Anthology of Dirofilariasis in Russia (1915–2017)
Abstract
1. Introduction
2. Epidemiology of Dirofilariasis in the Ex-USSR
2.1. Age-Wise and Sex-Wise of Cases
2.2. Seasonality of Human Dirofilariasis
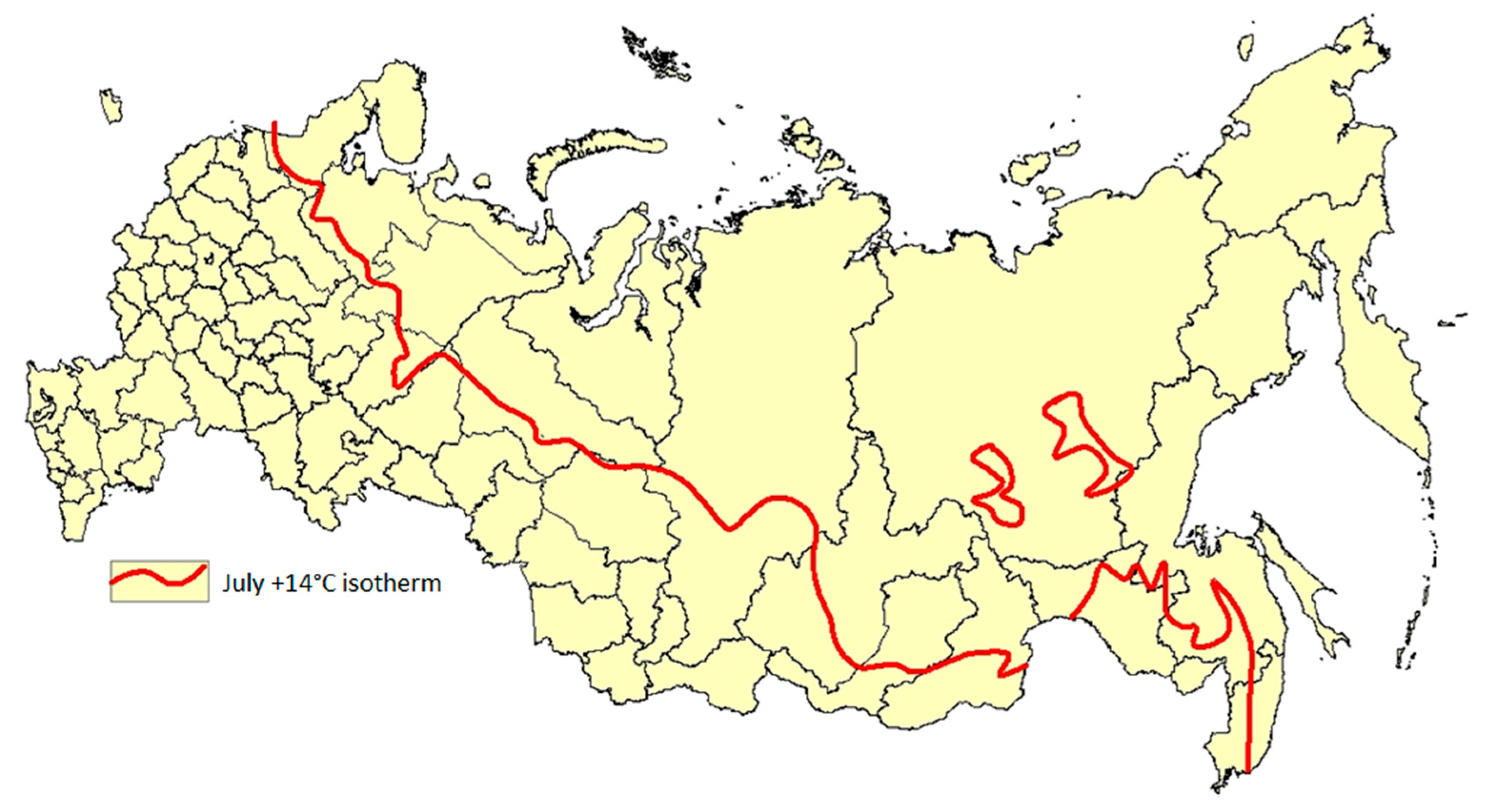
2.3. Geographical Confinement of Human Dirofilariasis
2.4. Detection and Diagnosis of Human Dirofilariasis Cases
2.5. Localization of Dirofilaria
2.6. Clinical Manifestations of Human Dirofilariasis
2.7. Dirofilariasis in Dogs
2.8. Dirofilaria in Vectors
2.9. Epidemiological Classification of Human Dirofilariasis Cases
3. Control and Prevention of Dirofilariasis in the Russian Federation
- T °C average actual is an average daily air temperature;
- T °C (14 °C) is the lowest temperature threshold below which Dirofilaria development within a mosquito vector does not occur;
- 130 degrees/days is “a sum of effective temperatures” (SET) or “growing degree day” (adopted in several models in the West) needed for Dirofilaria larvae to reach infectivity. The concept of SET was originally developed and successfully applied in Russia in the control of malaria [65]. This approach was applied to other vector-borne diseases as well, for example, for cutaneous leishmaniasis [66].
4. Conclusions
Author Contributions
Ethics Approval
Funding
Availability of Data and Materials
Conflicts of Interest
Abbreviations
| PCR | Polymerase Chain Reaction |
| USSR | Union of Soviet Socialist Republics |
| WHO | World Health Organization |
References
- Avdiukhina, T.I.; Supriaga, V.G.; Postnova, V.F.; Kuimova, R.T.; Mironova, N.I.; Murashov, N.E.; Putintseva, E.V. Dirofilariasis in the countries of the CIS: An analysis of the cases over the years 1915–1996. Meditsinskaia Parazitologiia Parazitarnye Bolezni 1997, 4, 3–7. [Google Scholar]
- Skryabin, K.I.; Shichobalova, N.P. Filaria of Animals and Man; Selkhozgiz: Moscow, Russia, 1948; p. 608. [Google Scholar]
- Ermakova, L.A.; Nagorny, S.A.; Krivorotova, E.Y.; Pshenichnaya, N.Y.; Matina, O.N. Dirofilaria repens in the Russian Federation: Current epidemiology, diagnosis, and treatment from a federal reference center perspective. Int. J. Infect. Dis. 2014, 23, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Darchenkova, N.N.; Supriaga, V.G.; Guzeeva, M.V.; Morozov, E.N.; Zhukova, L.A.; Sergiev, V.P. Prevalence of human dirofilariasis in Russia. Meditsinskaia Parazitologiia Parazitarnye Bolezni 2009, 2, 3–7. [Google Scholar]
- Sergiev, V.P.; Supriaga, V.G.; Bronshtein, A.M.; Ganushkina, L.A.; Rakova, V.M.; Morozov, E.N.; Fedianina, L.V.; Frolova, A.A.; Morozova, L.F.; Ivanova, I.B.; et al. Results of studies of human dirofilariasis in Russia. Meditsinskaia Parazitologiia Parazitarnye Bolezni 2014, 3, 3–9. [Google Scholar]
- Ermakova, L.; Nagorny, S.; Pshenichnaya, N.; Ambalov, Y.; Boltachiev, K. Clinical and laboratory features of human dirofilariasis in Russia. IDCases 2017, 9, 112–115. [Google Scholar] [CrossRef]
- Moskvina, T.V.; Ermolenko, A.V. Dirofilariasis in Russian Federation: A big problem with large distribution. Russ. Open Med. J. 2018, 7. [Google Scholar] [CrossRef]
- Kramer, L.H.; Kartashev, V.V.; Grandi, G.; Morchon, R.; Nagornii, S.A.; Karanis, P.; Simon, F. Human subcutaneous dirofilariasis, Russia. Emerg. Infect. Dis. 2007, 13, 150–152. [Google Scholar] [CrossRef]
- Avdiukhina, T.I.; Postnova, V.F.; Abrosimova, L.M.; Kovtunov, A.I.; Arakel’ian, S.E.; Murashov, N.E.; Plenkina, L.V.; Grigor’eva, E.S.; Korotkov, V.B.; Petukhova, G.A.; et al. Dirofilariasis (D. repens) in the Russian Federation and some of the Commonwealth of Independent States countries: Situation and trends. Meditsinskaia Parazitologiia Parazitarnye Bolezni 2003, 4, 44–48. [Google Scholar]
- Bartkova, A.D.; Poliakova, L.F.; Ermolenko, A.V. Human dirofilariasis in the Primorye Territory. Meditsinskaia Parazitologiia Parazitarnye Bolezni 2011, 1, 47–48. [Google Scholar]
- Kartashev, V.; Afonin, A.; Gonzalez-Miguel, J.; Sepulveda, R.; Simon, L.; Morchon, R.; Simon, F. Regional warming and emerging vector-borne zoonotic dirofilariosis in the Russian Federation, Ukraine, and other post-Soviet states from 1981 to 2011 and projection by 2030. Biomed. Res. Int. 2014, 2014, 858936. [Google Scholar] [CrossRef]
- Furmanov, I.P. Dirofilariasis cases in Turkmenistan. J. Public Health Turkm. 1991, 3, 41–43. [Google Scholar]
- Gavrilov, A.A. Prevalence of D. repens microfilaria in dogs. J. Public Health Kazakhstan 1977, 5, 84–88. [Google Scholar]
- Velichko, V.I.; Tulekova, B.A. Dirofilariasis in Kazakhstan. J. Public Health Kazakhstan 1988, 5, 65–68. [Google Scholar]
- Ivanova, I.B.; Trotsenko, O.E.; Karavianskaia, T.N.; Ganushkina, L.A. Human dirofilariasis in the city of Khabarovsk. Meditsinskaia Parazitologiia Parazitarnye Bolezni 2013, 3, 18–20. [Google Scholar]
- Rosolovskii, A.P.; P’Ianykh, V.A.; Ignat’eva, V.I.; Matina, O.N.; Shevchuk, E.A.; Danilova, E.P.; Tverdokhlebova, T.I.; Nagornyi, S.A.; Ermakova, L.A.; Krivorotova, E. Dirofilariasis in the Nizhny Novgorod Region. Meditsinskaia Parazitologiia Parazitarnye Bolezni 2013, 1, 34–35. [Google Scholar]
- Derzhavina, T.; Boldyreva, V.V.; Sheveleva, O.F.; Gribova, T.I.; Chernysheva, A.A.; Chernikova, E.A. A dirofilariasis focus in the Tula Region in 2011. Meditsinskaia Parazitologiia Parazitarnye Bolezni 2012, 2, 50. [Google Scholar]
- Figurnov, V.A.; Moiseenko, G.A.; Moshkonova, O.K.; Godovaniuk, A.; Sycheva, L.A. The first case of detected dirofilariasis in the Amursk Region. Meditsinskaia Parazitologiia Parazitarnye Bolezni 2009, 1, 58. [Google Scholar]
- Kazachkov, E.L.; Gorsheneva, V.M.; Faizullina, I.E. A case of dirofilariasis in the town of Magnitogorsk of the Cheliabinsk region. Meditsinskaia Parazitologiia Parazitarnye Bolezni 2004, 2, 55–57. [Google Scholar]
- Safronova, E.; Vorob’ev, A.A.; Latyshevskaia, N.I.; Ermilov, V.V.; Chulkova, G.B. Dirofilariasis in the Volgograd region—A new disease in the region. Meditsinskaia Parazitologiia Parazitarnye Bolezni 2004, 2, 51–54. [Google Scholar]
- Kiselev, V.S. Dirofilariasis in residents of the Ulanovsk region. Meditsinskaia Parazitologiia Parazitarnye Bolezni 2003, 1, 27–28. [Google Scholar]
- Tolkunova, I.I.; Ulacevich, R.A.; Iakovleva, E.G. Recording a case of dirofilariasis in residents of Novosibirsk. Meditsinskaia Parazitologiia Parazitarnye Bolezni 2003, 1, 26. [Google Scholar]
- Ushakov, A.V.; Stepanova, T.F.; Strugova, A.S. Incidence of dirofilariasis in the city of Tiumen. Meditsinskaia Parazitologiia Parazitarnye Bolezni 2002, 4, 52–53. [Google Scholar]
- Azarova, N.A.; Preider, V.P. Dirofilariasis in the city of Barnaul, Altai Territory. Meditsinskaia Parazitologiia Parazitarnye Bolezni 1998, 4, 49–50. [Google Scholar]
- Rumiantseva, E.E.; Voronok, V.M.; Mariutina, L.A. Case of dirofilariasis in the Maritime Territory. Meditsinskaia Parazitologiia Parazitarnye Bolezni 1985, 6, 75. [Google Scholar]
- Krivorotova, E.Y. Xenomonitoring of the Dirofilariasis in the South and North-West of the Russian Federation. Parazitologiia 2016, 50, 357–364. [Google Scholar]
- Dorofeev Iu, A.; Dashevskii, V.V.; Koltaniuk, M.N.; Sirotiuk, N.P.; Zhulaeva, T.E. Cases of dirofilariasis in inhabitants of the Crimea. Meditsinskaia Parazitologiia Parazitarnye Bolezni 1997, 4, 7–8. [Google Scholar]
- Dissanaike, A.S.; Abeyewickreme, W.; Wijesundera, M.D.; Weerasooriya, M.V.; Ismail, M.M. Human dirofilariasis caused by Dirofilaria (Nochtiella) repens in Sri Lanka. Parassitologia 1997, 39, 375–382. [Google Scholar]
- Bronshtein, A.M.; Supriaga, V.G.; Stavrovskii, B.I.; Sabgaida, T.P.; Luchshev, V.I.; Korotkova, G.I.; Legon’kov Iu, A.; Firsova, R.A.; Darchenkova, N.N.; Starkova, T.V.; et al. Human dirofilariasis in the Moscow region. Meditsinskaia Parazitologiia Parazitarnye Bolezni 2003, 3, 51–56. [Google Scholar]
- Nagornyi, S.A.; Beskrovnaia Iu, G.; Vaserin Iu, I.; Chernikova, E.A. Biological characteristics of the causative agent of dirofilariasis in dogs in the Rostov Region. Meditsinskaia Parazitologiia Parazitarnye Bolezni 2009, 3, 7–10. [Google Scholar]
- Pampiglione, S.; Rivasi, F.; Canestri-Trotti, G. Pitfalls and difficulties in histological diagnosis of human dirofilariasis due to Dirofilaria (Nochtiella) repens. Diagn. Microbiol. Infect. Dis. 1999, 34, 57–64. [Google Scholar] [CrossRef]
- Morozov, E.N.; Supriaga, V.G.; Rakova, V.M.; Morozova, L.F.; Zhukova, L.A. Human dirofilariasis: Clinical and diagnostic signs and diagnostic methods. Meditsinskaia Parazitologiia Parazitarnye Bolezni 2014, 2, 13–17. [Google Scholar]
- Supriaga, V.G.; Tsybina, T.N.; Denisova, T.N.; Morozov, E.N.; Romanenko, N.A.; Starkova, T.V. The first case of diagnosis of dirofilariasis from the microfilariae detected in the human subcutaneous tumor punctate. Meditsinskaia Parazitologiia Parazitarnye Bolezni 2004, 4, 6–8. [Google Scholar]
- Supriaga, V.G.; Starkova, T.V.; Korotkova, G.I. Clinical and parasitological diagnosis of human dirofilariasis. Meditsinskaia Parazitologiia Parazitarnye Bolezni 2002, 1, 53–55. [Google Scholar]
- Sonin, M.D. Basis of Nematodology, Filariasis of Animal and Men; Nauka: Moscow, Russia, 1975; p. 200. [Google Scholar]
- Sergiev, V.P.; Supriaga, V.G.; Morozov, E.N.; Zhukova, L.A. Human dirofilariasis: Diagnosis and the pattern of pathogen-host relations. Meditsinskaia Parazitologiia Parazitarnye Bolezni 2009, 3, 3–6. [Google Scholar]
- Supryaga, V.G.; Rakova, V.M.; Morozov, E.N. Current Ideas on Obligate and Facultative Relationships between Man and the Dirofilariasis Pathogen Dirofilaria (N.) Repens. Meditsinskaia Parazitologiia Parazitarnye Bolezni 2016, 2, 3–7. [Google Scholar]
- Fontanelli Sulekova, L.; Gabrielli, S.; De Angelis, M.; Milardi, G.L.; Magnani, C.; Di Marco, B.; Taliani, G.; Cancrini, G. Dirofilaria repens microfilariae from a human node fine-needle aspirate: A case report. BMC Infect. Dis. 2016, 16, 248. [Google Scholar] [CrossRef]
- Fedianina, L.V.; Shatova, S.M.; Rakova, V.M.; Shaĭtanov, V.M.; Lebedeva, M.N.; Frolova, A.A.; Morozov, E.N.; Morozova, L.F. Microfilaraemia in human dirofilariasis caused by Dirofilaria repens Raiet et Henry, 1911. A case report. Meditsinskaia Parazitologiia Parazitarnye Bolezni 2013, 2, 3–7. [Google Scholar]
- Fedianina, L.V.; Frolova, A.A.; Pliushcheva, G.L.; Chernyshenko, A.I.; Morozov, E.N.; Rakova, V.M. Cases confirming the concept that the human being is a facultative host of Dirofilaria repens. Meditsinskaia Parazitologiia Parazitarnye Bolezni 2011, 4, 37–38. [Google Scholar]
- Fedianina, L.V. Human dirofilariasis in Russia. Meditsinskaia Parazitologiia Parazitarnye Bolezni 2010, 2, 43–44. [Google Scholar]
- Vakalis, N.; Spanakos, G.; Patsoula, E.; Vamvakopoulos, N.C. Improved detection of Dirofilaria repens DNA by direct polymerase chain reaction. Parasitol. Int. 1999, 48, 145–150. [Google Scholar] [CrossRef]
- Lysenko, A. The general and specific features of human larval helminthiases. Meditsinskaia Parazitologiia Parazitarnye Bolezni 1998, 2, 27–31. [Google Scholar]
- Postnova, V.F.; Kovtunov, A.I.; Abrosimova, L.M.; Avdiukhina, T.I.; Mishina, L.I.; Pogorel’chuk, T.; Oleinik, V.A.; Beshko, N.I. New cases of human dirofilariasis. Meditsinskaia Parazitologiia Parazitarnye Bolezni 1997, 1, 6–9. [Google Scholar]
- Samoilovich, L.N.; Zhmak, Z.O. A case of human dirofilariasis. Meditsinskaia Parazitologiia Parazitarnye Bolezni 1966, 35, 376. [Google Scholar]
- Kamal’dinova, D.G.; An, A.S. A case of human dirofilariasis. Meditsinskaia Parazitologiia Parazitarnye Bolezni 1967, 36, 356. [Google Scholar]
- Borisova, M.A.; Sirotiuk, N.P.; Tsygankova, O.D.; Zhulaeva, T.E. Manifestations of a human infestation with Dirofilaria repens Raillet et Henry, 1911. Meditsinskaia Parazitologiia Parazitarnye Bolezni 1986, 5, 86. [Google Scholar]
- Mel’nichenko, A.P.; Prosvetova, T.A. 2D case of human drofilariasis in the Poltava region. Meditsinskaia Parazitologiia Parazitarnye Bolezni 1971, 40, 238–239. [Google Scholar]
- Pashkova, T.P.; Tokin, A.N. A case of human dirofilariasis. Meditsinskaia Parazitologiia Parazitarnye Bolezni 1968, 37, 244. [Google Scholar]
- Beshko, N.I.; Pogorel’chuk, T.; Man’kovskaia, N.N.; Oleinik, V.A. Human infestation by the nematode Dirofilaria repens. Meditsinskaia Parazitologiia Parazitarnye Bolezni 1994, 1, 58. [Google Scholar]
- Kovalev, N.E.; Zueva, V.K.; Mareich, O.I. Human dirofilariasis. Meditsinskaia Parazitologiia Parazitarnye Bolezni 1971, 40, 741–742. [Google Scholar]
- Supriaga, V.G.; Usishchev, P.V.; Opryshko, M.V. Infection of humans with a nematode Dirofilaria repens Railliet et Henry, 1911. Meditsinskaia Parazitologiia Parazitarnye Bolezni 1985, 1, 85–86. [Google Scholar]
- Mukvoz, L.G.; Belozerskaia, N.I. 2 clinical cases of human dirofilariasis in the town of Zaporozhie. Meditsinskaia Parazitologiia Parazitarnye Bolezni 1975, 44, 615. [Google Scholar]
- Supriaga, V.G.; Darchenkova, N.N.; Bronshtein, A.M.; Lebedeva, M.N.; Iastreb, V.B.; Ivanova, T.N.; Guzeeva, M.V.; Timoshenko, N.I.; Rakova, V.M.; Zhukova, L.A. Dirofilariasis in the Moscow Region, a low disease transmission risk area. Meditsinskaia Parazitologiia Parazitarnye Bolezni 2011, 1, 3–7. [Google Scholar]
- Bogacheva, A.S.; Ganushkina, L.A.; Lopatina, Y.V. Bloodsucking Mosquitoes (Diptera: Culicidae) in, the Tula Region Are Potential Vectors of Dirofilarias. Meditsinskaia Parazitologiia Parazitarnye Bolezni 2015, 4, 18–22. [Google Scholar]
- Bogacheva, A.S.; Ganushkina, L.A.; Lopatina, Y.V. Infection of Blood-Sucking Mosquitoes (Diptera: Culicidae) with Dirofilariae (Spirurida, Onchocercidae) in the Tula Region. Meditsinskaia Parazitologiia Parazitarnye Bolezni 2016, 2, 8–12. [Google Scholar]
- Ganushkina, L.A.; Rakova, V.M.; Ivanova, I.B.; Supriaga, V.G.; Sergiev, V.P. Entomological monitoring of an area to assess Dirofilaria transmission risk. Meditsinskaia Parazitologiia Parazitarnye Bolezni 2014, 3, 9–12. [Google Scholar]
- Shuikina, E.E.; Sergiev, V.P.; Supriaga, V.G.; Arakel’ian, R.S.; Darchenkova, N.N.; Arkhipov, I.A. Formation of the synanthropic foci of dirofilariasis in Russia. Meditsinskaia Parazitologiia Parazitarnye Bolezni 2009, 4, 9–11. [Google Scholar]
- Varlamova, A.I.; Arkhipov, I.A. Circulation of Dirofilaria Repens (Railliet Et Henry, 1911) in the Arid Zone of Southern Russia. Meditsinskaia Parazitologiia Parazitarnye Bolezni 2016, 2, 28–31. [Google Scholar]
- Baneth, G.; Volansky, Z.; Anug, Y.; Favia, G.; Bain, O.; Goldstein, R.E.; Harrus, S. Dirofilaria repens infection in a dog: Diagnosis and treatment with melarsomine and doramectin. Vet. Parasitol. 2002, 105, 173–178. [Google Scholar] [CrossRef]
- Arakelian, R.S.; Galimzianov Kh, M.; Kulagin, V.V.; Riabikina, M.A.; Chernukhin, D.A. Use of Dironet for prophylaxis in Dirofilaria immitis-infested working dogs in the Astrakhan region. Meditsinskaia Parazitologiia Parazitarnye Bolezni 2011, 4, 41–42. [Google Scholar]
- Nagornyi, S.A.; Ermakova, L.A.; Krivorotova, E. The specific features of the epidemiology and epizootology of dirofilariasis in Rostov-on-Don and the Rostov region. Meditsinskaia Parazitologiia Parazitarnye Bolezni 2012, 4, 46–48. [Google Scholar]
- Rakova, V.M. Molecular Biology Diagnosis of Dirofilariasis in Definitive Host and in Vector; Sechenov University: Moscow, Russia, 2013. [Google Scholar]
- Morozova, L.F. Geographical Information System in Epidemiological Surveillance for Parasite Diseases; Sechenov University: Moscow, Russia, 2014. [Google Scholar]
- Mironova, V.; Shartova, N.; Beljaev, A.; Varentsov, M.; Grishchenko, M. Effects of Climate Change and Heterogeneity of Local Climates on the Development of Malaria Parasite (Plasmodium vivax) in Moscow Megacity Region. Int. J. Environ. Res. Public Health 2019, 16, 694. [Google Scholar] [CrossRef] [PubMed]
- Ponirovsky, E.N.; Strelkova, M.V.; Sergiev, V.P.; Baranets, M.S. The mapping of focal areas as the basis for preventing zoonotic cutaneous leischmaniasis. Meditsinskaia Parazitologiia Parazitarnye Bolezni 2016, 1, 57–59. [Google Scholar]
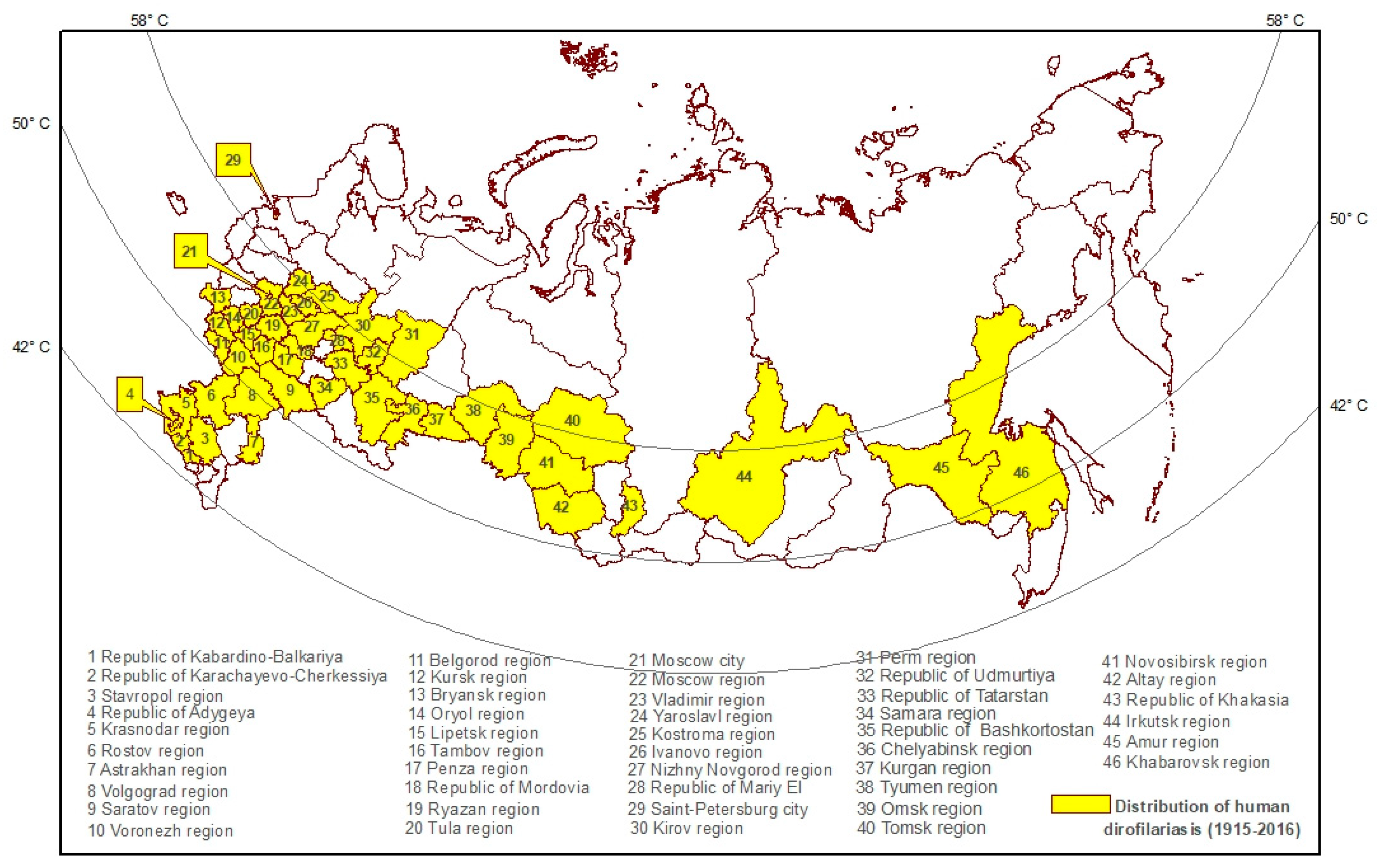
| 1915–1944 | 1945–1954 | 1955–1964 | 1965–1974 | 1975–1980 | 1981–1985 | 1986–1990 | 1991–1995 | 1996–1998 * | 1999–2001 * | 2002–2008 * | 2008–2016 * |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 9 | 5 | 12 | 21 | 13 | 25 | 13 | 21 | 28 | 114 | 303 | 636 |
| Zone | Host | Vectors (Infected) | Reference | ||||
|---|---|---|---|---|---|---|---|
| Definitive—Dog (%) (Prevalence) | Facultative—Human (Number of Patients) | Aedes (%) | Culex (%) | Anopheles (%) | |||
| 1 | Low | 7.7 (service) | 64 | 2.6 | 3.6 | 3.7 | Rakova, 2013 [63] |
| 2 | Moderate | 7.3 (service) | 36 | 4.8 | 6.2 | <1.0 | Ivanova, 2013 [15] |
| 4.2 (stray) | |||||||
| 3 | Stable | 25.6 | 244 | 18.2 | 10.8 | 1.3 | Nagornyi et al., 2012 [62] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondrashin, A.V.; Morozova, L.F.; Stepanova, E.V.; Turbabina, N.A.; Maksimova, M.S.; Morozov, E.N. Anthology of Dirofilariasis in Russia (1915–2017). Pathogens 2020, 9, 275. https://doi.org/10.3390/pathogens9040275
Kondrashin AV, Morozova LF, Stepanova EV, Turbabina NA, Maksimova MS, Morozov EN. Anthology of Dirofilariasis in Russia (1915–2017). Pathogens. 2020; 9(4):275. https://doi.org/10.3390/pathogens9040275
Chicago/Turabian StyleKondrashin, Anatoly V., Lola F. Morozova, Ekaterina V. Stepanova, Natalia A. Turbabina, Maria S. Maksimova, and Evgeny N. Morozov. 2020. "Anthology of Dirofilariasis in Russia (1915–2017)" Pathogens 9, no. 4: 275. https://doi.org/10.3390/pathogens9040275
APA StyleKondrashin, A. V., Morozova, L. F., Stepanova, E. V., Turbabina, N. A., Maksimova, M. S., & Morozov, E. N. (2020). Anthology of Dirofilariasis in Russia (1915–2017). Pathogens, 9(4), 275. https://doi.org/10.3390/pathogens9040275





