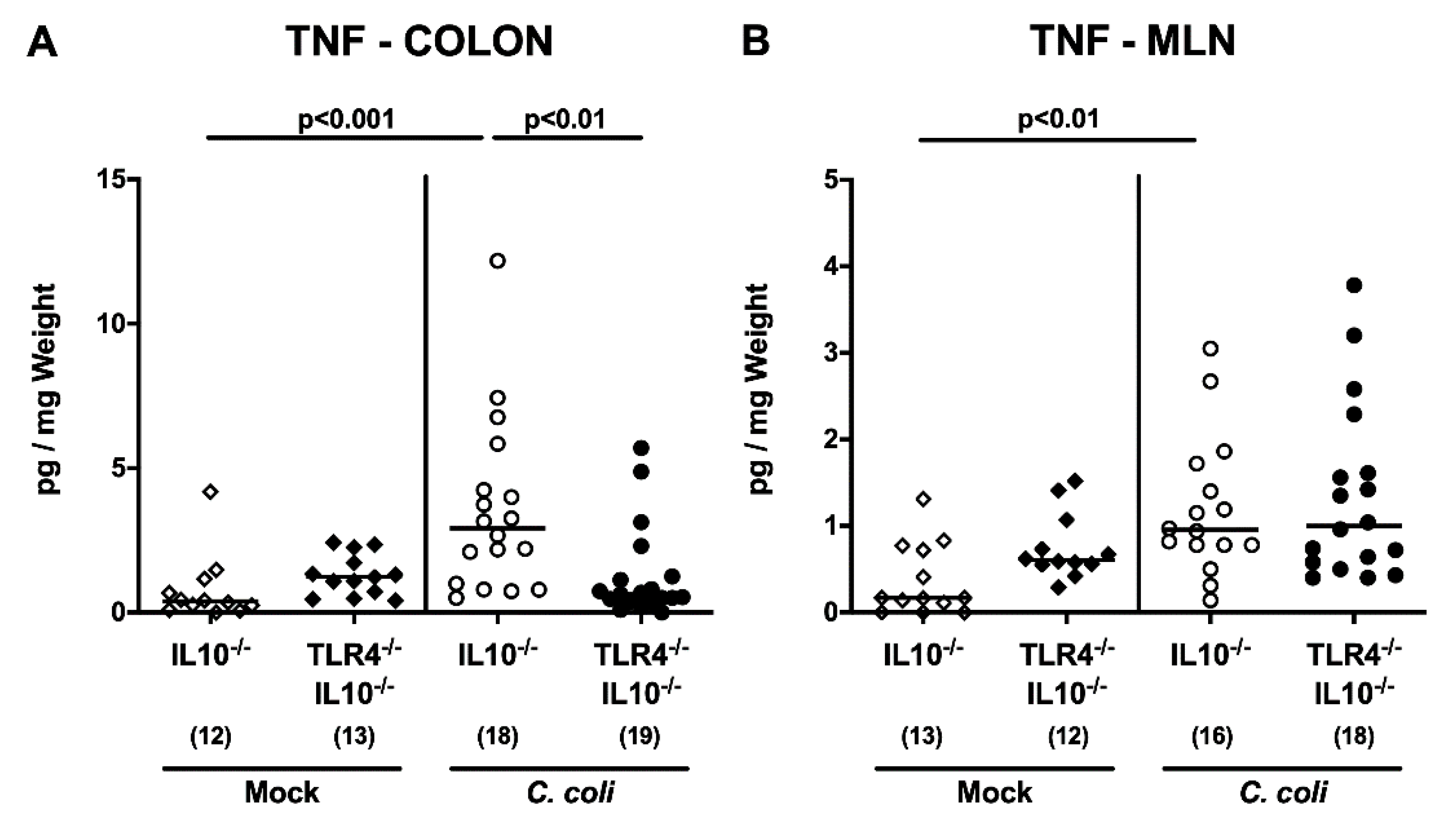
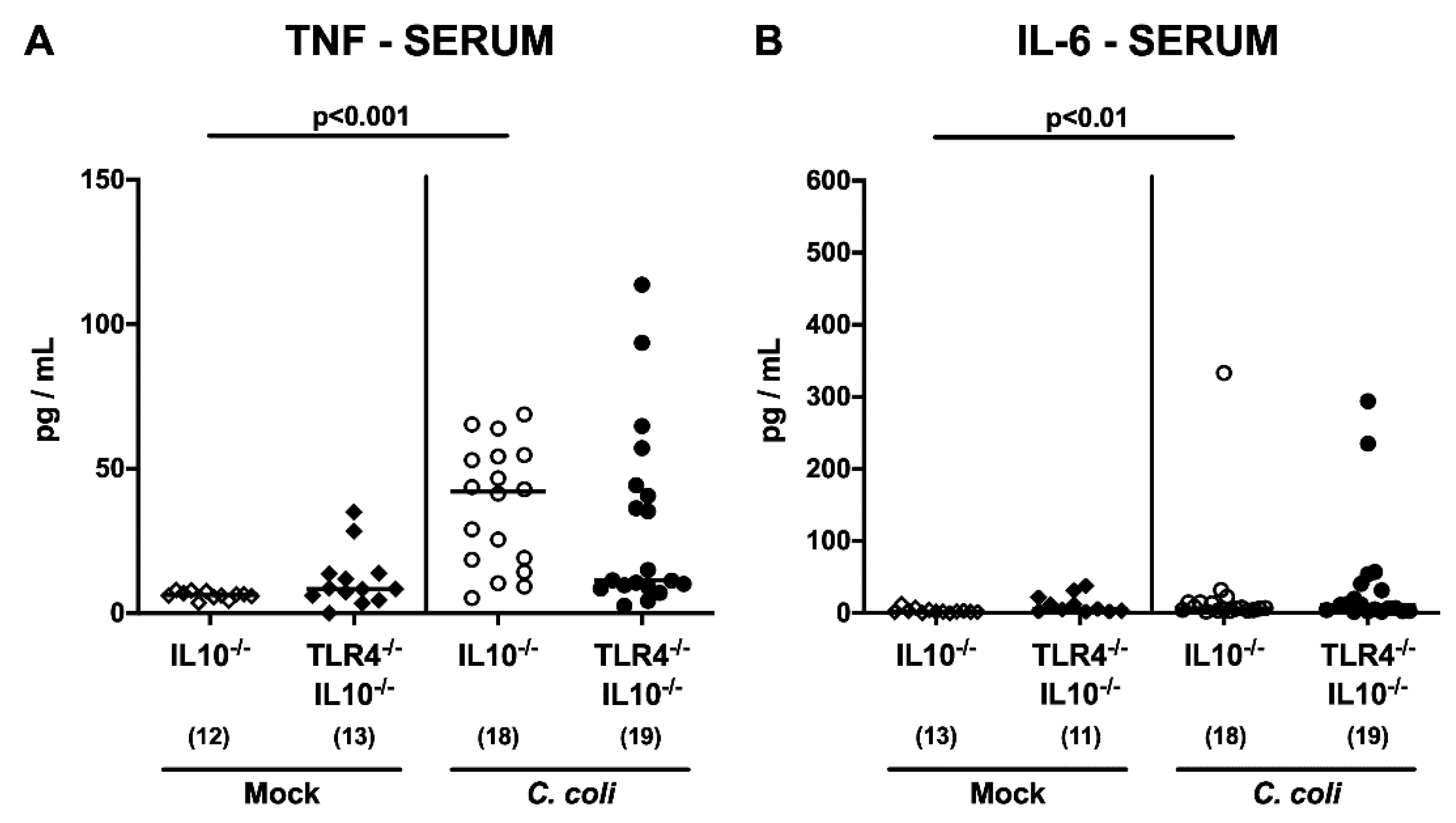
2.5. Intestinal and Systemic Pro-Inflammatory Cytokine Secretion Following C. coli Infection of Human Microbiota-Associated TLR4-Deficient IL10-/- Mice
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Generation of Secondary Abiotic Mice
4.3. Reassociation of Secondary Abiotic Mice with a Human Gut Microbiota by Fecal Microbiota Transplantation
4.4. C. coli Infection and Gastrointestinal Colonization
4.5. Cultural and Culture-Independent (i.e., Molecular) Survey of the Human Donor Suspensions and Gut Microbiota
4.6. Clinical Conditions
4.7. Sampling Procedures
4.8. Immunohistochemistry
4.9. Pro-Inflammatory Cytokines in Intestinal and Serum Samples
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sheppard, S.K.; Maiden, M.C. The evolution of Campylobacter jejuni and Campylobacter coli. Cold Spring Harb. Perspect. Biol. 2015, 7, a018119. [Google Scholar] [CrossRef]
- World Health Organization. Campylobacter. Available online: https://www.who.int/news-room/fact-sheets/detail/campylobacter (accessed on 2 January 2020).
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2018 Zoonoses Report. EFSA J. 2019, 17, e05926. [Google Scholar] [CrossRef]
- Alter, T.; Bereswill, S.; Glünder, G.; Haag, L.-M.; Hänel, I.; Heimesaat, M.; Lugert, R.; Rautenschlein, S.; Weber, R.; Zautner, A.; et al. Die Campylobacteriose des Menschen. Bundesgesundheitsblatt Gesundh. Gesundh. 2011, 54, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.I.; Caldwell, M.B.; Lee, E.C.; Guerry, P.; Trust, T.J.; Ruiz-Palacios, G.M. Pathophysiology of Campylobacter enteritis. Microbiol. Rev. 1986, 50, 81–94. [Google Scholar] [CrossRef]
- Backert, S.; Tegtmeyer, N.; Cróinín, T.Ó.; Boehm, M.; Heimesaat, M.M. Chapter 1-Human campylobacteriosis. In Campylobacter; Klein, G., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 1–25. [Google Scholar]
- Kist, M.; Bereswill, S. Campylobacter jejuni. Contrib. Microbiol. 2001, 8, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Allos, B.M. Association between Campylobacter infection and Guillain-Barre syndrome. J. Infect. Dis. 1997, 176 (Suppl. 2), S125–S128. [Google Scholar] [CrossRef]
- Facciolà, A.; Riso, R.; Avventuroso, E.; Visalli, G.; Delia, S.A.; Laganà, P. Campylobacter: From microbiology to prevention. J. Prev. Med. Hyg. 2017, 58, E79–E92. [Google Scholar] [PubMed]
- Abreu, M.T.; Arditi, M. Innate immunity and toll-like receptors: Clinical implications of basic science research. J. Pediatr. 2004, 144, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, K.; Takeuchi, O.; Kawai, T.; Sanjo, H.; Ogawa, T.; Takeda, Y.; Takeda, K.; Akira, S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J. Immunol. 1999, 162, 3749–3752. [Google Scholar] [PubMed]
- Pridmore, A.C.; Jarvis, G.A.; John, C.M.; Jack, D.L.; Dower, S.K.; Read, R.C. Activation of toll-like receptor 2 (TLR2) and TLR4/MD2 by Neisseria is independent of capsule and lipooligosaccharide (LOS) sialylation but varies widely among LOS from different strains. Infect. Immun. 2003, 71, 3901–3908. [Google Scholar] [CrossRef]
- Preston, A.; Mandrell, R.E.; Gibson, B.W.; Apicella, M.A. The lipooligosaccharides of pathogenic gram-negative bacteria. Crit. Rev. Microbiol. 1996, 22, 139–180. [Google Scholar] [CrossRef] [PubMed]
- Rietschel, E.T.; Kirikae, T.; Schade, F.U.; Mamat, U.; Schmidt, G.; Loppnow, H.; Ulmer, A.J.; Zahringer, U.; Seydel, U.; Di Padova, F.; et al. Bacterial endotoxin: Molecular relationships of structure to activity and function. FASEB J. 1994, 8, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Haag, L.M.; Fischer, A.; Otto, B.; Plickert, R.; Kuhl, A.A.; Gobel, U.B.; Bereswill, S.; Heimesaat, M.M. Campylobacter jejuni induces acute enterocolitis in gnotobiotic IL-10-/- mice via Toll-like-receptor-2 and -4 signaling. PLoS ONE 2012, 7, e40761. [Google Scholar] [CrossRef] [PubMed]
- Stahl, M.; Ries, J.; Vermeulen, J.; Yang, H.; Sham, H.P.; Crowley, S.M.; Badayeva, Y.; Turvey, S.E.; Gaynor, E.C.; Li, X.; et al. A novel mouse model of Campylobacter jejuni gastroenteritis reveals key pro-inflammatory and tissue protective roles for Toll-like receptor signaling during infection. PLoS Pathog. 2014, 10, e1004264. [Google Scholar] [CrossRef]
- Rathinam, V.A.; Appledorn, D.M.; Hoag, K.A.; Amalfitano, A.; Mansfield, L.S. Campylobacter jejuni-induced activation of dendritic cells involves cooperative signaling through Toll-like receptor 4 (TLR4)-MyD88 and TLR4-TRIF axes. Infect. Immun. 2009, 77, 2499–2507. [Google Scholar] [CrossRef]
- de Zoete, M.R.; Keestra, A.M.; Roszczenko, P.; van Putten, J.P. Activation of human and chicken toll-like receptors by Campylobacter spp. Infect. Immun. 2010, 78, 1229–1238. [Google Scholar] [CrossRef]
- Bereswill, S.; Fischer, A.; Plickert, R.; Haag, L.-M.; Otto, B.; Kühl, A.A.; Dashti, J.I.; Zautner, A.E.; Muñoz, M.; Loddenkemper, C.; et al. Novel Murine Infection Models Provide Deep Insights into the “Ménage à Trois” of Campylobacter jejuni, Microbiota and Host Innate Immunity. PLoS ONE 2011, 6, e20953. [Google Scholar] [CrossRef]
- Mortensen, N.P.; Kuijf, M.L.; Ang, C.W.; Schiellerup, P.; Krogfelt, K.A.; Jacobs, B.C.; van Belkum, A.; Endtz, H.P.; Bergman, M.P. Sialylation of Campylobacter jejuni lipo-oligosaccharides is associated with severe gastro-enteritis and reactive arthritis. Microbes Infect. 2009, 11, 988–994. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef]
- d’Hennezel, E.; Abubucker, S.; Murphy, L.O.; Cullen, T.W. Total Lipopolysaccharide from the Human Gut Microbiome Silences Toll-Like Receptor Signaling. mSystems 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Poltorak, A.; He, X.; Smirnova, I.; Liu, M.Y.; Van Huffel, C.; Du, X.; Birdwell, D.; Alejos, E.; Silva, M.; Galanos, C.; et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science 1998, 282, 2085–2088. [Google Scholar] [CrossRef] [PubMed]
- Rakoff-Nahoum, S.; Paglino, J.; Eslami-Varzaneh, F.; Edberg, S.; Medzhitov, R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 2004, 118, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Masanta, W.O.; Heimesaat, M.M.; Bereswill, S.; Tareen, A.M.; Lugert, R.; Gross, U.; Zautner, A.E. Modification of intestinal microbiota and its consequences for innate immune response in the pathogenesis of campylobacteriosis. Clin. Dev. Immunol. 2013, 2013, 526860. [Google Scholar] [CrossRef]
- Warren, H.S.; Fitting, C.; Hoff, E.; Adib-Conquy, M.; Beasley-Topliffe, L.; Tesini, B.; Liang, X.; Valentine, C.; Hellman, J.; Hayden, D.; et al. Resilience to bacterial infection: Difference between species could be due to proteins in serum. J. Infect. Dis. 2010, 201, 223–232. [Google Scholar] [CrossRef]
- Robertson, S.A.; Care, A.S.; Skinner, R.J. Interleukin 10 regulates inflammatory cytokine synthesis to protect against lipopolysaccharide-induced abortion and fetal growth restriction in mice. Biol. Reprod. 2007, 76, 738–748. [Google Scholar] [CrossRef]
- da Silva, A.M.T.; Kaulbach, H.C.; Chuidian, F.S.; Lambert, D.R.; Suffredini, A.F.; Danner, R.L. Shock and Multiple-Organ Dysfunction after Self-Administration of Salmonella Endotoxin. N. Engl. J. Med. 1993, 328, 1457–1460. [Google Scholar] [CrossRef]
- Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Novel Clinical Campylobacter jejuni Infection Models Based on Sensitization of Mice to Lipooligosaccharide, a Major Bacterial Factor Triggering Innate Immune Responses in Human Campylobacteriosis. Microorganisms 2020, 8, 482. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Alutis, M.; Grundmann, U.; Fischer, A.; Tegtmeyer, N.; Böhm, M.; Kühl, A.A.; Göbel, U.B.; Backert, S.; Bereswill, S. The role of serine protease HtrA in acute ulcerative enterocolitis and extra-intestinal immune responses during Campylobacter jejuni infection of gnotobiotic IL-10 deficient mice. Front. Cell Infect. Microbiol. 2014, 4, 77. [Google Scholar] [CrossRef]
- Tam, C.C.; O’Brien, S.J.; Adak, G.K.; Meakins, S.M.; Frost, J.A. Campylobacter coli - an important foodborne pathogen. J. Infect. 2003, 47, 28–32. [Google Scholar] [CrossRef]
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global Epidemiology of Campylobacter Infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef] [PubMed]
- Culebro, A.; Machado, M.P.; Carriço, J.A.; Rossi, M. Origin, evolution, and distribution of the molecular machinery for biosynthesis of sialylated lipooligosaccharide structures in Campylobacter coli. Sci. Rep. 2018, 8, 3028. [Google Scholar] [CrossRef] [PubMed]
- Culebro, A.; Revez, J.; Pascoe, B.; Friedmann, Y.; Hitchings, M.D.; Stupak, J.; Sheppard, S.K.; Li, J.; Rossi, M. Sequence Diversity within the Biosynthesis Locus and Common Biochemical Features of Campylobacter coli Lipooligosaccharides. J. Bacteriol. 2016, 198, 2829–2840. [Google Scholar] [CrossRef] [PubMed]
- Klančnik, A.; Pogačar, M.Š.; Raspor, P.; Abram, M.; Možina, S.S.; Vučković, D. Virulence genes and cytokine profile in systemic murine Campylobacter coli infection. Virulence 2015, 6, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Genger, C.; Kløve, S.; Mousavi, S.; Bereswill, S.; Heimesaat, M.M. The conundrum of colonization resistance against Campylobacter reloaded: The gut microbota composition in conventional mice does not prevent from Campylobacter coli infection. Eur. J. Microbiol. Immun. (Bp) 2020. [Google Scholar] [CrossRef]
- Ortega-Cava, C.F.; Ishihara, S.; Rumi, M.A.K.; Kawashima, K.; Ishimura, N.; Kazumori, H.; Udagawa, J.; Kadowaki, Y.; Kinoshita, Y. Strategic Compartmentalization of Toll-Like Receptor 4 in the Mouse Gut. J. Immunol. 2003, 170, 3977. [Google Scholar] [CrossRef]
- Schmausser, B.; Andrulis, M.; Endrich, S.; Lee, S.K.; Josenhans, C.; Müller-Hermelink, H.K.; Eck, M. Expression and subcellular distribution of toll-like receptors TLR4, TLR5 and TLR9 on the gastric epithelium in Helicobacter pylori infection. Clin. Exp. Immunol. 2004, 136, 521–526. [Google Scholar] [CrossRef]
- Haase, R.; Kirschning, C.J.; Sing, A.; Schrottner, P.; Fukase, K.; Kusumoto, S.; Wagner, H.; Heesemann, J.; Ruckdeschel, K. A dominant role of Toll-like receptor 4 in the signaling of apoptosis in bacteria-faced macrophages. J. Immunol. 2003, 171, 4294–4303. [Google Scholar] [CrossRef]
- Kibe, R.; Sakamoto, M.; Yokota, H.; Benno, Y. Characterization of the inhabitancy of mouse intestinal bacteria (MIB) in rodents and humans by real-time PCR with group-specific primers. Microbiol. Immunol. 2007, 51, 349–357. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Bereswill, S.; Fischer, A.; Fuchs, D.; Struck, D.; Niebergall, J.; Jahn, H.K.; Dunay, I.R.; Moter, A.; Gescher, D.M.; et al. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J. Immunol. 2006, 177, 8785–8795. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Fischer, A.; Siegmund, B.; Kupz, A.; Niebergall, J.; Fuchs, D.; Jahn, H.K.; Freudenberg, M.; Loddenkemper, C.; Batra, A.; et al. Shift towards pro-inflammatory intestinal bacteria aggravates acute murine colitis via Toll-like receptors 2 and 4. PLoS ONE 2007, 2, e662. [Google Scholar] [CrossRef] [PubMed]
- Heimesaat, M.M.; Fischer, A.; Jahn, H.K.; Niebergall, J.; Freudenberg, M.; Blaut, M.; Liesenfeld, O.; Schumann, R.R.; Gobel, U.B.; Bereswill, S. Exacerbation of murine ileitis by Toll-like receptor 4 mediated sensing of lipopolysaccharide from commensal Escherichia coli. Gut 2007, 56, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Heimesaat, M.M.; Danker, K.; Struck, D.; Lohmann, U.; Plickert, R.; Bereswill, S.; Fischer, A.; Dunay, I.R.; Wolk, K.; et al. Interleukin (IL)-23 mediates Toxoplasma gondii–induced immunopathology in the gut via matrixmetalloproteinase-2 and IL-22 but independent of IL-17. J. Exp. Med. 2009, 206, 3047–3059. [Google Scholar] [CrossRef] [PubMed]
- Erridge, C.; Duncan, S.H.; Bereswill, S.; Heimesaat, M.M. The Induction of Colitis and Ileitis in Mice Is Associated with Marked Increases in Intestinal Concentrations of Stimulants of TLRs 2, 4, and 5. PLoS ONE 2010, 5, e9125. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Nogai, A.; Bereswill, S.; Plickert, R.; Fischer, A.; Loddenkemper, C.; Steinhoff, U.; Tchaptchet, S.; Thiel, E.; Freudenberg, M.A.; et al. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut 2010, 59, 1079–1087. [Google Scholar] [CrossRef]
- Bereswill, S.; Munoz, M.; Fischer, A.; Plickert, R.; Haag, L.M.; Otto, B.; Kuhl, A.A.; Loddenkemper, C.; Gobel, U.B.; Heimesaat, M.M. Anti-inflammatory effects of resveratrol, curcumin and simvastatin in acute small intestinal inflammation. PLoS ONE 2010, 5, e15099. [Google Scholar] [CrossRef] [PubMed]
- Haag, L.M.; Fischer, A.; Otto, B.; Plickert, R.; Kuhl, A.A.; Gobel, U.B.; Bereswill, S.; Heimesaat, M.M. Intestinal microbiota shifts towards elevated commensal Escherichia coli loads abrogate colonization resistance against Campylobacter jejuni in mice. PLoS ONE 2012, 7, e35988. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Boelke, S.; Fischer, A.; Haag, L.-M.; Loddenkemper, C.; Kühl, A.A.; Göbel, U.B.; Bereswill, S. Comprehensive Postmortem Analyses of Intestinal Microbiota Changes and Bacterial Translocation in Human Flora Associated Mice. PLoS ONE 2012, 7, e40758. [Google Scholar] [CrossRef]
- Haag, L.M.; Fischer, A.; Otto, B.; Grundmann, U.; Kuhl, A.A.; Gobel, U.B.; Bereswill, S.; Heimesaat, M.M. Campylobacter jejuni infection of infant mice: Acute enterocolitis is followed by asymptomatic intestinal and extra-intestinal immune responses. Eur. J. Microbiol. Immunol. (Bp) 2012, 2, 2–11. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Bereswill, S. Murine infection models for the investigation of Campylobacter jejuni—Host interactions and pathogenicity. Berl. Munch Tierarztl. Wochenschr. 2015, 128, 98–103. [Google Scholar]
- Fiebiger, U.; Bereswill, S.; Heimesaat, M.M. Dissecting the Interplay Between Intestinal Microbiota and Host Immunity in Health and Disease: Lessons Learned from Germfree and Gnotobiotic Animal Models. Eur. J. Microbiol. Immunol. (Bp) 2016, 6, 253–271. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.-C.; Walter, J.; Finlay, B.B. Human Microbiota-Associated Mice: A Model with Challenges. Cell Host Microbe 2016, 19, 575–578. [Google Scholar] [CrossRef] [PubMed]
- von Klitzing, E.; Ekmekciu, I.; Kuhl, A.A.; Bereswill, S.; Heimesaat, M.M. Intestinal, extra-intestinal and systemic sequelae of Toxoplasma gondii induced acute ileitis in mice harboring a human gut microbiota. PLoS ONE 2017, 12, e0176144. [Google Scholar] [CrossRef] [PubMed]
- von Klitzing, E.; Ekmekciu, I.; Bereswill, S.; Heimesaat, M.M. Acute ileitis facilitates infection with multidrug resistant Pseudomonas aeruginosa in human microbiota-associated mice. Gut Pathog. 2017, 9, 4. [Google Scholar] [CrossRef]
- von Klitzing, E.; Ekmekciu, I.; Bereswill, S.; Heimesaat, M.M. Intestinal and Systemic Immune Responses upon Multi-drug Resistant Pseudomonas aeruginosa Colonization of Mice Harboring a Human Gut Microbiota. Front. Microbiol. 2017, 8, 2590. [Google Scholar] [CrossRef]
- Escher, U.; Giladi, E.; Dunay, I.R.; Bereswill, S.; Gozes, I.; Heimesaat, M.M. Anti-inflammatory Effects of the Octapeptide NAP in Human Microbiota-Associated Mice Suffering from Subacute Ileitis. Eur. J. Microbiol. Immunol. (Bp) 2018, 8, 34–40. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Escher, U.; Grunau, A.; Fiebiger, U.; Bereswill, S. Peroral Low-Dose Toxoplasma gondii Infection of Human Microbiota-Associated Mice - A Subacute Ileitis Model to Unravel Pathogen-Host Interactions. Eur. J. Microbiol. Immunol. (Bp) 2018, 8, 53–61. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Mrazek, K.; Bereswill, S. Murine Fecal Microbiota Transplantation Alleviates Intestinal and Systemic Immune Responses in Campylobacter jejuni Infected Mice Harboring a Human Gut Microbiota. Front. Immunol. 2019, 10, 2272. [Google Scholar] [CrossRef]
- Bereswill, S.; Escher, U.; Grunau, A.; Kuhl, A.A.; Dunay, I.R.; Tamas, A.; Reglodi, D.; Heimesaat, M.M. Pituitary Adenylate Cyclase-Activating Polypeptide-A Neuropeptide as Novel Treatment Option for Subacute Ileitis in Mice Harboring a Human Gut Microbiota. Front. Immunol. 2019, 10, 554. [Google Scholar] [CrossRef]
- Ekmekciu, I.; von Klitzing, E.; Fiebiger, U.; Escher, U.; Neumann, C.; Bacher, P.; Scheffold, A.; Kuhl, A.A.; Bereswill, S.; Heimesaat, M.M. Immune Responses to Broad-Spectrum Antibiotic Treatment and Fecal Microbiota Transplantation in Mice. Front. Immunol. 2017, 8, 397. [Google Scholar] [CrossRef]
- von Klitzing, E.; Bereswill, S.; Heimesaat, M.M. Multidrug-Resistant Pseudomonas Aeruginosa Induce Systemic Pro-Inflammatory Immune Responses in Colonized Mice. Eur. J. Microbiol. Immunol. (Bp) 2017, 7, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Heimesaat, M.M.; Haag, L.M.; Fischer, A.; Otto, B.; Kuhl, A.A.; Gobel, U.B.; Bereswill, S. Survey of extra-intestinal immune responses in asymptomatic long-term Campylobacter jejuni-infected mice. Eur. J. Microbiol. Immunol. (Bp) 2013, 3, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Alutis, M.E.; Grundmann, U.; Fischer, A.; Hagen, U.; Kuhl, A.A.; Gobel, U.B.; Bereswill, S.; Heimesaat, M.M. The Role of Gelatinases in Campylobacter Jejuni Infection of Gnotobiotic Mice. Eur. J. Microbiol. Immunol. (Bp) 2015, 5, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Alutis, M.E.; Grundmann, U.; Hagen, U.; Fischer, A.; Kuhl, A.A.; Gobel, U.B.; Bereswill, S.; Heimesaat, M.M. Matrix Metalloproteinase-2 Mediates Intestinal Immunopathogenesis in Campylobacter Jejuni-Infected Infant Mice. Eur. J. Microbiol. Immunol. (Bp) 2015, 5, 188–198. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Lugert, R.; Fischer, A.; Alutis, M.; Kuhl, A.A.; Zautner, A.E.; Tareen, A.M.; Gobel, U.B.; Bereswill, S. Impact of Campylobacter jejuni cj0268c knockout mutation on intestinal colonization, translocation, and induction of immunopathology in gnotobiotic IL-10 deficient mice. PLoS ONE 2014, 9, e90148. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kløve, S.; Genger, C.; Mousavi, S.; Weschka, D.; Bereswill, S.; Heimesaat, M.M. Toll-Like Receptor-4 Dependent Intestinal and Systemic Sequelae Following Peroral Campylobacter coli Infection of IL10 Deficient Mice Harboring a Human Gut Microbiota. Pathogens 2020, 9, 386. https://doi.org/10.3390/pathogens9050386
Kløve S, Genger C, Mousavi S, Weschka D, Bereswill S, Heimesaat MM. Toll-Like Receptor-4 Dependent Intestinal and Systemic Sequelae Following Peroral Campylobacter coli Infection of IL10 Deficient Mice Harboring a Human Gut Microbiota. Pathogens. 2020; 9(5):386. https://doi.org/10.3390/pathogens9050386
Chicago/Turabian StyleKløve, Sigri, Claudia Genger, Soraya Mousavi, Dennis Weschka, Stefan Bereswill, and Markus M. Heimesaat. 2020. "Toll-Like Receptor-4 Dependent Intestinal and Systemic Sequelae Following Peroral Campylobacter coli Infection of IL10 Deficient Mice Harboring a Human Gut Microbiota" Pathogens 9, no. 5: 386. https://doi.org/10.3390/pathogens9050386
APA StyleKløve, S., Genger, C., Mousavi, S., Weschka, D., Bereswill, S., & Heimesaat, M. M. (2020). Toll-Like Receptor-4 Dependent Intestinal and Systemic Sequelae Following Peroral Campylobacter coli Infection of IL10 Deficient Mice Harboring a Human Gut Microbiota. Pathogens, 9(5), 386. https://doi.org/10.3390/pathogens9050386





