Innovative Alternatives for Continuous In Vitro Culture of Babesia bigemina in Medium Free of Components of Animal Origin
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Bovine Erythrocytes
2.3. Culture Media
2.4. Parasite and In Vitro Culture
2.5. Selection of an Animal Component-Free Culture Medium
2.6. Effect of Lipids on In Vitro Proliferation of B. bigemina
2.7. In Vitro Proliferation of B. bigemina in a Perfusion Bioreactor
2.8. Statistical Analysis
3. Results
3.1. Selection of an Animal Component-Free Culture Medium
3.2. Effect of the Supplementation with Chemically Defined Lipid on Proliferation of B. bigemina
3.3. In Vitro Proliferation of B. bigemina in a Perfusion Bioreactor System
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bock, R.; Jackson, L.; De Vos, A.; Jorgensen, W. Bovine babesiosis. Parasitology 2004, 129, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Grisi, L.; Leite, R.C.; Martins, J.R.; Barros, A.T.; Andreotti, R.; Cançado, P.H.; Pérez de León, A.A.; Pereira, J.B.; Villela, H.S. Reassessment of the potential economic impact of cattle parasites in Brazil. Rev. Bras. Parasitol. Vet. 2014, 23, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Florín-Christensen, M.; Suarez, C.E.; Rodriguez, A.E.; Flores, D.A.; Schnittger, L. Vaccines against bovine babesiosis: Where we are now and possible roads ahead. Parasitology 2014, 141, 1563–1592. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.G.; Ristic, M. Babesia bovis: Continuous cultivation in a microaerophilus stationary phase culture. Science 1980, 207, 1218–1220. [Google Scholar] [CrossRef]
- Rodríguez-Vivas, R.I.; Trees, J.A. Utilización del cultivo in vitro de Babesia bovis para evaluar la efectividad de babesicidas. Rev. Biomed. 1994, 5, 133–139. [Google Scholar]
- Jackson, L.A.; Waldron, S.J.; Weier, H.M.; Nicoll, C.L.; Cooke, B.M. Babesia bovis: Culture of laboratory-adapted parasite lines and clinical isolates in a chemically defined medium. Exp. Parasitol. 2001, 99, 168–174. [Google Scholar] [CrossRef]
- Mosqueda, J.; McElwain, T.F.; Stiller, D.; Palmer, G.H. Babesia bovis merozoite surface antigen 1 and rhoptry-associated protein 1 are expressed in sporozoites, and specific antibodies inhibit sporozoite attachment to erythrocytes. Infect. Immun. 2002, 70, 1599–1603. [Google Scholar] [CrossRef][Green Version]
- Silva, M.G.; Knowles, D.P.; Mazuz, M.L.; Cooke, B.M.; Suarez, C.E. Stable transformation of Babesia bigemina and Babesia bovis using a single transfection plasmid. Sci. Rep. 2018, 1, 1–9. [Google Scholar] [CrossRef]
- Suarez, C.E.; McElwain, T.F. Transfection systems for Babesia bovis: A review of methods for the transient and stable expression of exogenous genes. Vet. Parasitol. 2010, 4, 205–215. [Google Scholar] [CrossRef]
- Schuster, F.L. Cultivation of Babesia and Babesia-like blood parasites: Agents of an emerging zoonotic disease. Clin. Microbiol. Rev. 2012, 15, 365–373. [Google Scholar] [CrossRef]
- Vega, C.A.; Buening, G.M.; Green, T.J.; Carson, C.A. In vitro cultivation of Babesia bigemina. Am. J. Vet. Res. 1985, 46, 416–420. [Google Scholar] [PubMed]
- Rojas-Martínez, C.; Rodríguez-Vivas, R.I.; Figueroa, M.J.V.; Acosta, V.K.Y.; Gutiérrez, R.E.J.; Bautista-Garfias, C.R.; Lira-Amaya, J.J.; Polanco-Martínez, D.J.; Álvarez, M.J.A. Babesia bigemina: Advances in continuous in vitro culture using serum-free medium supplemented with insulin, transferrin, selenite, and putrescine. Parasitol. Int. 2018, 6, 294–301. [Google Scholar] [CrossRef] [PubMed]
- da Costa-Silva, T.A.; da Silva, M.C.; Frazzatti-Gallina, N.; Pereira-Chioccola, V.L. Toxoplasma gondii antigens: Recovery analysis of tachyzoites cultivated in Vero cell maintained in serum free medium. Exp. Parasitol. 2012, 4, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, M. Growth characteristics of canine pathogenic viruses in MDCK cells cultured in RPMI 1640 medium without animal protein. Vaccine 2006, 11, 1744–1748. [Google Scholar] [CrossRef]
- Trabelsi, K.; Ben Zakour, M.; Kallel, H. Purification of rabies virus produced in Vero cells grown in serum free medium. Vaccine 2019, 47, 7052–7060. [Google Scholar] [CrossRef]
- Frazzati-Gallina, N.M.; Paoli, R.L.; Mourão-Fuches, R.M.; Jorge, S.A.; Pereira, C.A. Higher production of rabies virus in serum-free medium cell cultures on microcarriers. J. Biotechnol. 2001, 1, 67–72. [Google Scholar] [CrossRef]
- Kallel, H.; Jouini, A.; Majoul, S.; Rourou, S. Evaluation of various serum and animal protein free media for the production of a veterinary rabies vaccine in BHK-21 cells. J. Biotechnol. 2002, 3, 195–204. [Google Scholar] [CrossRef]
- Merten, O.W. Development of serum-free media for cell growth and production of viruses/viral vaccines-safety issues of animal products used in serum-free media. Dev. Biol. 2002, 111, 233–257. [Google Scholar]
- Kim, D.K.; Choi, H.; Nishida, H.; Oh, J.Y.; Gregory, C.; Lee, R.H.; Yu, J.M.; Watanabe, J.; An, S.Y.; Bartosh, T.J.; et al. Scalable Production of a Multifunctional Protein (TSG-6). That Aggregates with Itself and the CHO Cells That Synthesize It. PLoS ONE 2016, 1, e0147553. [Google Scholar] [CrossRef]
- Gélinas, J.F.; Davies, L.A.; Gill, D.R.; Hyde, S.C. Assessment of selected media supplements to improve F/HN lentiviral vector production yields. Sci. Rep. 2017, 1, 10198. [Google Scholar] [CrossRef]
- Rojas, M.C.; Rodríguez-Vivas, R.I.; Figueroa, M.J.V.; Acosta, V.K.Y.; Gutiérrez, R.E.J.; Álvarez, M.J.A. In vitro culture of Babesia bovis in a bovine serum-free culture medium supplemented with insulin, transferrin, and selenite. Exp. Parasitol. 2016, 170, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, M.J.V.; Canto, A.G.J.; Juarez, F.J.; Ruiz, L.F. Cultivo in vitro de Babesia bovis: Establecimiento y condiciones óptimas de multiplicación. Rev. Mex. Cienc. Pecu. 1984, 46, 46–52. [Google Scholar]
- Rojas-Martínez, C.; Rodríguez-Vivas, R.I.; Figueroa, M.J.V.; Acosta, V.K.Y.; Gutiérrez, R.E.J.; Álvarez, M.J.A. Putrescine: Essential factor for in vitro proliferation of Babesia bovis. Exp. Parasitol. 2017, 175, 79–84. [Google Scholar] [CrossRef]
- Müller, I.B.; Das Gupta, R.; Lüersen, K.; Wrenger, C.; Walter, R.D. Assessing the polyamine metabolism of Plasmodium falciparum as chemotherapeutic target. Mol. Biochem. Parasitol. 2008, 1, 1–7. [Google Scholar] [CrossRef]
- Cook, T.; Roos, D.; Morada, M.; Zhu, G.; Keithly, J.; Feagin, J.; Wu, G.; Yarlett, N. Divergent polyamine metabolism in the Apicomplexa. Microbiology 2007, 153, 1123–1130. [Google Scholar] [CrossRef]
- Brayton, K.; Lau, A.; Herndon, D.; Hannick, L.; Kappmeyer, L.; Berens, S.; Bidwell, S.; Brown, W.; Crabtree, J.; Fadrosh, D.; et al. Genome sequence of Babesia bovis and comparative analysis of apicomplexan hemoprotozoa. PLoS Pathog. 2007, 10, 1401–1413. [Google Scholar] [CrossRef]
- Müller, S.; Coombs, G.; Walter, R. Targeting polyamines of parasitic protozoa in chemotherapy. Trends Parasitol. 2001, 17, 242–249. [Google Scholar] [CrossRef]
- van der Valk, J.; Brunner, D.; de Smet, K.; Fex Svenningsen, A.; Honegger, P.; Knudsen, L.E.; Lindl, T.; Noraberg, J.; Price, A.; Scarino, M.; et al. Optimization of chemically defined cell culture media replacing fetal bovine serum in mammalian in vitro. Toxicol. In Vitro 2010, 24, 1053–1063. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Serricchio, M.; Striepen, B.; Bütikofer, P. Lipid synthesis in protozoan parasites: A comparison between kinetoplastids and apicomplexans. Prog. Lipid. Res. 2013, 4, 488–512. [Google Scholar] [CrossRef]
- Mi-Ichi, F.; Kita, K.; Mitamura, T. Intraerythrocytic Plasmodium falciparum utilize a broad range of serum-derived fatty acids with limited modification for their growth. Parasitology 2006, 133, 399–410. [Google Scholar] [CrossRef]
- Vial, H.J.; Thuet, M.J.; Philippot, J.R. Phospholipid biosynthesis in synchronous Plasmodium falciparum cultures. J. Protozool. 1982, 29, 258–263. [Google Scholar] [CrossRef]
- Asahi, H.; Kanazawa, T.; Hirayama, N.; Kajihara, Y. Investigating serum factors promoting erythrocytic growth of Plasmodium falciparum. Exp. Parasitol. 2005, 109, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, L.L.; Howard, R.J.; Aikawa, M.; Taraschi, T.F. Modification of host cell membrane lipid composition by the intra-erythrocytic human malaria parasite Plasmodium falciparum. Biochem. J. 1991, 274, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Gratraud, P.; Huws, E.; Falkard, B.; Adjalley, S.; Fidock, D.A.; Berry, L.; Jacobs, W.R., Jr.; Baird, M.S.; Vial, H.; Kremer, L. Oleic acid biosynthesis in Plasmodium falciparum: Characterization of the stearoyl-CoA desaturase and investigation as a potential therapeutic target. PLoS ONE 2009, 9, e6889. [Google Scholar] [CrossRef] [PubMed]
- Vial, H.J.; Thuet, M.J.; Broussal, J.L.; Philippot, J.R. Phospholipid biosynthesis by Plasmodium knowlesi-infected erythrocytes: The incorporation of phospohlipid precursors and the identification of previously undetected metabolic pathways. J. Parasitol. 1982, 68, 379–391. [Google Scholar] [CrossRef]
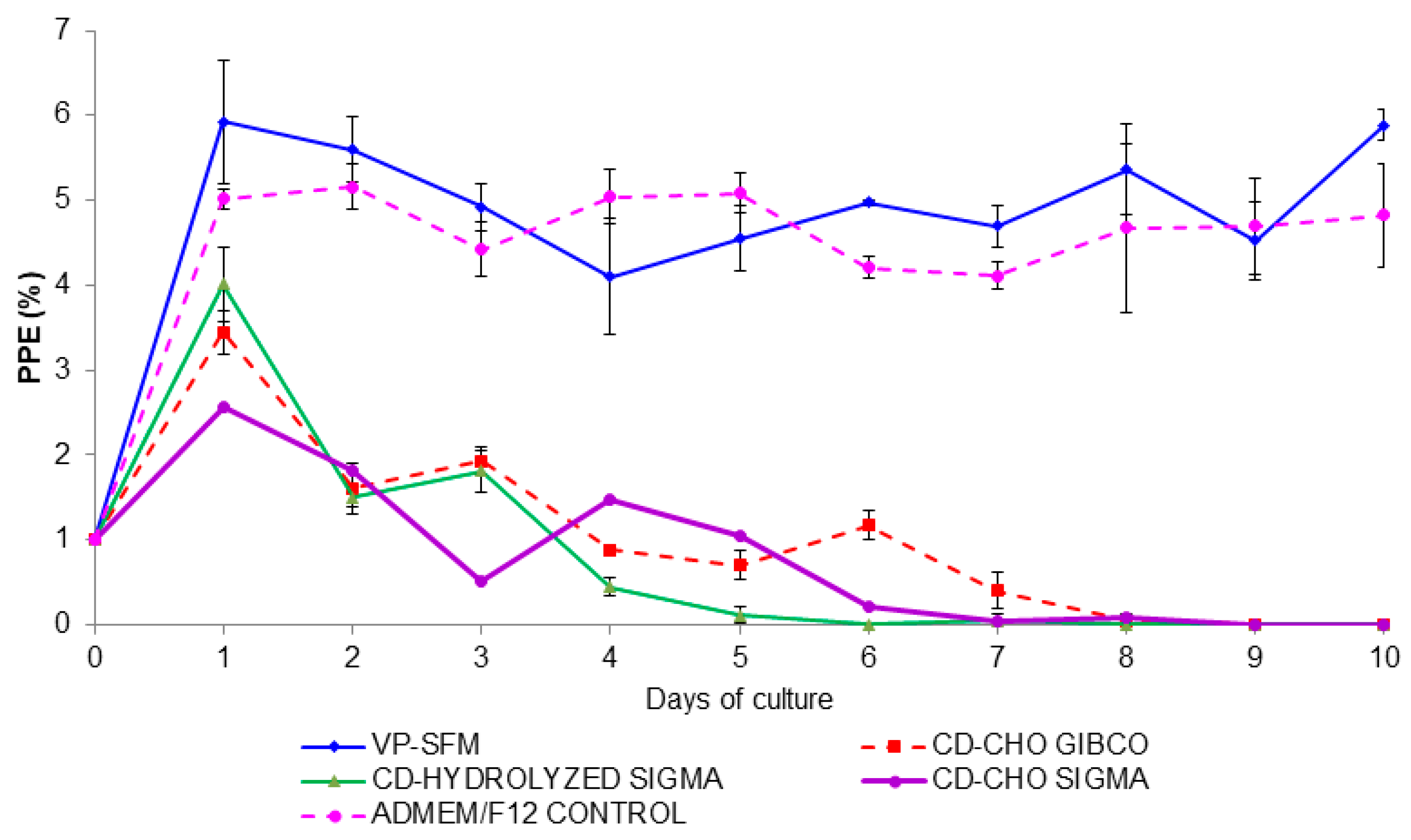
| Medium | Serum-Free | Animal-Free Components | Protein-Free |
|---|---|---|---|
| ADMEM/F12 | Yes | No | No |
| CD-CHO (Gibco®), | Yes | Yes | Yes |
| CD-CHO (Sigma-Aldrich) | Yes | Yes | Yes |
| CD-Hydrolyzed (Sigma-Aldrich) | Yes | Yes | Yes |
| VP-SFM (Gibco®) | Yes | Yes | No |
| Treatment | Lipids Mixture Concentration (mg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Myristic | Palmitic | Palmitoleic | Stearic | Oleic | Linolenic | Stearic | Arachidonic | Cholesterol | |
| I | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.04 | 4.4 |
| II | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.02 | 2.2 |
| III | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.01 | 1.1 |
| IV | 0.025 | 0.025 | 0.025 | 0.025 | 0.025 | 0.025 | 0.025 | 0.005 | 0.55 |
| V | 0.0125 | 0.0125 | 0.0125 | 0.0125 | 0.0125 | 0.0125 | 0.0125 | 0.0025 | 0.275 |
| Treatment Lipid | Days of Culture | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | |
| I | 1.0% | 2.43% | 5.71% | 8.00% | 7.92% | 8.96% | 8.03% |
| II | 1.0% | 4.93% | 7.19% * | 9.85% * | 10.33% * | 10.43% * | 11.35% * |
| II | 1.0% | 4.77% | 5.03% | 7.91% | 8.70% | 8.60% | 9.04% |
| IV | 1.0% | 4.20% | 5.08% | 7.06% | 7.77% | 5.25% | 8.10% |
| V | 1.0% | 3.12% | 3.74% | 5.49% | 5.61% | 8.60% | 7.06% |
| VP-SFM | 1.0% | 1.77% | 4.40% | 4.99% | 6.07% | 7.42% | 7.43% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez Martínez, J.A.; Figueroa Millán, J.V.; Ueti, M.W.; Rojas-Martínez, C. Innovative Alternatives for Continuous In Vitro Culture of Babesia bigemina in Medium Free of Components of Animal Origin. Pathogens 2020, 9, 343. https://doi.org/10.3390/pathogens9050343
Álvarez Martínez JA, Figueroa Millán JV, Ueti MW, Rojas-Martínez C. Innovative Alternatives for Continuous In Vitro Culture of Babesia bigemina in Medium Free of Components of Animal Origin. Pathogens. 2020; 9(5):343. https://doi.org/10.3390/pathogens9050343
Chicago/Turabian StyleÁlvarez Martínez, Jesús A., Julio V. Figueroa Millán, Massaro W. Ueti, and Carmen Rojas-Martínez. 2020. "Innovative Alternatives for Continuous In Vitro Culture of Babesia bigemina in Medium Free of Components of Animal Origin" Pathogens 9, no. 5: 343. https://doi.org/10.3390/pathogens9050343
APA StyleÁlvarez Martínez, J. A., Figueroa Millán, J. V., Ueti, M. W., & Rojas-Martínez, C. (2020). Innovative Alternatives for Continuous In Vitro Culture of Babesia bigemina in Medium Free of Components of Animal Origin. Pathogens, 9(5), 343. https://doi.org/10.3390/pathogens9050343





