Ecology of Neglected Rodent-Borne American Orthohantaviruses
Abstract
1. Introduction
2. Host Diversity
3. Orthohantavirus Communities
4. Transmission among Rodents
5. Risk of Spillover to Humans
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Virus Strain/Genotype | Rodent Host | Genome Strands Sequenced | Virus Isolation | ||
|---|---|---|---|---|---|
| S | M | L | |||
| Andes virus | Oligoryzomys longicaudatus | X | X | X | |
| Oligoryzomys chacoensis | X | X | |||
| Oligoryzomys flavescens | X | X | |||
| Abrothrix longipilis | X | ||||
| Loxodontomys micopus | X | ||||
| Rattus rattus | X | ||||
| Araraquara virus | Bolomys lasiurus | X | X | ||
| Oxymycterus judex | X | X | |||
| Akodon montensis | X | ||||
| Juquitiba virus | Oligoryzomys fornesi | X | X | ||
| Oxymycterus nasutus | X | X | |||
| Oligoryzomys nigripes | X | X | |||
| Maciel virus | Bolomys obscurus | X | |||
| Pergamino virus | Akodon azarae | X | X | ||
| Oxymycterus rufus | X | ||||
| Tunari virus | Unknown | ||||
| Castelo dos Sonhos virus | Oligoryzomys uriaritensis | X | |||
| Lechiguanas virus | Oligoryzomys flavescens | X | |||
| Oligoryzomys nigripes | X | X | |||
| Bermejo virus | Oligoryzomys chacoensis | X | |||
| Oran virus | Oligoryzomys longicaudatus | X | |||
| Bayou virus | Oryzomys palustris | X | X | X | |
| Catacamas virus | Oryzomys couesi | X | X | X | |
| Playa de Oro virus | Oryzomys couesi | X | X | ||
| Sigmodon mascotensis | X | X | |||
| Black Creek Canal virus | Sigmodon hispidus | X | X | X | X |
| Muleshoe virus | Sigmodon hispidus | X | X | ||
| Caño Delgadito virus | Sigmodon alstoni | X | X | X | |
| Choclo virus | Oligoryzomys fulvescens (costaricensis) | X | X | ||
| Jabora virus | Akodon montensis | X | |||
| Carrizal virus | Reithrodontomys sumichrasti | X | X | X | |
| El Moro Canyon virus | Reithrodontomys megalotis | X | X | ||
| Reithrodontomys sumichrasti | X | X | |||
| Neotoma mexicana | X | ||||
| Rio Segundo virus | Reithrodontomys mexicanus | X | |||
| Huitzilac virus | Reithrodontomys megalotis | X | X | X | |
| Laguna Negra virus | Calomys laucha | X | X | X | |
| Maripa virus | Oligoryzomys fulvescens | X | X | ||
| Zygodontomys brevicauda | X | X | |||
| Rio Mamoré virus | Oligoryzomys microtis | X | X | X | |
| Anajatuba virus | Oligoryzomys fornesi | X | |||
| Rio Mearim virus | Holochilus sciureus | X | |||
| Maporal virus | Oligoryzomys fulvescens (delicatus) | X | X | X | |
| Montano virus | Peromyscus beatae | X | X | X | |
| Necocli virus | Zygodontomys brevicauda (cherriei) | X | X | ||
| Calabazo virus | Zygodontomys brevicauda (cherriei) | X | X | ||
| Prospect Hill virus | Microtus pennsylvanicus | X | |||
| Isla Vista virus | Microtus californicus | X | X | ||
| Peromyscus californicus | X | X | |||
| Bloodland Lake virus | Microtus ochrogaster | X | |||
| New York virus | Peromyscus leucopus | X | X | X | |
| Monongahela virus | Peromyscus maniculatus nubiterrae | X | X | ||
| Peromyscus leucopus | X | X | |||
| Blue River virus | Peromyscus leucopus | X | |||
| Sin Nombre virus | Peromyscus maniculatus | X | X | X | |
| Peromyscus californicus | X | X | |||
| Peromyscus attwateri | X | X | |||
| Peromyscus eremicus | X | ||||
| Peromyscus laceianus | X | X | |||
| Reithrodontomys fulvescens | X | X | |||
| Limestone Canyon virus | Peromyscus boylii | X | X | ||
| Peromyscus hylocetes | X | X | |||
| Peromyscus leucopus | X | X | |||
| Peromyscus levipes | X | X | |||
| Peromyscus melanotis | X | X | |||
| Peromyscus ochraventer | X | X | |||
| Peromyscus spicilegus | X | X | |||
References
- Anthony, S.J.; Epstein, J.H.; A Murray, K.; Navarrete-Macias, I.; Zambrana-Torrelio, C.; Solovyov, A.; Ojeda-Flores, R.; Arrigo, N.C.; Islam, A.; Khan, S.A.; et al. A strategy to estimate unknown viral diversity in mammals. mBio 2013, 4, e00598-13. [Google Scholar] [CrossRef]
- Parrish, C.R.; Holmes, E.C.; Morens, D.M.; Park, E.-C.; Burke, D.S.; Calisher, C.H.; Laughlin, C.A.; Saif, L.J.; Daszak, P. Cross-species virus transmission and the emergence of new epidemic diseases. Microbiol. Mol. Biol. Rev. 2008, 72, 457–470. [Google Scholar] [CrossRef]
- Walsh, M.G.; Wiethoelter, A.; Haseeb, M.A. The impact of human population pressure on flying fox niches and the potential consequences for Hendra virus spillover. Sci. Rep. 2017, 7, 8226. [Google Scholar] [CrossRef]
- Hahn, M.B.; Gurley, E.S.; Epstein, J.H.; Islam, M.S.; Patz, J.A.; Daszak, P.; Luby, S.P. The role of landscape composition and configuration on Pteropus giganteus roosting ecology and nipah virus spillover risk in Bangladesh. Am. J. Trop. Med. Hyg. 2014, 90, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Gardner, L.; Chen, N.; Sarkar, S. Vector status of Aedes species determines geographical risk of autochthonous Zika virus establishment. PLoS Negl. Trop. Dis. 2017, 11, e0005487. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, R.S. Origin of the west nile virus responsible for an outbreak of encephalitis in the Northeastern United States. Science 1999, 286, 2333–2337. [Google Scholar] [CrossRef] [PubMed]
- Abudurexiti, A.; Adkins, S.; Alioto, D.; Alkhovsky, S.V.; Avšič-Županc, T.; Ballinger, M.; Bente, D.A.; Beer, M.; Bergeron, É.; Blair, C.; et al. Taxonomy of the order Bunyavirales: Update 2019. Arch. Virol. 2019, 164, 1949–1965. [Google Scholar] [CrossRef]
- Maes, P.; Adkins, S.; Alkhovsky, S.V.; Avšič-Županc, T.; Ballinger, M.; Bente, D.A.; Beer, M.; Bergeron, É.; Blair, C.; Briese, T.; et al. Taxonomy of the order Bunyavirales: Second update 2018. Arch. Virol. 2019, 164, 927–941. [Google Scholar] [CrossRef]
- Laenen, L.; Vergote, V.; Calisher, C.H.; Klempa, B.; Klingström, J.; Kuhn, J.H.; Maes, P. Hantaviridae: Current classification and future perspectives. Viruses 2019, 11, 788. [Google Scholar] [CrossRef]
- Arai, S.; Yanagihara, R. Genetic diversity and geographic distribution of bat-borne hantaviruses. In Bats and Viruses: Current Research and Future Trends; Corrales-Aguilar, E., Schwemmle, M., Eds.; Caister Academic Press: Norfolk, UK, 2020; pp. 59–86. [Google Scholar]
- Avšič-Županc, T.; Saksida, A.; Korva, M. Hantavirus infections. Clin. Microbiol. Infect. 2019, 21, e6–e16. [Google Scholar] [CrossRef]
- Lee, P.-W.; Amyx, H.L.; Yanagihara, R.; Gajdusek, D.C.; Goldgaber, D.; Gibbs, C.J. Partial characterization of prospect hill virus isolated from meadow voles in the United States. J. Infect. Dis. 1985, 152, 826–829. [Google Scholar] [CrossRef]
- Duchin, J.S.; Koster, F.T.; Peters, C.; Simpson, G.L.; Tempest, B.; Zaki, S.R.; Ksiazek, T.G.; Rollin, P.E.; Nichol, S.; Umland, E.T.; et al. Hantavirus pulmonary syndrome: A clinical description of 17 patients with a newly recognized disease. N. Engl. J. Med. 1994, 330, 949–955. [Google Scholar] [CrossRef] [PubMed]
- López, N.; Padula, P.; Rossi, C.; Lázaro, M.E.; Franze-Fernández, M.T. Genetic edentification of a new hantavirus causing severe pulmonary syndrome in Argentina. Virology 1996, 220, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.D.; Forshey, B.M.; Vallejo, E.; Agudo, R.; Vargas, J.; Blazes, D.L.; Guevara, C.; Laguna-Torres, V.A.; Halsey, E.S.; Kochel, T.J. Novel Strain of Andes virus associated with fatal human infection, Central Bolivia. Emerg. Infect. Dis. 2012, 18, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Bohlman, M.C.; Morzunov, S.P.; Meissner, J.; Taylor, M.B.; Ishibashi, K.; Rowe, J.; Levis, S.; Enria, D.; Jeor, S.C.S. Analysis of hantavirus genetic diversity in argentina: S segment-derived phylogeny. J. Virol. 2002, 76, 3765–3773. [Google Scholar] [CrossRef]
- Milazzo, M.L.; Cajimat, M.N.; Richter, M.H.; Bradley, R.D.; Fulhorst, C.F. Muleshoe virus and other hantaviruses associated with neotomine or sigmodontine rodents in Texas. Vector-Borne Zoonotic Dis. 2017, 17, 720–729. [Google Scholar] [CrossRef]
- Song, J.-W.; Baek, L.-J.; Gajdusek, D.; Yanagihara, R.; Gavrilovskaya, I.; Luft, B.; Mackow, E.; Hjelle, B. Isolation of pathogenic hantavirus from white-footed mouse (Peromyscus leucopus). Lancet 1994, 344, 1637. [Google Scholar] [CrossRef]
- Forbes, K.M.; Sironen, T.; Plyusnin, A. Hantavirus maintenance and transmission in reservoir host populations. Curr. Opin. Virol. 2018, 28, 1–6. [Google Scholar] [CrossRef]
- Douglass, R.J.; Semmens, W.J.; Mills, J.N.; Zanto, S.N.; Bond, C.W.; Wilson, T.; Van Horn, R. Longitudinal studies of Sin Nombre virus in deer mouse-dominated ecosystems of Montana. Am. J. Trop. Med. Hyg. 2001, 65, 33–41. [Google Scholar] [CrossRef]
- Douglass, R.; Calisher, C.H.; Wagoner, K.D.; Mills, J.N. Sin Nombre virus infection of deer mice in Montana: Characteristics of newly infected mice, incidence, and temporal pattern of infection. J. Wildl. Dis. 2007, 43, 12–22. [Google Scholar] [CrossRef]
- Botten, J.; Mirowsky, K.; Ye, C.; Gottlieb, K.; Saavedra, M.; Ponce, L.; Hjelle, B. Shedding and intracage transmission of sin nombre hantavirus in the deer mouse (Peromyscus maniculatus) model. J. Virol. 2002, 76, 7587–7594. [Google Scholar] [CrossRef] [PubMed]
- Ehlen, L.; Tödtmann, J.; Specht, S.; Kallies, R.; Papies, J.; Müller, M.A.; Junglen, S.; Drosten, C.; Eckerle, I. Epithelial cell lines of the cotton rat (Sigmodon hispidus) are highly susceptible in vitro models to zoonotic Bunya-, Rhabdo-, and Flaviviruses. Virol. J. 2016, 13, 74. [Google Scholar] [CrossRef] [PubMed]
- Vaheri, A.; Strandin, T.; Hepojoki, J.; Sironen, T.; Henttonen, H.; Mäkelä, S.; Mustonen, J. Uncovering the mysteries of hantavirus infections. Nat. Rev. Genet. 2013, 11, 539–550. [Google Scholar] [CrossRef]
- De Oliveira, R.C.; Guterres, A.; Fernandes, J.; D’Andrea, P.; Bonvicino, C.R.; de Lemos, E.R.S. Hantavirus reservoirs: Current status with an emphasis on data from Brazil. Viruses 2014, 6, 1929–1973. [Google Scholar] [CrossRef]
- Milazzo, M.L.; Duno, G.; Utrera, A.; Richter, M.H.; Duno, F.; de Manzione, N.; Fulhorst, C.F. Natural host relationships of hantaviruses native to Western Venezuela. Vector-Borne Zoonotic Dis. 2010, 10, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Plyusnin, A.; Morzunov, S.P. Virus evolution and genetic diversity of hantaviruses and their rodent hosts. In Hantaviruses; Schmaljohn, C.S., Nichol, S.T., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; Volume 256, pp. 47–75. [Google Scholar]
- Pitts, R.M.; Mauldin, M.R.; Thompson, C.W.; Choate, J.R. Evidence of hantavirus exposure in rodents from North Texas. West. N. Am. Nat. 2013, 73, 386–391. [Google Scholar] [CrossRef]
- Colombo, V.C.; Brignone, J.; Sen, C.; Previtali, M.A.; Martin, M.L.; Levis, S.; Monje, L.D.; Gonzalez-Ittig, R.; Beldomenico, P.M. Orthohantavirus genotype Lechiguanas in Oligoryzomys nigripes (Rodentia: Cricetidae): New evidence of host-switching. Acta Trop. 2019, 191, 133–138. [Google Scholar] [CrossRef]
- Milazzo, M.L.; Cajimat, M.N.; Romo, H.E.; Estrada-Franco, J.G.; Iñiguez-Dávalos, L.I.; Bradley, R.D.; Fulhorst, C.F. Geographic distribution of hantaviruses associated with neotomine and sigmodontine rodents, Mexico. Emerg. Infect. Dis. 2012, 18, 571–576. [Google Scholar] [CrossRef]
- Kuenzi, A.J.; Morrison, M.L.; Madhav, N.K.; Mills, J.N. Brush mouse (Peromyscus boylii) population dynamics and hantavirus infection during a warm, drought period in southern Arizona. J. Wildl. Dis. 2007, 43, 675–683. [Google Scholar] [CrossRef][Green Version]
- Song, W.; Torrez-Martinez, N.; Irwin, W.; Harrison, F.J.; Davis, R.; Ascher, M.; Jay, M.; Hjelle, B. Isla Vista virus: A genetically novel hantavirus of the California vole Microtus californicus. J. Gen. Virol. 1995, 76, 3195–3199. [Google Scholar] [CrossRef]
- Rosa, E.S.; Mills, J.N.; Padula, P.; ElKhoury, M.R.; Ksiazek, T.G.; Mendes, W.S.; Santos, E.D.; Araújo, G.C.; Martinez, V.P.; Rosa, J.F.; et al. Newly recognized hantaviruses associated with hantavirus pulmonary syndrome in Northern Brazil: Partial genetic characterization of viruses and serologic implication of likely reservoirs. Vector-Borne Zoonotic Dis. 2005, 5, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.-K.; Owen, R.D.; Sánchez-Hernández, C.; Romero-Almaraz, M.D.L.; Jonsson, C.B. Genetic characterization and phylogeny of a hantavirus from Western Mexico. Virus Res. 2008, 131, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Ruiz, C.; Díaz, F.J.; Rodas, J.D. Recent evidence of hantavirus circulation in the American tropic. Viruses 2014, 6, 1274–1293. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.D.; Utrera, A.; Fulhorst, C.F. The delicate pygmy rice rat (Oligoryzomys delicatus) is the principal host of maporal virus (family Bunyaviridae, genus Hantavirus). Vector-Borne Zoonotic Dis. 2011, 11, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.-P.; Lin, X.-D.; Wang, W.; Tian, J.-H.; Cong, M.-L.; Zhang, H.-L.; Wang, M.-R.; Zhou, R.-H.; Wang, J.-B.; Li, M.-H.; et al. Phylogeny and origins of hantaviruses harbored by bats, insectivores, and rodents. PLoS Pathog. 2013, 9, e1003159. [Google Scholar] [CrossRef] [PubMed]
- Arai, S.; Bennett, S.N.; Sumibcay, L.; Cook, J.A.; Song, J.-W.; Hope, A.; Parmenter, C.; Nerurkar, V.R.; Yates, T.L.; Yanagihara, R. Short report: Phylogenetically distinct hantaviruses in the masked shrew (Sorex cinereus) and dusky shrew (Sorex monticolus) in the United States. Am. J. Trop. Med. Hyg. 2008, 78, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Vapalahti, O.; Lundkvist, Å.; Fedorov, V.; Conroy, C.J.; Hirvonen, S.; Plyusnina, A.; Nemirov, K.; Fredga, K.; Cook, J.A.; Niemimaa, J.; et al. Isolation and characterization of a hantavirus from lemmus sibiricus: Evidence for host switch during hantavirus evolution. J. Virol. 1999, 73, 5586–5592. [Google Scholar] [CrossRef]
- Albariño, C.G.; Guerrero, L.W.; Chakrabarti, A.K.; Rollin, P.E.; Nichol, S.T. Complete genome sequences of Monongahela hantavirus from Pennsylvania, USA. Microbiol. Resour. Announc. 2018, 7, e00928-18. [Google Scholar] [CrossRef]
- Monroe, M.C.; Morzunov, S.P.; Johnson, A.M.; Bowen, M.D.; Artsob, H.; Yates, T.; Peters, C.; Rollin, P.E.; Ksiazek, T.G.; Nichol, S.T. Genetic diversity and distribution of Peromyscus-borne hantaviruses in North America. Emerg. Infect. Dis. 1999, 5, 75–86. [Google Scholar] [CrossRef]
- Sinclair, J.R.; Bell, M.; Nichol, S.T.; Montgomery, J.M.; Mills, J.N.; Ksiazek, T.G.; McCombs, K.; Hutson, C.L.; Carroll, D.S.; Comer, J.A.; et al. Two cases of hantavirus pulmonary syndrome in Randolph county, West Virginia: A coincidence of time and place? Am. J. Trop. Med. Hyg. 2007, 76, 438–442. [Google Scholar] [CrossRef]
- Rhodes, L., III. Hantavirus pulmonary syndrome associated with Monongahela virus, Pennsylvania. Emerg. Infect. Dis. 2000, 6, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Delfraro, A.; Tomé, L.; D’Elía, G.; Clara, M.; Achával, F.; Russi, J.C.; Rodonz, J.R.A. Juquitiba-like hantavirus from 2 honrelated rodent species, Uruguay. Emerg. Infect. Dis. 2008, 14, 1447–1451. [Google Scholar] [CrossRef] [PubMed]
- Saasa, N.; Sánchez-Hernández, C.; Romero-Almaraz, M.D.L.; Guerrero-Ibarra, E.; Almazán-Catalán, A.; Yoshida, H.; Miyashita, D.; Ishizuka, M.; Sanada, T.; Seto, T.; et al. Ecology of hantaviruses in Mexico: Genetic identification of rodent host species and spillover infection. Virus Res. 2012, 168, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Henderson, W.W.; Monroe, M.C.; Jeor, S.C.S.; Thayer, W.P.; Rowe, J.E.; Peters, C.; Nichol, S.T. Naturally occurring sin Nombre virus genetic reassortants. Virology 1995, 214, 602–610. [Google Scholar] [CrossRef]
- Firth, C.; Tokarz, R.; Simith, D.B.; Nunes, M.R.; Bhat, M.; Rosa, E.S.T.; Medeiros, D.B.A.; Palacios, G.; Vasconcelos, P.F.C.; Lipkin, W.I. Diversity and distribution of hantaviruses in South America. J. Virol. 2012, 86, 13756–13766. [Google Scholar] [CrossRef]
- Mills, J.N.; Childs, J.E. Ecologic studies of rodent reservoirs: Their relevance for human health. Emerg. Infect. Dis. 1998, 4, 529–537. [Google Scholar] [CrossRef]
- Rollin, P.E.; Ksiazek, T.G.; Elliott, L.H.; Ravkov, E.V.; Martin, M.L.; Morzunov, S.; Livingstone, W.; Monroe, M.; Glass, G.; Ruo, S.; et al. Isolation of black creek canal virus, a new hantavirus from Sigmodon hispidus in Florida. J. Med. Virol. 1995, 46, 35–39. [Google Scholar] [CrossRef]
- Ksiazek, T.G.; Martin, M.L.; Groves, M.G.; Nichol, S.T.; Peters, C.J.; Monroe, M.C.; Mills, J.N.; Rollin, P.E.; Johnson, A.M.; Wozniak, A.; et al. Isolation, genetic diversity, and geographic distribution of Bayou virus (Bunyaviridae: Hantavirus). Am. J. Trop. Med. Hyg. 1997, 57, 445–448. [Google Scholar] [CrossRef]
- Holsomback, T.S.; Van Nice, C.J.; Clark, R.N.; McIntyre, N.E.; Abuzeineh, A.A.; Salazar-Bravo, J. Socio-ecology of the marsh rice rat (Oryzomys palustris) and the spatio-temporal distribution of Bayou virus in coastal Texas. Geospat. Health 2013, 7, 289. [Google Scholar] [CrossRef][Green Version]
- Vincent, M.J.; Quiroz, E.; Gracia, F.; Sanchez, A.J.; Ksiazek, T.G.; Kitsutani, P.T.; Ruedas, L.A.; Tinnin, D.S.; Cáceres, L.; Garcia, A.; et al. Hantavirus pulmonary syndrome in Panama: Identification of novel hantaviruses and their likely reservoirs. Virology 2000, 277, 14–19. [Google Scholar] [CrossRef]
- Raboni, S.M.; Hoffmann, F.G.; Oliveira, R.C.; Teixeira, B.R.; Bonvicino, C.R.; Stella, V.; Carstensen, S.; Bordignon, J.; D’Andrea, P.; De Lemos, E.R.S.; et al. Phylogenetic characterization of hantaviruses from wild rodents and hantavirus pulmonary syndrome cases in the state of Parana (southern Brazil). J. Gen. Virol. 2009, 90, 2166–2171. [Google Scholar] [CrossRef] [PubMed]
- Matheus, S.; Lavergne, A.; De Thoisy, B.; Dussart, P.; Lacoste, V. Complete genome sequence of a novel hantavirus variant of Rio Mamoré virus, Maripa Virus, from French Guiana. J. Virol. 2012, 86, 5399. [Google Scholar] [CrossRef] [PubMed]
- De Thoisy, B.; Matheus, S.; Guitet, S.; Donato, D.; Clément, L.; Lavergne, A.; Brunaux, O.; Guidez, A.; Catzeflis, F.; Lacoste, V.; et al. Maripa hantavirus in French Guiana: Phylogenetic position and predicted spatial distribution of rodent hosts. Am. J. Trop. Med. Hyg. 2014, 90, 988–992. [Google Scholar] [CrossRef] [PubMed]
- Matheus, S.; Djossou, F.; Moua, D.; Bourbigot, A.M.; Hommel, D.; Lacoste, V.; Dussart, P.; Lavergne, A. Hantavirus pulmonary syndrome, French Guiana. Emerg. Infect. Dis. 2010, 16, 739–741. [Google Scholar] [CrossRef]
- Montoya-Ruiz, C.; Cajimat, M.N.B.; Milazzo, M.L.; Diaz, F.J.; Rodas, J.D.; Valbuena, G.; Fulhorst, C.F. Phylogenetic relationship of Necoclí virus to other South American hantaviruses (Bunyaviridae: Hantavirus). Vector-Borne Zoonotic Dis. 2015, 15, 438–445. [Google Scholar] [CrossRef]
- Loarie, S.R.; Duffy, P.B.; Hamilton, H.; Asner, G.P.; Field, C.B.; Ackerly, D.D. The velocity of climate change. Nature 2009, 462, 1052–1055. [Google Scholar] [CrossRef]
- Samson, F.; Knopf, F. Prairie conservation in North America. BioScience 1994, 44, 418–421. [Google Scholar] [CrossRef]
- Fuhlendorf, S.D.; Davis, C.A.; Elmore, R.D.; Goodman, L.E.; Hamilton, R.G. Perspectives on grassland conservation efforts: Should we rewild to the past or conserve for the future? Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170438. [Google Scholar] [CrossRef]
- Geluso, K.; Geluso, K.N.; Andersen, B.R. Distribution of the northern pygmy mouse (Baiomys taylori) in southwestern New Mexico, with notes on reproduction. Occas. Pap. Tex. Tech. Univ. Mus. 2017, 349, 12. [Google Scholar]
- Padula, P.; Figueroa, R.; Navarrete, M.; Pizarro, E.; Cadiz, R.; Bellomo, C.; Jofre, C.; Zaror, L.; Rodriguez, E.; Murua, R. Transmission study of Andes hantavirus infection in wild sigmodontine rodents. J. Virol. 2004, 78, 11972–11979. [Google Scholar] [CrossRef]
- Safronetz, D.; Drebot, M.A.; Artsob, H.; Cote, T.; Makowski, K.; Lindsay, R. Sin nombre virus shedding patterns in naturally infected deer mice (Peromyscus maniculatus) in relation to duration of infection. Vector-Borne Zoonotic Dis. 2008, 8, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Voutilainen, L.; Sironen, T.; Tonteri, E.; Bäck, A.T.; Razzauti, M.; Karlsson, M.; Wahlström, M.; Niemimaa, J.; Henttonen, H.; Lundkvist, Å. Life-long shedding of Puumala hantavirus in wild bank voles (Myodes glareolus). J. Gen. Virol. 2015, 96, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.; Hörnfeldt, B.; Evander, M.; Magnusson, M.; Olsson, G.; Ecke, F. Dynamics and drivers of hantavirus prevalence in rodent populations. Vector-Borne Zoonotic Dis. 2014, 14, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.N.; Ksiazek, T.G.; Peters, C.; Childs, J.E. Long-term studies of hantavirus reservoir populations in the Southwestern United States: A synthesis. Emerg. Infect. Dis. 1999, 5, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Yahnke, C.J.; Meserve, P.L.; Mills, J.N.; Ksiazek, T.G. Patterns of infection with Laguna Negra virus in wild populations of Calomys laucha in the central Paraguayan chaco. Am. J. Trop. Med. Hyg. 2001, 65, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Vadell, M.V.; Bellomo, C.; Martín, A.S.; Padula, P.; Villafañe, I.G. Hantavirus ecology in rodent populations in three protected areas of Argentina: Hantavirus ecology in protected areas. Trop. Med. Int. Health 2011, 16, 1342–1352. [Google Scholar] [CrossRef]
- Walsh, A.S.; Louis, T.A.; Glass, G.E. Detecting multiple levels of effect during survey sampling using a Bayesian approach: Point prevalence estimates of a hantavirus in hispid cotton rats (Sigmodon hispidus). Ecol. Model. 2007, 205, 29–38. [Google Scholar] [CrossRef]
- Emlen, S.; Oring, L. Ecology, sexual selection, and the evolution of mating systems. Science 1977, 197, 215–223. [Google Scholar] [CrossRef]
- Vestal, B.M.; Sehnell, G.D. Influence of environmental complexity and space on social interactions of mice (Mus musculus and Peromyscus leucopus). J. Comp. Psychol. 1986, 100, 143–154. [Google Scholar] [CrossRef]
- Hasler, J.F.; Banks, E. The behavioral and somatic effects of ovariectomy and replacement therapy in female collared lemmings (Dicrostonyx groenlandicus). Horm. Behav. 1976, 7, 59–74. [Google Scholar] [CrossRef]
- Ruffer, D.G. Sexual behaviour of the northern grasshopper mouse (Onychomys leucogaster). Anim. Behav. 1965, 13, 447–452. [Google Scholar] [CrossRef]
- Johnston, R.E. Chemical communication in rodents: From pheromones to individual recognition. J. Mammal. 2003, 84, 1141–1162. [Google Scholar] [CrossRef]
- Hjelle, B.; Torres-Pérez, F. Hantaviruses in the Americas and their role as emerging pathogens. Viruses 2010, 2, 2559–2586. [Google Scholar] [CrossRef]
- Ostfeld, R.S. Limiting resources and territoriality in microtine rodents. Am. Nat. 1985, 126, 1–15. [Google Scholar] [CrossRef]
- Wolff, J.; Peterson, J. An offspring-defense hypothesis for territoriality in female mammals. Ethol. Ecol. Evol. 1998, 10, 227–239. [Google Scholar] [CrossRef]
- Frank, D.H.; Heske, E.J. Seasonal changes in space use patterns in the southern grasshopper mouse, Onychomys torridus torridus. J. Mammal. 1992, 73, 292–298. [Google Scholar] [CrossRef]
- Priotto, J.; Steinmann, A.; Polop, J. Factors affecting home range size and overlap in Calomys venustus (Muridae: Sigmodontinae) in Argentine agroecosystems. Mamm. Biol. 2002, 67, 97–104. [Google Scholar] [CrossRef]
- Schradin, C.; Schubert, M.; Pillay, N. Winter huddling groups in the striped mouse. Can. J. Zool. 2006, 84, 693–698. [Google Scholar] [CrossRef]
- Madison, D.M.; Fitzgerald, R.W.; McShea, W.J. Dynamics of social nesting in overwintering meadow voles (Microtus pennsylvanicus): Possible consequences for population cycling. Behav. Ecol. Sociobiol. 1984, 15, 9–17. [Google Scholar] [CrossRef]
- Wolff, J.O.; Durr, D.S. Winter nesting behavior of Peromyscus leucopus and Peromyscus maniculatus. J. Mammal. 1986, 67, 409–412. [Google Scholar] [CrossRef]
- Vaughan, T.A. Vertebrates inhabiting pocket gopher burrows in Colorado. J. Mammal. 1961, 42, 171. [Google Scholar] [CrossRef]
- Stapp, P. Rodent communities in active and inactive colonies of black-tailed prairie dogs in shortgrass steppe. J. Mammal. 2007, 88, 241–249. [Google Scholar] [CrossRef]
- Kraft, J.P.; Stapp, P. Movements and burrow use by northern grasshopper mice as a possible mechanism of plague spread in prairie dog colonies. J. Mammal. 2013, 94, 1087–1093. [Google Scholar] [CrossRef]
- Diatta, G.; Duplantier, J.; Granjon, L.; Bâ, K.; Chauvancy, G.; Ndiaye, M.; Trape, J.-F. Borrelia infection in small mammals in West Africa and its relationship with tick occurrence inside burrows. Acta Trop. 2015, 152, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Kollath, D.R.; Teixeira, M.M.; Funke, A.; Miller, K.J.; Barker, B.M. Investigating the role of animal burrows on the ecology and distribution of Coccidioides spp. in Arizona soils. Mycopathologia 2019, 1–15. [Google Scholar] [CrossRef]
- Buck, C.L.; Barnes, B. Temperatures of hibernacula and changes in body composition of arctic ground squirrels over winter. J. Mammal. 1999, 80, 1264–1276. [Google Scholar] [CrossRef]
- Šumbera, R.; Chitaukali, W.N.; Elichová, M.; Kubová, J.; Burda, H. Microclimatic stability in burrows of an Afrotropical solitary bathyergid rodent, the silvery mole-rat (Heliophobius argenteocinereus). J. Zool. 2004, 263, 409–416. [Google Scholar] [CrossRef]
- Carver, S.; Mills, J.N.; Parmenter, C.A.; Parmenter, R.; Richardson, K.S.; Harris, R.; Douglass, R.J.; Kuenzi, A.J.; Luis, A.D. Toward a mechanistic understanding of environmentally forced zoonotic disease emergence: Sin Nombre hantavirus. BioScience 2015, 65, 651–666. [Google Scholar] [CrossRef]
- Yates, T.L.; Mills, J.N.; Parmenter, C.A.; Ksiazek, T.G.; Parmenter, R.; Castle, J.R.V.; Calisher, C.H.; Nichol, S.T.; Abbott, K.D.; Young, J.C.; et al. The ecology and evolutionary history of an emergent disease: hantavirus pulmonary syndrome. BioScience 2002, 52, 989–998. [Google Scholar] [CrossRef]
- Mills, J.N.; Schmidt, K.; Ellis, B.A.; Calderon, G.; Enria, D.A.; Ksiazek, T.G. A longitudinal study of hantavirus infection in three sympatric reservoir species in agroecosystems on the Argentine Pampa. Vector-Borne Zoonotic Dis. 2007, 7, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Adler, F.R.; Pearce-Duvet, J.M.C.; Dearing, M.D. How host population dynamics translate into time-lagged prevalence: An investigation of Sin Nombre virus in deer mice. Bull. Math. Biol. 2007, 70, 236–252. [Google Scholar] [CrossRef] [PubMed]
- Adler, F.R.; Clay, C.A.; Lehmer, E.M. The role of heterogeneity in the persistence and prevalence of Sin Nombre virus in deer mice. Am. Nat. 2008, 172, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Padula, P.; Edelstein, A.; Miguel, S.; Lopez, N.; Rossi, C.; Rabinovich, R. Hantavirus pulmonary syndrome outbreak in Argentina: Molecular evidence for person-to-person transmission of andes virus. Virology 1998, 241, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Toro, J.; Vega, J.D.; Khan, A.S.; Mills, J.N.; Padula, P.; Terry, W.; Yadón, Z.; Valderrama, R.; Ellis, B.A.; Pavletic, C.; et al. An outbreak of hantavirus pulmonary syndrome, Chile, 1997. Emerg. Infect. Dis. 1998, 4, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Valdebenito, C.; Calvo, M.; Vial, C.; Mansilla, R.; Marco, C.; Palma, R.E.; Vial, P.A.; Valdivieso, F.; Mertz, G.; Garrido, M.F. Person-to-person household and nosocomial transmission of andes hantavirus, Southern Chile, 2011. Emerg. Infect. Dis. 2014, 20, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Castillo, C.; Mardones, J.; Villagra, E. Prevalencia de anticuerpos anti-hantavirus en personal de salud en contacto directo con pacientes portadores del síndrome pulmonar por hantavirus: Temuco 1997 a 1999. Rev. Méd. Chile 2000, 128, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, J. Assessment of person-to-person transmission of hantavirus pulmonary syndrome in a Chilean hospital setting. J. Hosp. Infect. 1998, 40, 281–285. [Google Scholar] [CrossRef]
- Bharadwaj, M.; Hjelle, B.; Torrez-Martinez, N.; Botten, J. Rio Mamore virus: Genetic characterization of a newly recognized hantavirus of the pygmy rice rat, Oligoryzomys microtis, from Bolivia. Am. J. Trop. Med. Hyg. 1997, 57, 368–374. [Google Scholar] [CrossRef]
- Armstrong, L.R.; Zaki, S.R.; Goldoft, M.J.; Todd, R.L.; Khan, A.S.; Khabbaz, R.F.; Ksiazek, T.G.; Peters, C.J. Hantavirus pulmonary syndrome associated with entering or cleaning rarely used, rodent-infested structures. J. Infect. Dis. 1995, 172, 1166. [Google Scholar] [CrossRef]
- Munshi-South, J.; Kharchenko, K. Rapid, pervasive genetic differentiation of urban white-footed mouse (Peromyscus leucopus) populations in New York City: Genetics of urban white-footed mice. Mol. Ecol. 2010, 19, 4242–4254. [Google Scholar] [CrossRef]
- Harris, S.E.; Munshi-South, J. Signatures of positive selection and local adaptation to urbanization in white-footed mice (Peromyscus leucopus). Mol. Ecol. 2017, 26, 6336–6350. [Google Scholar] [CrossRef] [PubMed]
- Clément, J.; Maes, P.; Van Ranst, M. Hantaviruses in the old and new world. Perspect. Med. Virol. 2006, 16, 161–177. [Google Scholar]
- Wilson, A.C.; Fenton, B.; Malloch, G.L.; Boag, B.; Hubbard, S.; Begg, G. Urbanisation versus agriculture: A comparison of local genetic diversity and gene flow between wood mouse Apodemus sylvaticus populations in human-modified landscapes. Ecography 2015, 39, 87–97. [Google Scholar] [CrossRef]
- Kerins, J.L.; Koske, S.E.; Kazmierczak, J.; Austin, C.; Gowdy, K.; Dibernardo, A. Outbreak of Seoul virus among rats and rat owners—United States and Canada, 2017. Morb. Mortal. Wkly. Rep. 2018, 67, 131–134. [Google Scholar] [CrossRef]
- Childs, J.E.; Korch, G.W.; Glass, G.E.; Leduc, J.W.; Shah, K.V. Epizootiology of hantavirus infections in Baltimore: Isolation of a virus from Norway rats, and characteristics of infected rat populations. Am. J. Epidemiol. 1987, 126, 55–68. [Google Scholar] [CrossRef]
- Williams, S.H.; Che, X.; Paulick, A.; Guo, C.; Lee, B.; Muller, D.; Uhlemann, A.-C.; Lowy, F.D.; Corrigan, R.M.; Lipkin, W.I. New York City house mice (Mus musculus) as potential reservoirs for pathogenic bacteria and antimicrobial resistance determinants. mBio 2018, 9, e00624-18. [Google Scholar] [CrossRef]
- Williams, S.H.; Che, X.; Garcia, J.A.; Klena, J.D.; Lee, B.; Muller, D.; Ulrich, W.; Corrigan, R.M.; Nichol, S.; Jain, K.; et al. Viral diversity of house mice in New York City. mBio 2018, 9, e01354-17. [Google Scholar] [CrossRef]
- Delfraro, A.; Clara, M.; Tome, L.; Achával, F.; Levis, S.; Calderon, G.; Enria, D.; Lozano, M.; Russi, J.; Arbiza, J. Yellow pygmy rice rat (Oligoryzomys flavescens) and hantavirus pulmonary syndrome in Uruguay. Emerg. Infect. Dis. 2003, 9, 846–852. [Google Scholar] [CrossRef]
- Wilkinson, D.A.; Marshall, J.C.; French, N.P.; Hayman, D.T.S. Habitat fragmentation, biodiversity loss and the risk of novel infectious disease emergence. J. R. Soc. Interface 2018, 15, 20180403. [Google Scholar] [CrossRef]
- Rulli, M.C.; Santini, M.; Hayman, D.T.S.; D’Odorico, P. The nexus between forest fragmentation in Africa and Ebola virus disease outbreaks. Sci. Rep. 2017, 7, 41613. [Google Scholar] [CrossRef]
- Morzunov, S.P.; Feldmann, H.; Spiropoulou, C.F.; Semenova, V.A.; Rollin, P.E.; Ksiazek, T.G.; Peters, C.J.; Nichol, S.T. A newly recognized virus associated with a fatal case of hantavirus pulmonary syndrome in Louisiana. J. Virol. 1995, 69, 1980–1983. [Google Scholar] [CrossRef]
- Ravkov, E.V.; Rollin, P.E.; Ksiazek, T.G.; Peters, C.J.; Nichol, S.T. Genetic and serologic analysis of black creek canal virus and its association with human disease and Sigmodon hispidus infection. Virology 1995, 210, 482–489. [Google Scholar] [CrossRef]
- Palma, R.E.; Polop, J.J.; Owen, R.D.; Mills, J.N. Ecology of rodent-associated hantaviruses in the southern cone of South America: Argentina, Chile, Paraguay, and Uruguay. J. Wildl. Dis. 2012, 48, 267–281. [Google Scholar] [CrossRef]
- Salazar-Bravo, J.; Armién, B.; Suzán, G.; Armien, A.; A Ruedas, L.; Avila, M.; Zaldívar, Y.; Pascale, J.M.; Gracia, F.; Yates, T.L. Serosurvey of wild rodents for hantaviruses in Panama, 2000–2002. J. Wildl. Dis. 2004, 40, 103–109. [Google Scholar] [CrossRef]
- Levis, S.; Morzunov, S.P.; Rowe, J.E.; Enria, D.; Pini, N.; Calderon, G.; Sabattini, M.; Jeor, S.C.S. Genetic diversity and epidemiology of hantaviruses in Argentina. J. Infect. Dis. 1998, 177, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, C.B.; Figueiredo, L.T.M.; Vapalahti, O. A global perspective on hantavirus ecology, epidemiology, and disease. Clin. Microbiol. Rev. 2010, 23, 412–441. [Google Scholar] [CrossRef] [PubMed]
- Kitsutani, P.T.; Denton, R.W.; Fritz, C.L.; Murray, R.A.; Todd, R.L.; Pape, W.J.; Frampton, J.W.; Young, J.C.; Khan, A.S.; Peters, C.J.; et al. Acute Sin Nombre hantavirus infection without pulmonary syndrome, United States. Emerg. Infect. Dis. 1999, 5, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Bennett, S.N.; Dizney, L.; Sumibcay, L.; Arai, S.; Ruedas, L.A.; Song, J.-W.; Yanagihara, R. Host switch during evolution of a genetically distinct hantavirus in the American shrew mole (Neurotrichus gibbsii). Virology 2009, 388, 8–14. [Google Scholar] [CrossRef]
- Kang, H.J.; Bennett, S.N.; Hope, A.G.; Cook, J.A.; Yanagihara, R. Shared ancestry between a newfound mole-borne hantavirus and hantaviruses harbored by cricetid rodents. J. Virol. 2011, 85, 7496–7503. [Google Scholar] [CrossRef]
- Arai, S.; Song, J.-W.; Sumibcay, L.; Bennett, S.N.; Nerurkar, V.R.; Parmenter, C.; Cook, J.A.; Yates, T.L.; Yanagihara, R. Hantavirus in Northern short-tailed shrew, United States. Emerg. Infect. Dis. 2007, 13, 1420–1423. [Google Scholar] [CrossRef]
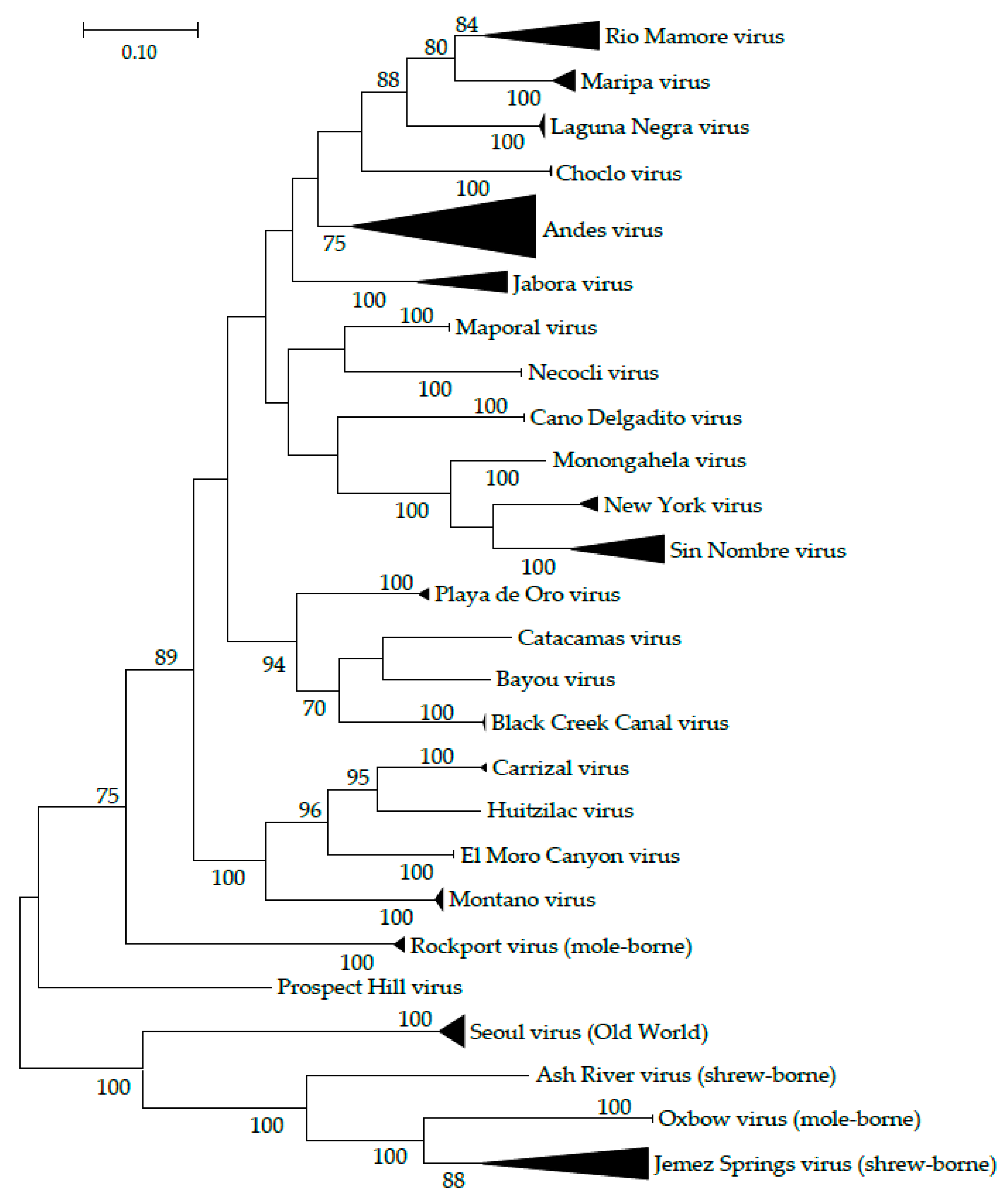
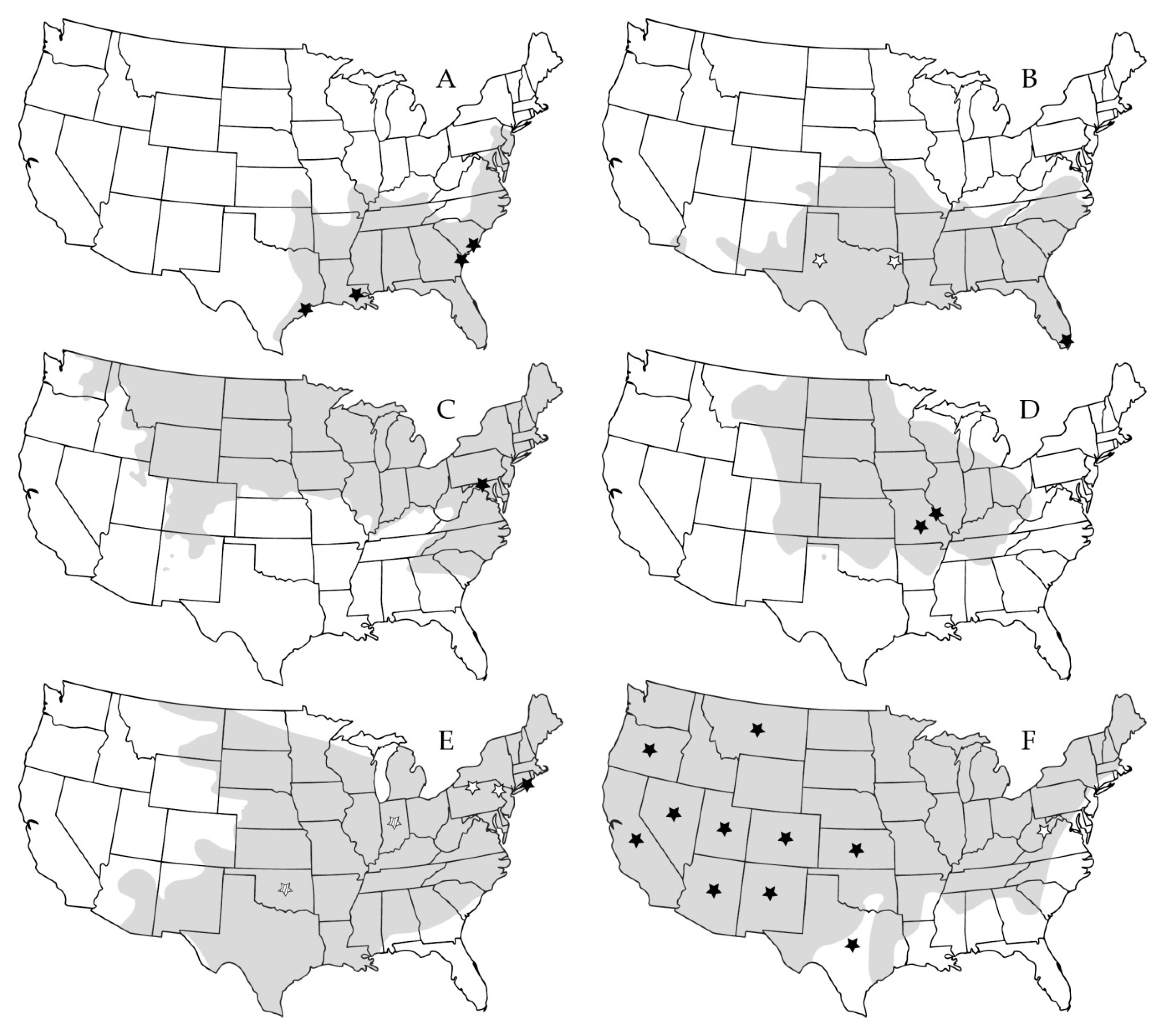
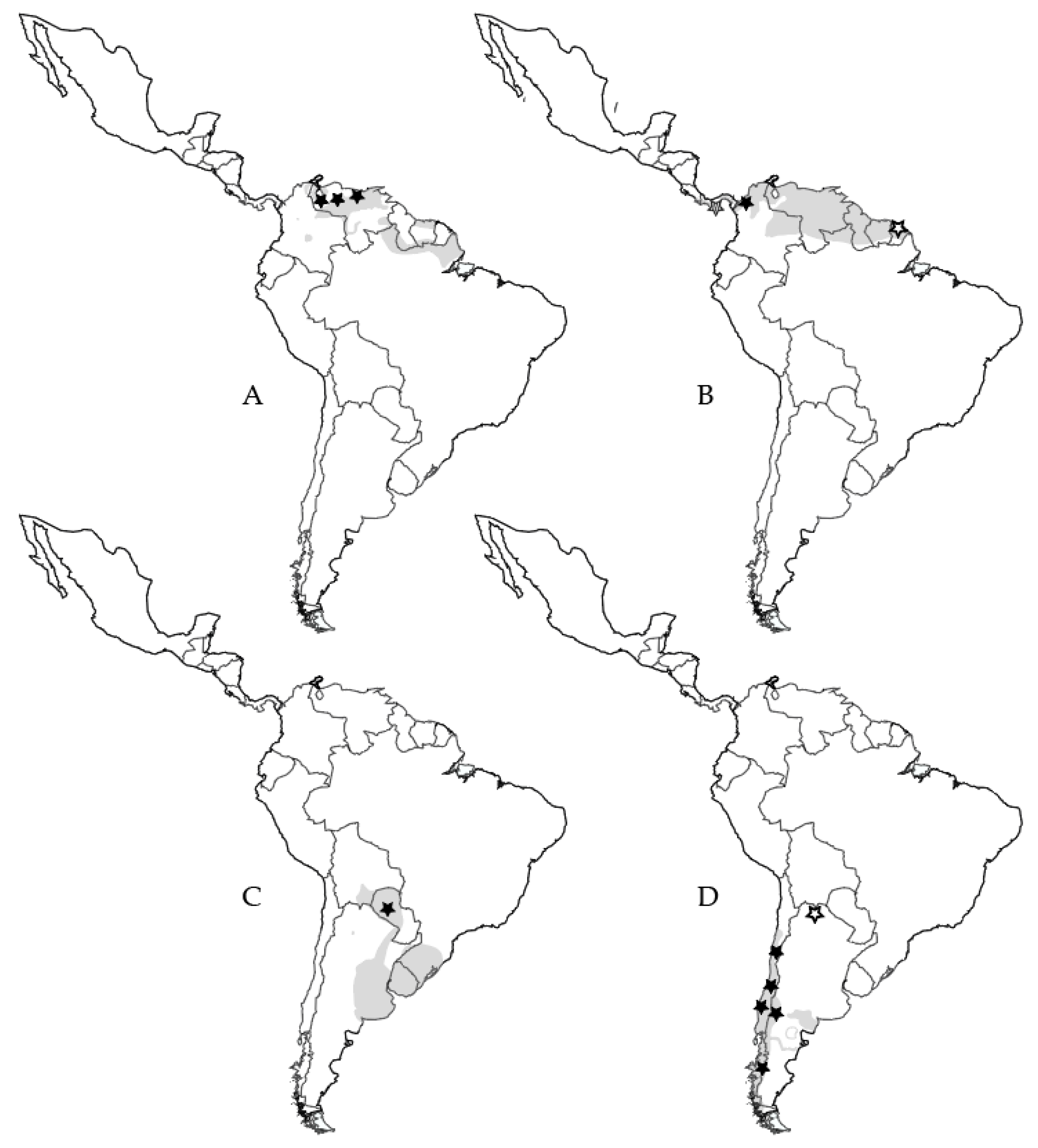
| Virus Species | Virus Strain | Virus Abbreviation | No. GenBank Submissions (Nov 9 2019) | Year Described | Human Disease | Discovery Source |
|---|---|---|---|---|---|---|
| Andes orthohantavirus | Andes virus | ANDV | 285 | 1996 | Yes | HCPS |
| Castelo dos Sonhos virus | CASV | 11 | 1999 | Yes | HCPS | |
| Lechiguanas virus | LECV/LECHV | 26 | 1997 | Yes | HCPS | |
| Oran virus | ORNV | 11 | 1998 | Yes | HCPS | |
| Bayou orthohantavirus | Bayou virus | BAYV | 13 | 1995 | Yes | HCPS |
| Catacamas virus | CATV | 3 | 2006 | No | Rodent | |
| Black Creek Canal orthohantavirus | Black Creek Canal virus | BCCV | 8 | 1995 | Yes | Rodent |
| Caño Delgadito orthohantavirus | Caño Delgadito virus | CADV | 17 | 1997 | No 1 | Rodent |
| Choclo orthohantavirus | Choclo virus | CHOV | 12 | 2000 | Yes | HCPS |
| El Moro Canyon orthohantavirus | Carrizal virus | CARV | 9 | 2012 | No | Rodent |
| El Moro Canyon virus | ELMCV | 35 | 1994 | No 1 | Rodent | |
| Huitzilac virus | HUIV | 4 | 2012 | No | Rodent | |
| Laguna Negra orthohantavirus | Laguna Negra virus | LANV | 35 | 1997 | Yes | HCPS |
| Maripa virus | MARV | 16 | 2012 | Yes | HCPS | |
| Rio Mamoré virus | RIOMV | 15 | 1997 | Yes | Rodent | |
| Maporal orthohantavirus | Maporal virus | MAPV | 10 | 2004 | No | Rodent |
| Montano orthohantavirus | Montano virus | MTNV | 60 | 2012 | No | Rodent |
| Necocli orthohantavirus | Necocli virus | NECV | 10 | 2011 | No | Rodent |
| Prospect Hill orthohantavirus | Prospect Hill virus | PHV | 24 | 1985 | No | Rodent |
| Sin Nombre orthohantavirus | New York virus | NYV | 4 | 1995 2 | Yes | HCPS |
| Sin Nombre virus | SNV | 228 | 1994 | Yes | HCPS |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mull, N.; Jackson, R.; Sironen, T.; Forbes, K.M. Ecology of Neglected Rodent-Borne American Orthohantaviruses. Pathogens 2020, 9, 325. https://doi.org/10.3390/pathogens9050325
Mull N, Jackson R, Sironen T, Forbes KM. Ecology of Neglected Rodent-Borne American Orthohantaviruses. Pathogens. 2020; 9(5):325. https://doi.org/10.3390/pathogens9050325
Chicago/Turabian StyleMull, Nathaniel, Reilly Jackson, Tarja Sironen, and Kristian M. Forbes. 2020. "Ecology of Neglected Rodent-Borne American Orthohantaviruses" Pathogens 9, no. 5: 325. https://doi.org/10.3390/pathogens9050325
APA StyleMull, N., Jackson, R., Sironen, T., & Forbes, K. M. (2020). Ecology of Neglected Rodent-Borne American Orthohantaviruses. Pathogens, 9(5), 325. https://doi.org/10.3390/pathogens9050325






