Neospora caninum: Structure and Fate of Multinucleated Complexes Induced by the Bumped Kinase Inhibitor BKI-1294
Abstract
1. Introduction
2. Results
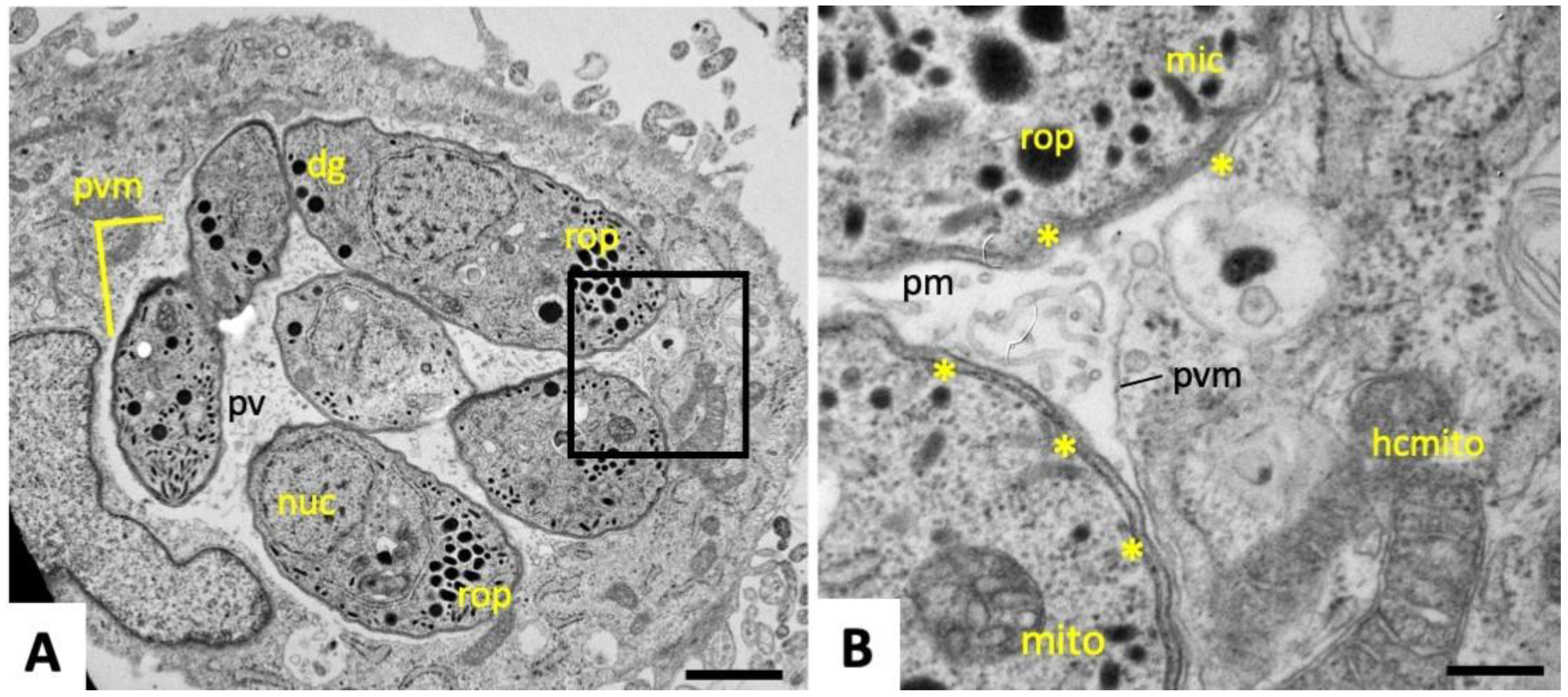
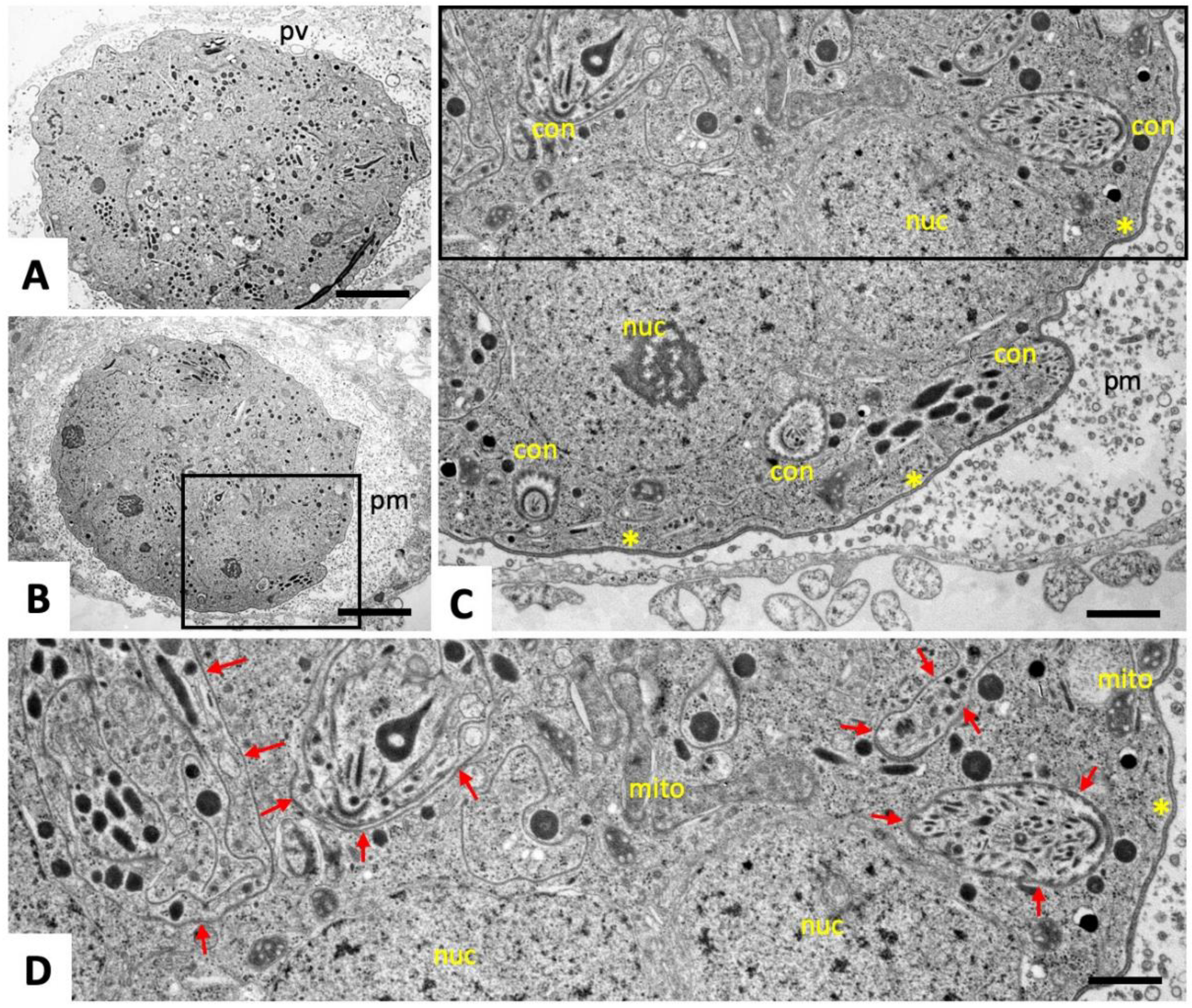
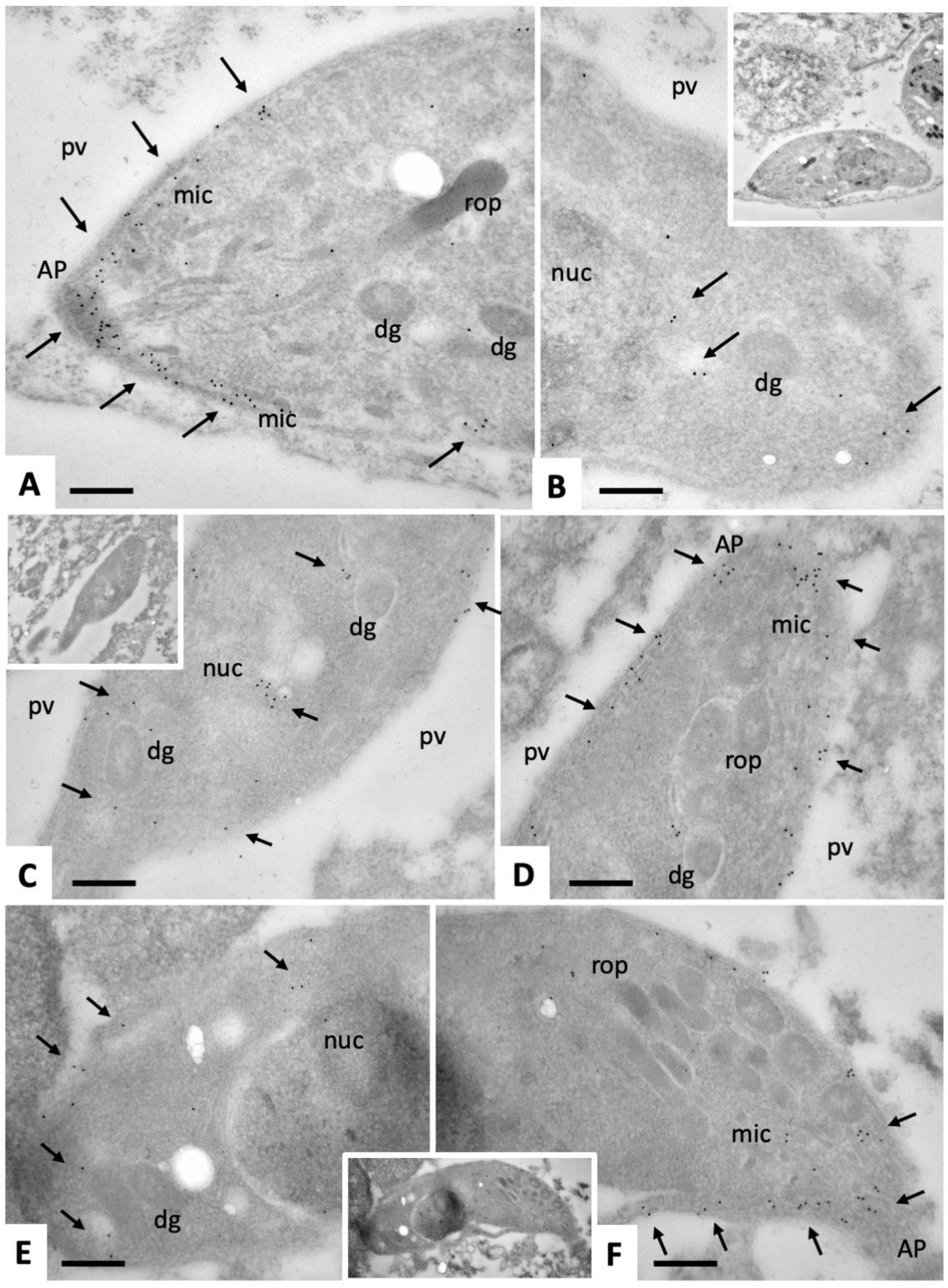
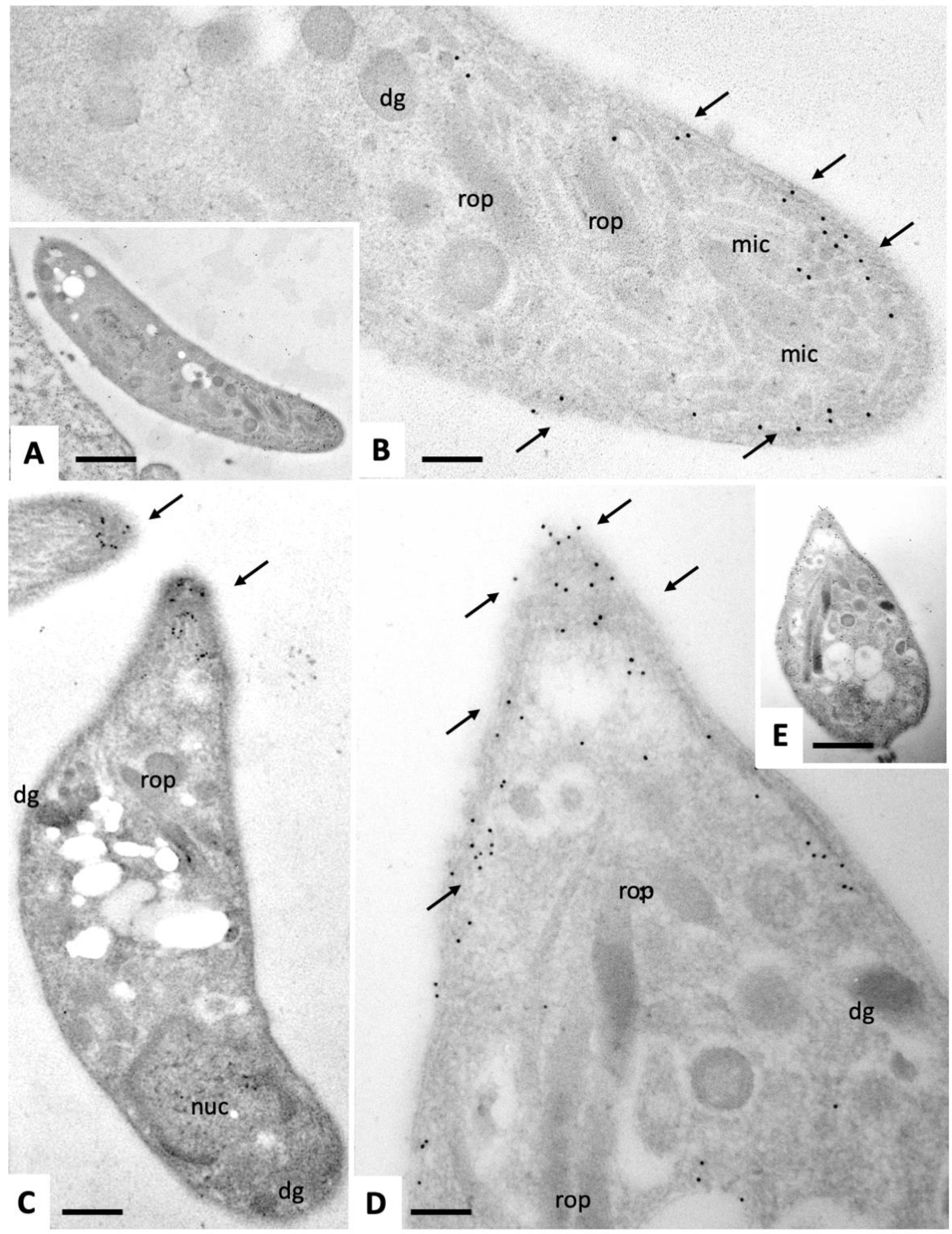
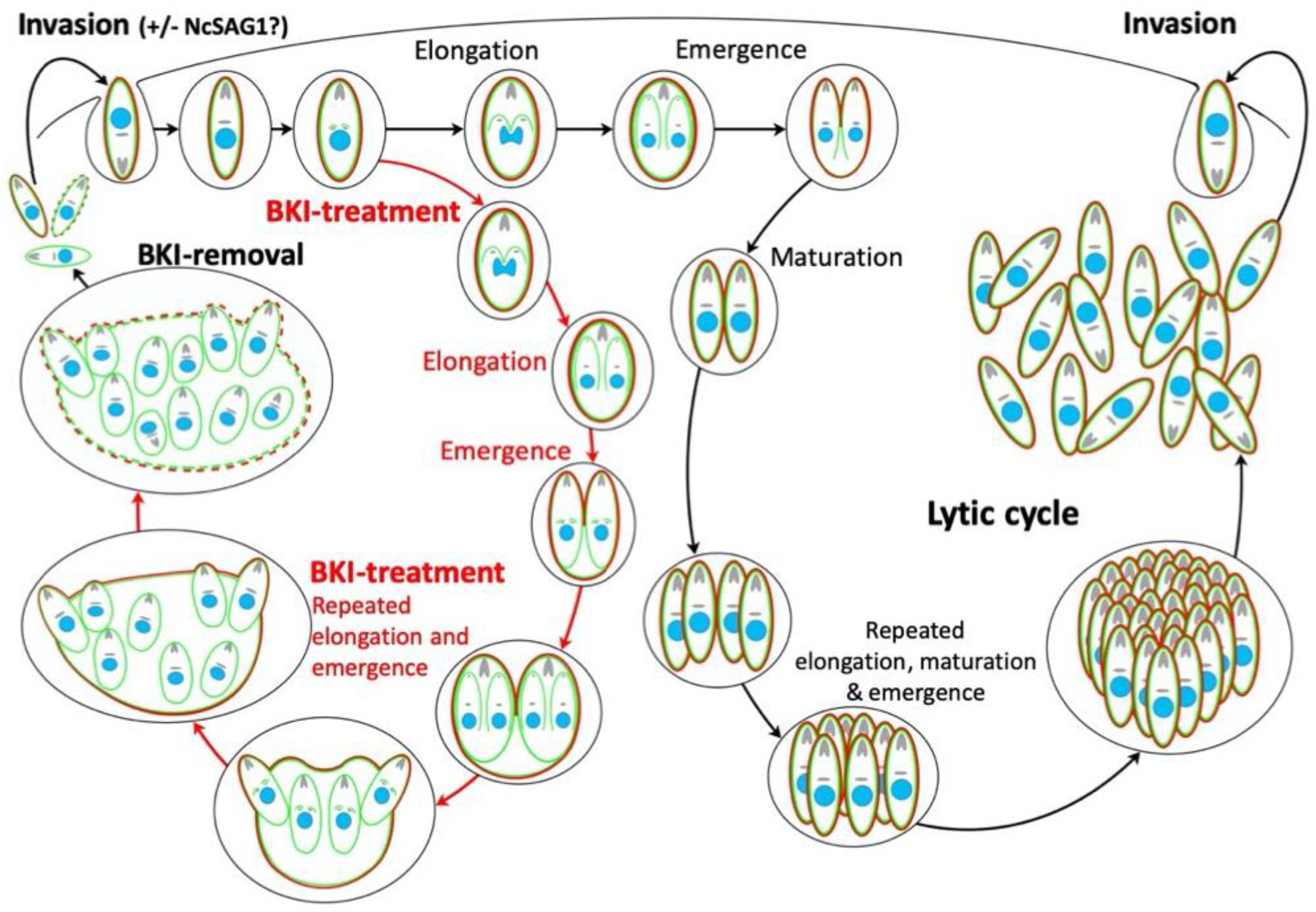
2.1. Ultrastructural Characteristics of Multinucleated Complexes (MNCs)
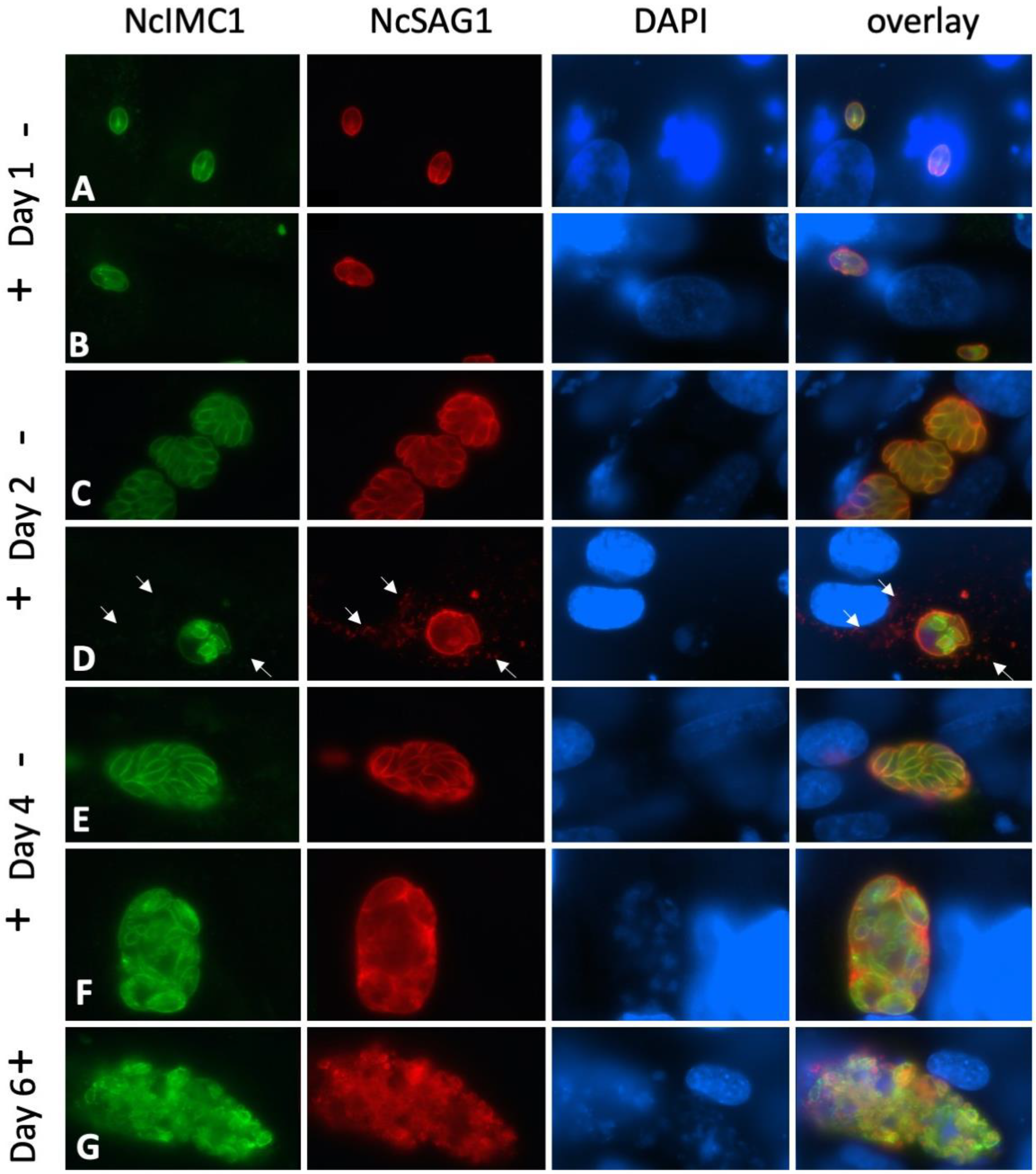
2.2. Expression of NcSAG1 and NcIMC1 during the Formation of MNCs and Subsequent Drug Release
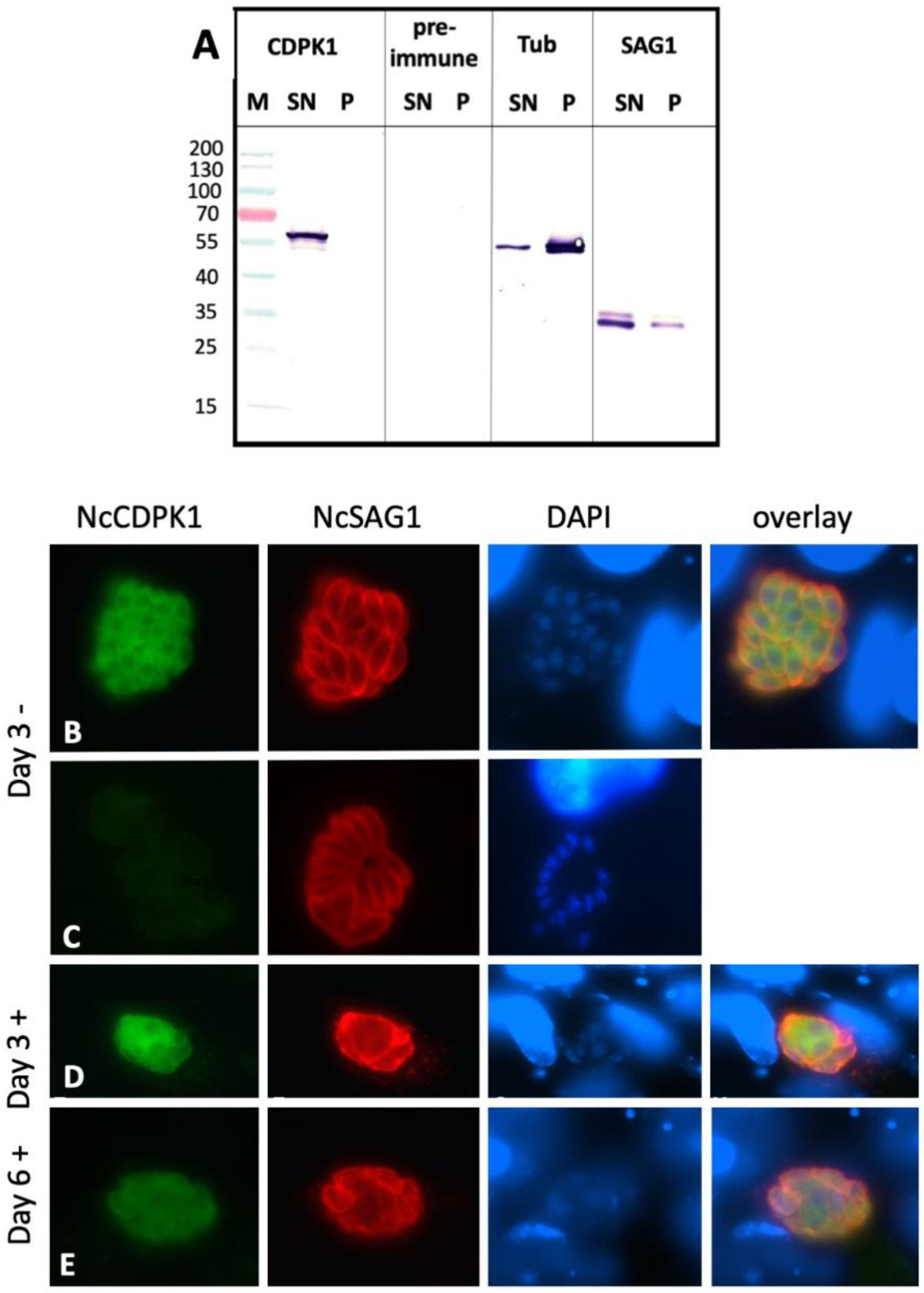
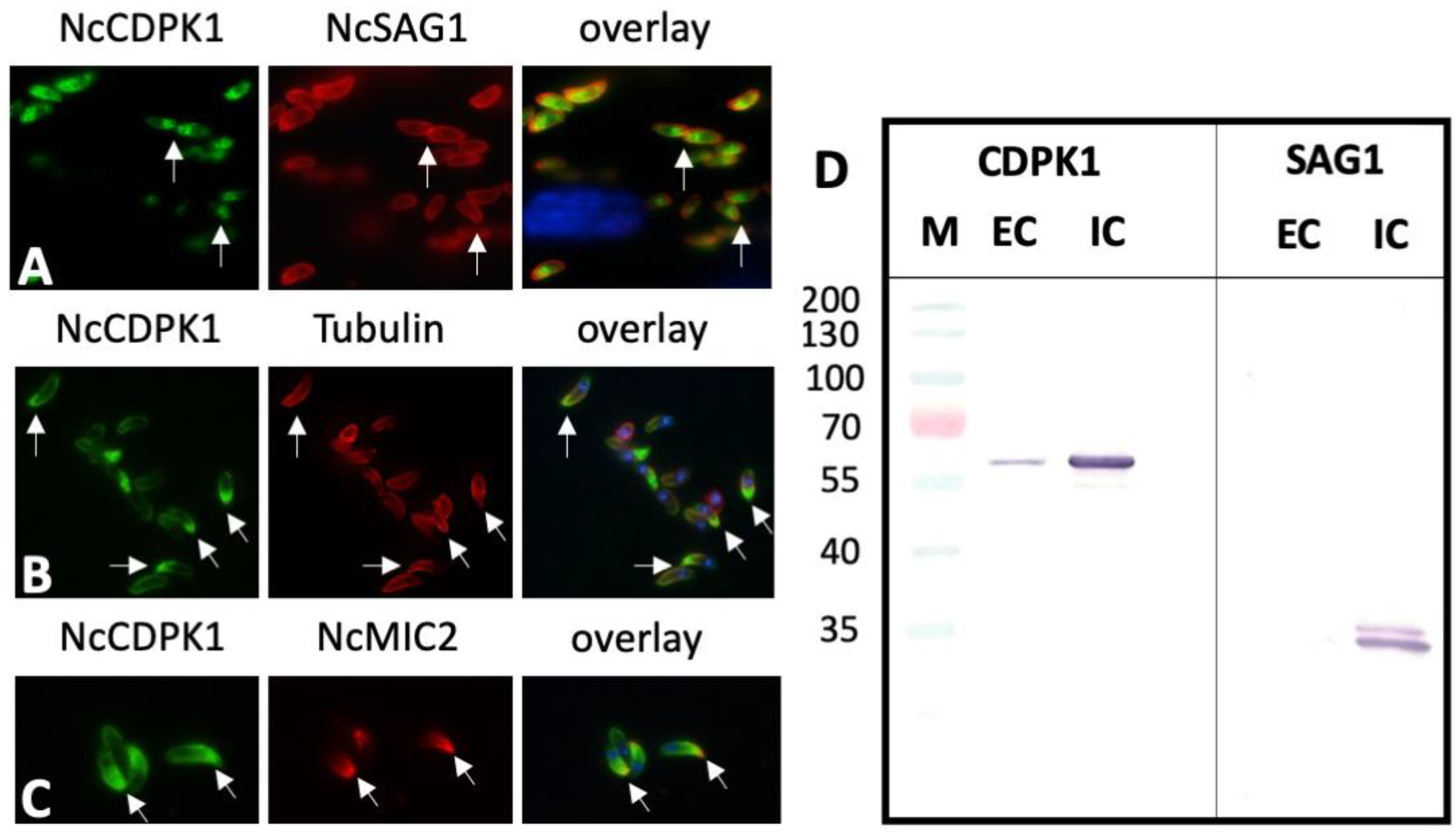
2.3. Expression of NcCDPK1 during the Formation of MNCs, Tachyzoite Egress, and Extracellular Maintenance of Tachyzoites
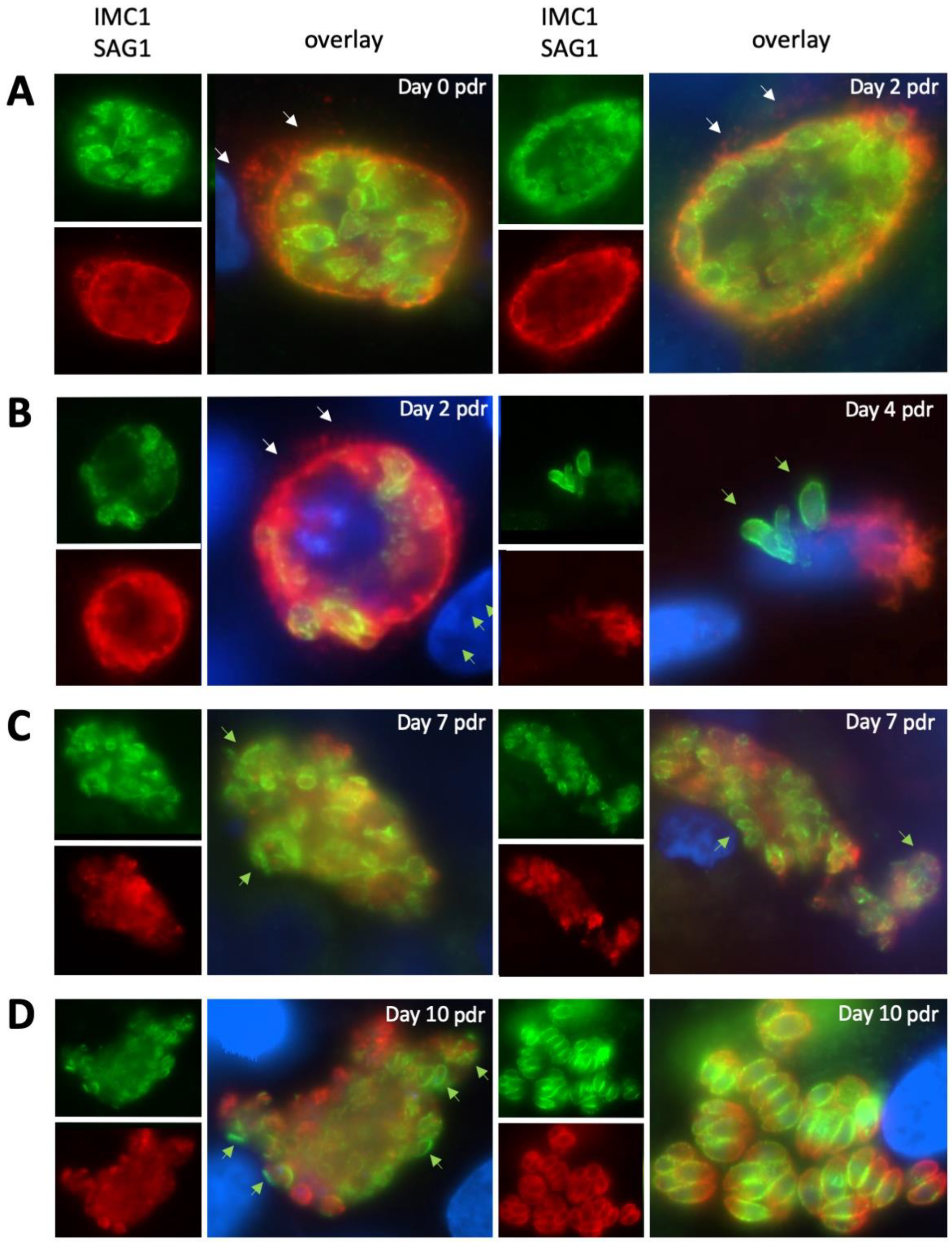
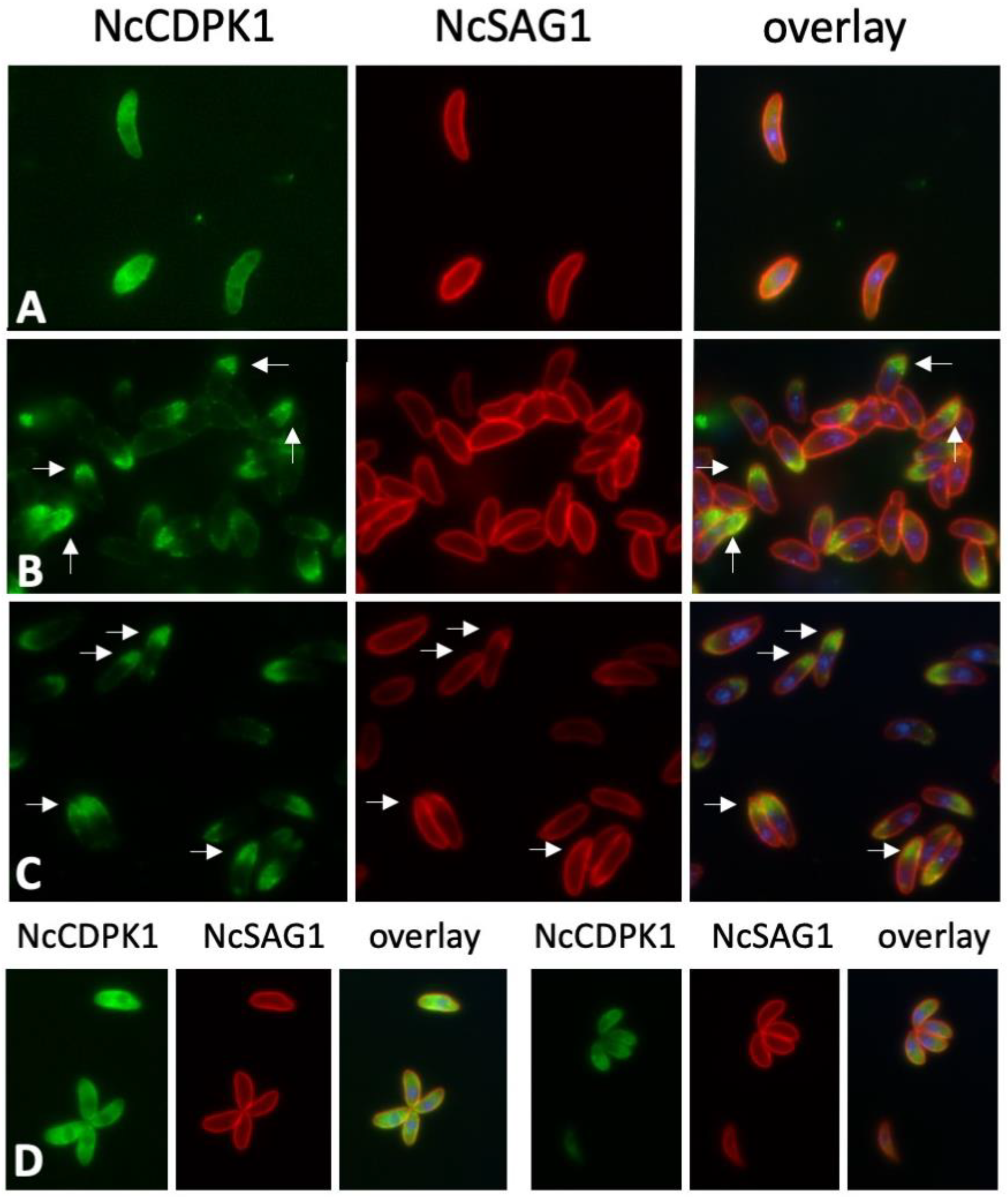
2.4. Expression of NcCDPK1 during the Formation of BKI-1294 Induced MNCs, Egress, and Extracellular Maintenance
3. Discussion
4. Materials and Methods
4.1. Tissue Culture Media, Biochemicals, and Drugs
4.2. Host Cell Cultivation and Parasite Maintenance
4.3. Analysis of N. caninum Tachyzoite Triton-X-100 and Secreted Fractions by Western Blots
4.4. Immunofluorescence (IF) Labeling of Intracellular Tachyzoites
4.5. IF-labeling of Extracellular Tachyzoites
4.6. Transmission Electron Microscopy (TEM)
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goodswen, S.J.; Kennedy, P.; Ellis, J. A review of the infection, genetics, and evolution of Neospora caninum: From the past to the present. Infect. Genet. Evol. 2013, 13, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, A.; Aguado-Martínez, A.; Müller, J. Approaches for the vaccination and treatment of Neospora caninum infections in mice and ruminant models. Parasitol. 2015, 143, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Reichel, M.; McAllister, M.; Pomroy, W.; Campero, C.; Ortega-Mora, L.M.; Ellis, J. Control options for Neospora caninum – is there anything new or are we going backwards? Parasitology 2014, 141, 1455–1470. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.C.; Cooke, B.M.; Doerig, C.; Saeij, J.P. Toxoplasma and Plasmodium protein kinases: Roles in invasion and host cell remodelling. Int. J. Parasitol. 2011, 42, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Saavedra, D.; Gabaldón, T.; Barton, G.J.; Langsley, G.; Doerig, C. The kinomes of apicomplexan parasites. Microbes Infect. 2012, 14, 796–810. [Google Scholar] [CrossRef] [PubMed]
- Van Voorhis, W.C.; Doggett, J.S.; Parsons, M.; Hulverson, M.A.; Choi, R.; Arnold, S.L.; Riggs, M.W.; Hemphill, A.; Howe, D.K.; Mealey, R.H.; et al. Extended-spectrum antiprotozoal bumped kinase inhibitors: A review. Exp. Parasitol. 2017, 180, 71–83. [Google Scholar] [CrossRef]
- Ojo, K.K.; Larson, E.T.; Keyloun, K.R.; Castaneda, L.J.; E DeRocher, A.; Inampudi, K.K.; E Kim, J.; Arakaki, T.L.; Murphy, R.C.; Zhang, L.; et al. Toxoplasma gondii calcium-dependent protein kinase 1 is a target for selective kinase inhibitors. Nat. Struct. Mol. Boil. 2010, 17, 602–607. [Google Scholar] [CrossRef]
- Johnson, S.M.; Murphy, R.C.; Geiger, J.A.; DeRocher, A.E.; Zhang, Z.; Ojo, K.K.; Larson, E.T.; Perera, B.G.K.; Dale, E.J.; He, P.; et al. Development ofToxoplasma gondiiCalcium-Dependent Protein Kinase 1 (TgCDPK1) Inhibitors with Potent Anti-ToxoplasmaActivity. J. Med. Chem. 2012, 55, 2416–2426. [Google Scholar] [CrossRef]
- Lourido, S.; Tang, K.; Sibley, L.D. Distinct signalling pathways control Toxoplasma egress and host-cell invasion. EMBO J. 2012, 31, 4524–4534. [Google Scholar] [CrossRef]
- Ojo, K.K.; Reid, M.C.; Siddaramaiah, L.K.; Müller, J.; Winzer, P.; Zhang, Z.; Keyloun, K.R.; Vidadala, R.S.R.; Merritt, E.A.; Hol, W.G.J.; et al. Neospora caninum Calcium-Dependent Protein Kinase 1 Is an Effective Drug Target for Neosporosis Therapy. PLoS ONE 2014, 9, e92929. [Google Scholar] [CrossRef]
- Winzer, P.; Müller, J.; Aguado-Martínez, A.; Rahman, M.; Balmer, V.; Manser, V.; Ortega-Mora, L.M.; Ojo, K.K.; Fan, E.; Maly, D.J.; et al. In Vitro and In Vivo Effects of the Bumped Kinase Inhibitor 1294 in the Related Cyst-Forming Apicomplexans Toxoplasma gondii and Neospora caninum. Antimicrob. Agents Chemother. 2015, 59, 6361–6374. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Aguado-Martínez, A.; Ortega-Mora, L.M.; Moreno-Gonzalo, J.; Ferre, I.; Hulverson, M.A.; Choi, R.; McCloskey, M.C.; Barrett, L.K.; Maly, D.J.; et al. Development of a murine vertical transmission model for Toxoplasma gondii oocyst infection and studies on the efficacy of bumped kinase inhibitor (BKI)-1294 and the naphthoquinone buparvaquone against congenital toxoplasmosis. J. Antimicrob. Chemother. 2017, 72, 2334–2341. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Sanchez, R.; Ferre, I.; Re, M.; Ramos, J.J.; Regidor-Cerrillo, J.; Pizarro Diaz, M.; Gonzalez-Huecas, M.; Tabanera, E.; Benavides, J.; Hemphill, A.; et al. Treatment with bumped kinase inhibitor 1294 is safe and leads to significant protection against abortion and vertical transmission in sheep experimentally Iifected with Toxoplasma gondii during pregnancy. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Aguado-Martínez, A.; Balmer, V.; Maly, D.J.; Fan, E.; Ortega-Mora, L.M.; Ojo, K.K.; Van Voorhis, W.C.; Hemphill, A. Two Novel Calcium-Dependent Protein Kinase 1 Inhibitors Interfere with Vertical Transmission in Mice Infected with Neospora caninum Tachyzoites. Antimicrob. Agents Chemother. 2017, 61, e02324-16. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, R.; Ferre, I.; Re, M.; Vázquez, P.; Ferrer, L.M.; Blanco-Murcia, J.; Regidor-Cerrillo, J.; Díaz, M.P.; González-Huecas, M.; Tabanera, E.; et al. Safety and efficacy of the bumped kinase inhibitor BKI-1553 in pregnant sheep experimentally infected with Neospora caninum tachyzoites. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Ojo, K.K.; Dangoudoubiyam, S.; Verma, S.K.; Scheele, S.; DeRocher, A.E.; Yeargan, M.; Choi, R.; Smith, T.R.; Rivas, K.L.; Hulverson, M.A.; et al. Selective inhibition of Sarcocystis neurona calcium-cependent protein kinase 1 for equine protozoal myeloencephalitis therapy. Int. J. Parasitol. 2016, 46, 871–880. [Google Scholar] [CrossRef]
- Esposito, M.; Moores, S.; Naguleswaran, A.; Müller, J.; Hemphill, A. Induction of tachyzoite egress from cells infected with the protozoan Neospora caninum by nitro- and bromo-thiazolides, a class of broad-spectrum anti-parasitic drugs. Int. J. Parasitol. 2007, 37, 1143–1152. [Google Scholar] [CrossRef]
- Howe, D.K.; Crawford, A.C.; Lindsay, D.; Sibley, L.D. The p29 and p35 Immunodominant Antigens ofNeospora caninum Tachyzoites Are Homologous to the Family of Surface Antigens of Toxoplasma gondii. Infect. Immun. 1998, 66, 5322–5328. [Google Scholar] [CrossRef]
- Sonda, S.; Fuchs, N.; Connolly, B.; Fernandez, P.; Gottstein, B.; Hemphill, A. The major 36 kDa Neospora caninum tachyzoite surface protein is closely related to the major Toxoplasma gondii surface antigen. Mol. Biochem. Parasitol. 1998, 97, 97–108. [Google Scholar] [CrossRef]
- Sinnott, F.A.; Monte, L.G.; Collares, T.F.; Silveira, R.M.; Borsuk, S. Review on the immunological and molecular diagnosis of neosporosis (years 2011–2016). Veter- Parasitol. 2017, 239, 19–25. [Google Scholar] [CrossRef]
- Ouologuem, D.; Roos, D.S. Dynamics of the Toxoplasma gondii inner membrane complex. J. Cell Sci. 2014, 127, 3320–3330. [Google Scholar] [CrossRef] [PubMed]
- Stommel, E.; Ely, K.H.; Schwartzman, J.D.; Kasper, L.H. Toxoplasma gondii:Dithiol-Induced Ca2+Flux Causes Egress of Parasites from the Parasitophorous Vacuole. Exp. Parasitol. 1997, 87, 88–97. [Google Scholar] [CrossRef] [PubMed]
- McCoy, J.M.; Whitehead, L.; Van Dooren, G.G.; Tonkin, C.J. TgCDPK3 Regulates Calcium-Dependent Egress of Toxoplasma gondii from Host Cells. PLOS Pathog. 2012, 8, e1003066. [Google Scholar] [CrossRef]
- Han, H.; Zhu, S.; Jiang, L.; Li, Y.; Dong, H.; Zhao, Q.; Kong, C.; Huang, B. Molecular characterization and analysis of a novel calcium-dependent protein kinase from Eimeria tenella. Parasitol. 2013, 140, 746–755. [Google Scholar] [CrossRef]
- Jiménez-Meléndez, A.; Ojo, K.K.; Wallace, A.M.; Smith, T.R.; Hemphill, A.; Balmer, V.; Regidor-Cerrillo, J.; Ortega-Mora, L.M.; Hehl, A.B.; Fan, E.; et al. In vitro efficacy of bumped kinase inhibitors against Besnoitia besnoiti tachyzoites. Int. J. Parasitol. 2017, 47, 811–821. [Google Scholar] [CrossRef]
- Hemphill, A.; Gottstein, B.; Kaufmann, H. Adhesion and invasion of bovine endothelial cells by Neospora caninum. Parasitol. 1996, 112, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, A. Subcellular localization and functional characterization of Nc-p43, a major Neospora caninum tachyzoite surface protein. Infect. Immun. 1996, 64, 4279–4287. [Google Scholar] [CrossRef]
- Naguleswaran, A.; Cannas, A.; Keller, N.; Vonlaufen, N.; Schares, G.; Conraths, F.J.; Björkman, C.; Hemphill, A. Neospora caninum Microneme Protein NcMIC3: Secretion, Subcellular Localization, and Functional Involvement in Host Cell Interaction. Infect. Immun. 2001, 69, 6483–6494. [Google Scholar] [CrossRef][Green Version]
- Alaeddine, F.; Hemphill, A.; Debache, K.; Guionaud, C. Molecular cloning and characterization of NcROP2Fam-1, a member of the ROP2 family of rhoptry proteins in Neospora caninum that is targeted by antibodies neutralizing host cell invasion in vitro. Parasitol. 2013, 140, 1033–1050. [Google Scholar] [CrossRef][Green Version]
- Eberhard, N.; Balmer, V.; Müller, J.; Müller, N.; Winter, R.; Pou, S.; Nilsen, A.; Riscoe, M.; Francisco, S.; Leitao, A.; et al. Activities of Endochin-Like Quinolones Against in vitro Cultured Besnoitia besnoiti Tachyzoites. Front. Veter- Sci. 2020, 7, 96. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winzer, P.; Anghel, N.; Imhof, D.; Balmer, V.; Ortega-Mora, L.-M.; Ojo, K.K.; Van Voorhis, W.C.; Müller, J.; Hemphill, A. Neospora caninum: Structure and Fate of Multinucleated Complexes Induced by the Bumped Kinase Inhibitor BKI-1294. Pathogens 2020, 9, 382. https://doi.org/10.3390/pathogens9050382
Winzer P, Anghel N, Imhof D, Balmer V, Ortega-Mora L-M, Ojo KK, Van Voorhis WC, Müller J, Hemphill A. Neospora caninum: Structure and Fate of Multinucleated Complexes Induced by the Bumped Kinase Inhibitor BKI-1294. Pathogens. 2020; 9(5):382. https://doi.org/10.3390/pathogens9050382
Chicago/Turabian StyleWinzer, Pablo, Nicoleta Anghel, Dennis Imhof, Vreni Balmer, Luis-Miguel Ortega-Mora, Kayode K. Ojo, Wesley C. Van Voorhis, Joachim Müller, and Andrew Hemphill. 2020. "Neospora caninum: Structure and Fate of Multinucleated Complexes Induced by the Bumped Kinase Inhibitor BKI-1294" Pathogens 9, no. 5: 382. https://doi.org/10.3390/pathogens9050382
APA StyleWinzer, P., Anghel, N., Imhof, D., Balmer, V., Ortega-Mora, L.-M., Ojo, K. K., Van Voorhis, W. C., Müller, J., & Hemphill, A. (2020). Neospora caninum: Structure and Fate of Multinucleated Complexes Induced by the Bumped Kinase Inhibitor BKI-1294. Pathogens, 9(5), 382. https://doi.org/10.3390/pathogens9050382






