Kaposi’s Sarcoma-Associated Herpesvirus and Host Interaction by the Complement System
Abstract
1. Introduction
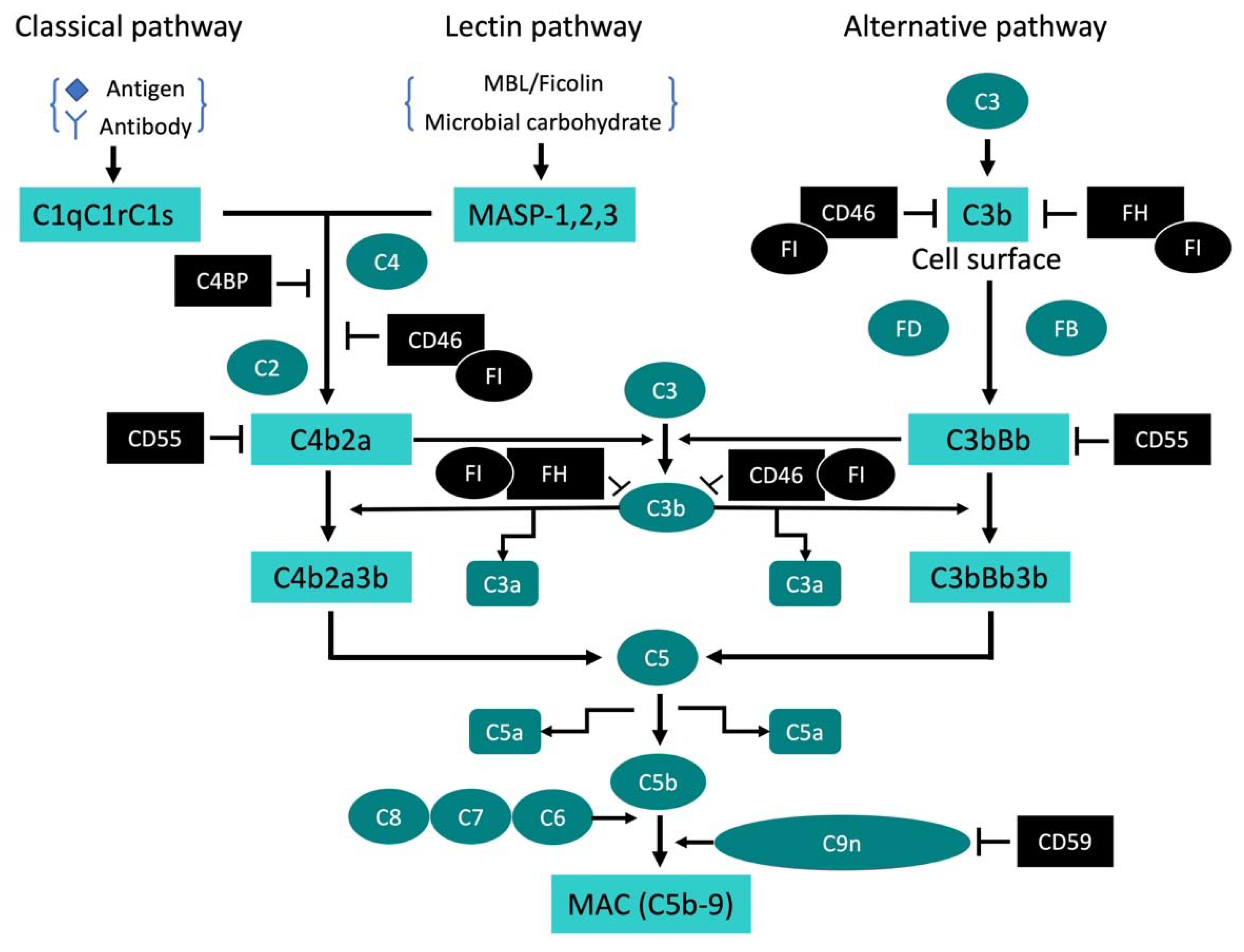
2. Complement System
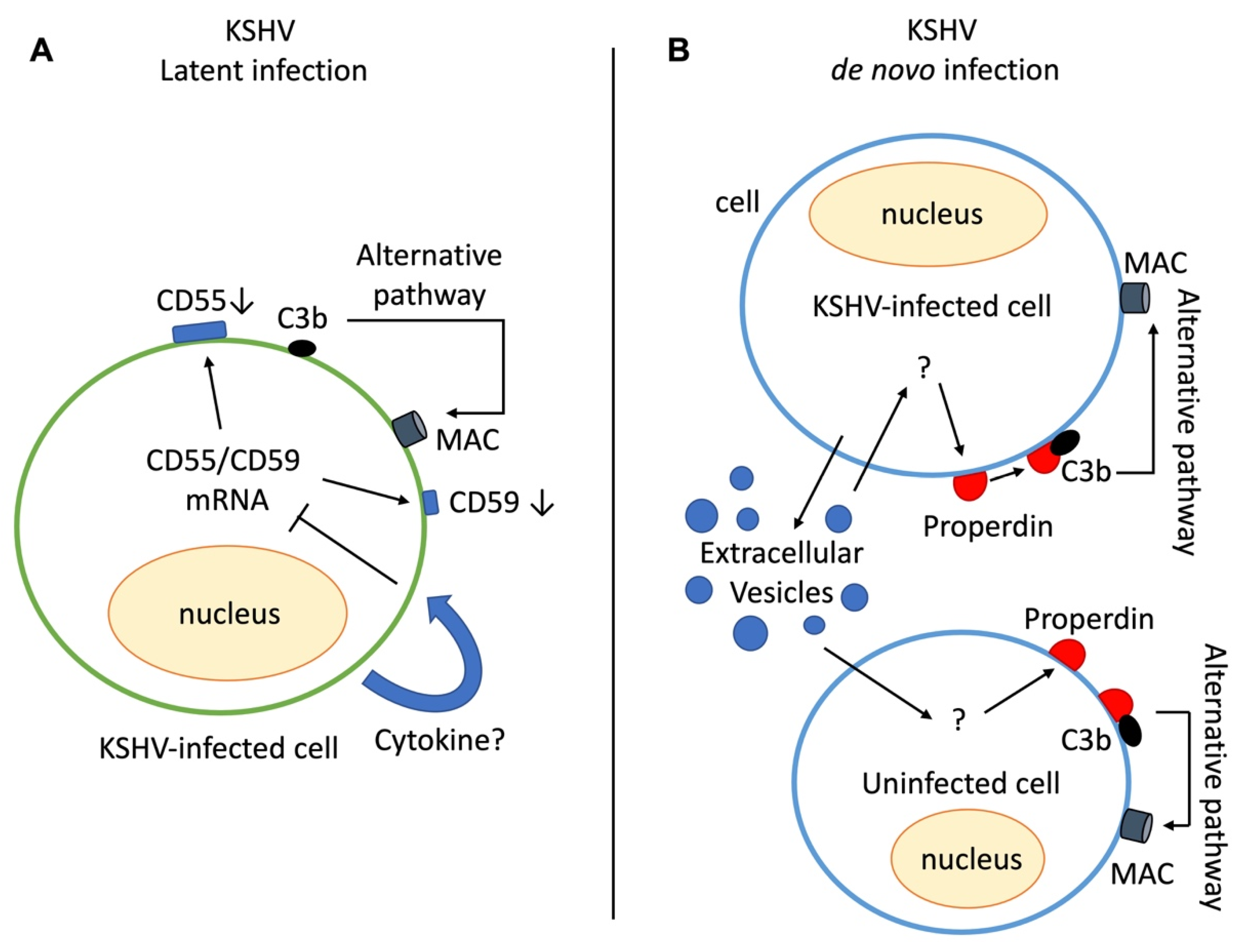
3. Evasion of the Complement System by KSHV
4. Activation of the Complement System in KSHV-Infected Cells
4.1. Complement Activation in the KS Cellular Model
4.2. Complement Activation in Primary Effusion Lymphoma
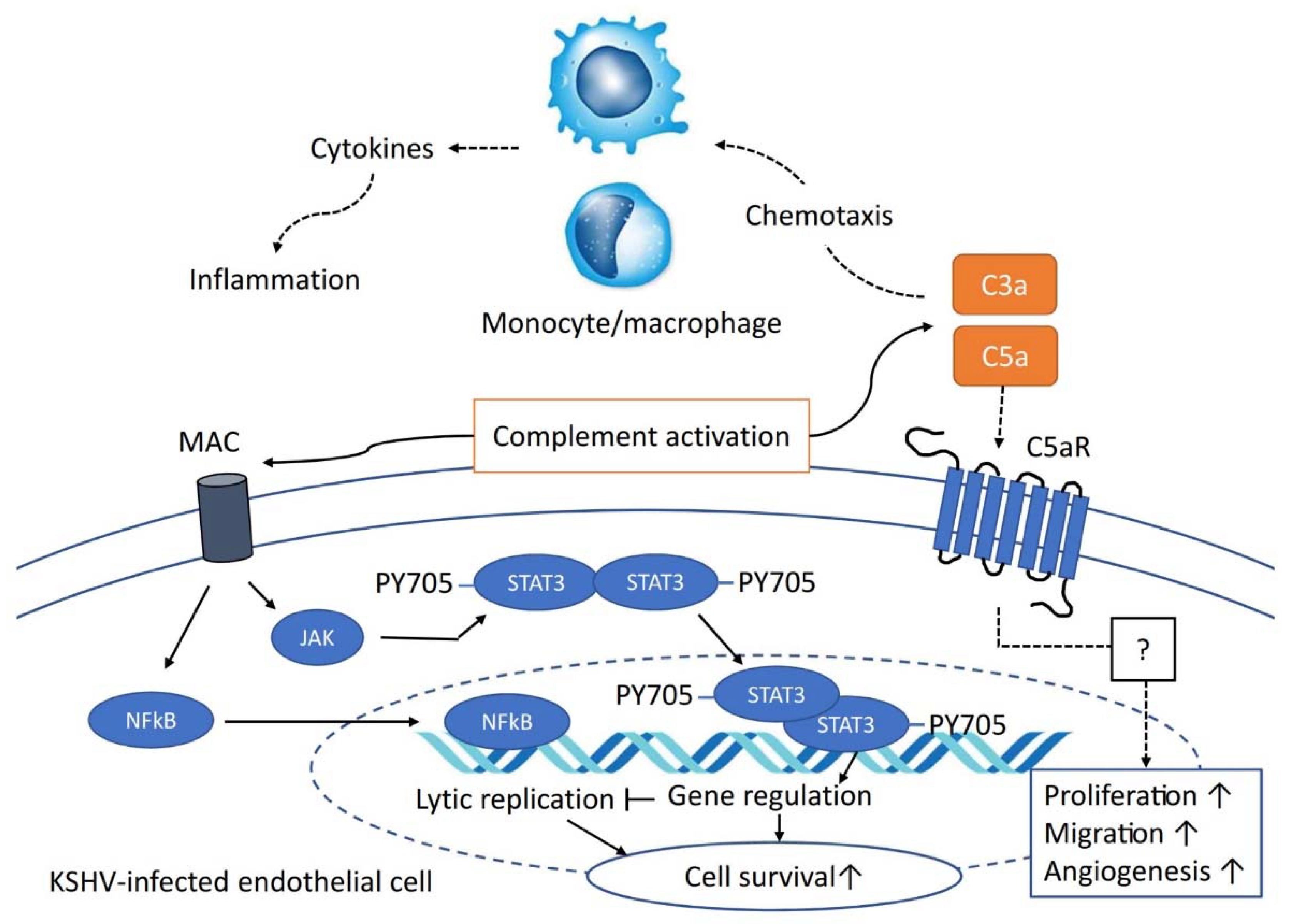
5. The Role of the Complement System in KSHV-Infected Cells
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Chang, Y.; Cesarman, E.; Pessin, M.S.; Lee, F.; Culpepper, J.; Knowles, D.M.; Moore, P.S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 1994, 266, 1865–1869. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.S.; Chang, Y. Detection of herpesvirus-like DNA sequences in Kaposi’s sarcoma in patients with and those without HIV infection. N. Engl. J. Med. 1995, 332, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Oksenhendler, E.; Boulanger, E.; Galicier, L.; Du, M.Q.; Dupin, N.; Diss, T.C.; Hamoudi, R.; Daniel, M.T.; Agbalika, F.; Boshoff, C.; et al. High incidence of Kaposi sarcoma-associated herpesvirus-related non-Hodgkin lymphoma in patients with HIV infection and multicentric Castleman disease. Blood 2002, 99, 2331–2336. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Stebbing, J.; Bazeos, A.; Hatzimichael, E.; Mandalia, S.; Nelson, M.; Gazzard, B.; Bower, M. The role of immune suppression and HHV-8 in the increasing incidence of HIV-associated multicentric Castleman’s disease. Ann. Oncol. 2009, 20, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Krown, S.E.; Lee, J.Y.; Dittmer, D.P.; Consortium, A.M. More on HIV-associated Kaposi’s sarcoma. N. Engl. J. Med. 2008, 358, 535–536. [Google Scholar] [CrossRef]
- Douglas, J.L.; Gustin, J.K.; Moses, A.V.; Dezube, B.J.; Pantanowitz, L. Kaposi Sarcoma Pathogenesis: A Triad of Viral Infection, Oncogenesis and Chronic Inflammation. Transl. Biomed. 2010, 1, 172. [Google Scholar]
- Maillard, P.; Krawczynski, K.; Nitkiewicz, J.; Bronnert, C.; Sidorkiewicz, M.; Gounon, P.; Dubuisson, J.; Faure, G.; Crainic, R.; Budkowska, A. Nonenveloped nucleocapsids of hepatitis C virus in the serum of infected patients. J. Virol. 2001, 75, 8240–8250. [Google Scholar] [CrossRef]
- Nascimento, E.J.; Silva, A.M.; Cordeiro, M.T.; Brito, C.A.; Gil, L.H.; Braga-Neto, U.; Marques, E.T. Alternative complement pathway deregulation is correlated with dengue severity. PLoS ONE 2009, 4, e6782. [Google Scholar] [CrossRef]
- Smith, J.G.; Nemerow, G.R. Complement Seals a Virus to Block Infection. Cell Host Microbe 2019, 25, 482–483. [Google Scholar] [CrossRef]
- Stoermer, K.A.; Morrison, T.E. Complement and viral pathogenesis. Virology 2011, 411, 362–373. [Google Scholar] [CrossRef]
- Lee, M.S.; Jones, T.; Song, D.Y.; Jang, J.H.; Jung, J.U.; Gao, S.J. Exploitation of the complement system by oncogenic Kaposi’s sarcoma-associated herpesvirus for cell survival and persistent infection. PLoS Pathog. 2014, 10, e1004412. [Google Scholar] [CrossRef]
- Carroll, M.V.; Sim, R.B. Complement in health and disease. Adv. Drug Deliv. Rev. 2011, 63, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Porter, R.R.; Reid, K.B. Activation of the complement system by antibody-antigen complexes: The classical pathway. Adv. Protein Chem. 1979, 33, 1–71. [Google Scholar] [CrossRef] [PubMed]
- Kishore, U.; Reid, K.B. Modular organization of proteins containing C1q-like globular domain. Immunopharmacology 1999, 42, 15–21. [Google Scholar] [CrossRef]
- Ziccardi, R.J. The first component of human complement (C1): Activation and control. Springer Semin. Immunopathol. 1983, 6, 213–230. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, R.F.; Palomaki, G.E.; Neveux, L.M.; Navolotskaia, O.; Ledue, T.B.; Craig, W.Y. Reference distributions for complement proteins C3 and C4: A practical, simple and clinically relevant approach in a large cohort. J. Clin. Lab. Anal. 2004, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lynch, N.J.; Roscher, S.; Hartung, T.; Morath, S.; Matsushita, M.; Maennel, D.N.; Kuraya, M.; Fujita, T.; Schwaeble, W.J. L-ficolin specifically binds to lipoteichoic acid, a cell wall constituent of Gram-positive bacteria, and activates the lectin pathway of complement. J. Immunol. 2004, 172, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Eisen, S.; Dzwonek, A.; Klein, N.J. Mannose-binding lectin in HIV infection. Future Virol. 2008, 3, 225–233. [Google Scholar] [CrossRef]
- Van Asbeck, E.C.; Hoepelman, A.I.; Scharringa, J.; Herpers, B.L.; Verhoef, J. Mannose binding lectin plays a crucial role in innate immunity against yeast by enhanced complement activation and enhanced uptake of polymorphonuclear cells. BMC Microbiol. 2008, 8, 229. [Google Scholar] [CrossRef]
- Pangburn, M.K.; Muller-Eberhard, H.J. Initiation of the alternative complement pathway due to spontaneous hydrolysis of the thioester of C3. Ann. N. Y. Acad. Sci. 1983, 421, 291–298. [Google Scholar] [CrossRef]
- Geller, A.; Yan, J. The Role of Membrane Bound Complement Regulatory Proteins in Tumor Development and Cancer Immunotherapy. Front. Immunol. 2019, 10, 1074. [Google Scholar] [CrossRef] [PubMed]
- Kouser, L.; Abdul-Aziz, M.; Nayak, A.; Stover, C.M.; Sim, R.B.; Kishore, U. Properdin and factor h: Opposing players on the alternative complement pathway “see-saw”. Front. Immunol. 2013, 4, 93. [Google Scholar] [CrossRef] [PubMed]
- Pryzdial, E.L.; Isenman, D.E. A reexamination of the role of magnesium in the human alternative pathway of complement. Mol. Immunol. 1986, 23, 87–96. [Google Scholar] [CrossRef]
- Lesher, A.M.; Nilsson, B.; Song, W.C. Properdin in complement activation and tissue injury. Mol. Immunol. 2013, 56, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Irmscher, S.; Doring, N.; Halder, L.D.; Jo, E.A.H.; Kopka, I.; Dunker, C.; Jacobsen, I.D.; Luo, S.; Slevogt, H.; Lorkowski, S.; et al. Kallikrein Cleaves C3 and Activates Complement. J. Innate Immun. 2018, 10, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Amara, U.; Rittirsch, D.; Flierl, M.; Bruckner, U.; Klos, A.; Gebhard, F.; Lambris, J.D.; Huber-Lang, M. Interaction between the coagulation and complement system. Adv. Exp. Med. Biol. 2008, 632, 71–79. [Google Scholar] [CrossRef]
- Kondos, S.C.; Hatfaludi, T.; Voskoboinik, I.; Trapani, J.A.; Law, R.H.; Whisstock, J.C.; Dunstone, M.A. The structure and function of mammalian membrane-attack complex/perforin-like proteins. Tissue Antigens 2010, 76, 341–351. [Google Scholar] [CrossRef]
- Brown, E.J. Interaction of gram-positive microorganisms with complement. Curr. Top. Microbiol. Immunol. 1985, 121, 159–187. [Google Scholar] [CrossRef]
- Lambris, J.D.; Ricklin, D.; Geisbrecht, B.V. Complement evasion by human pathogens. Nat. Rev. Microbiol. 2008, 6, 132–142. [Google Scholar] [CrossRef]
- Packman, C.H.; Rosenfeld, S.I.; Jenkins, D.E., Jr.; Thiem, P.A.; Leddy, J.P. Complement lysis of human erythrocytes. Differeing susceptibility of two types of paroxysmal nocturnal hemoglobinuria cells to C5b-9. J. Clin. Investig. 1979, 64, 428–433. [Google Scholar] [CrossRef]
- Kunchithapautham, K.; Rohrer, B. Sublytic membrane-attack-complex (MAC) activation alters regulated rather than constitutive vascular endothelial growth factor (VEGF) secretion in retinal pigment epithelium monolayers. J. Biol. Chem. 2011, 286, 23717–23724. [Google Scholar] [CrossRef] [PubMed]
- Kilgore, K.S.; Schmid, E.; Shanley, T.P.; Flory, C.M.; Maheswari, V.; Tramontini, N.L.; Cohen, H.; Ward, P.A.; Friedl, H.P.; Warren, J.S. Sublytic concentrations of the membrane attack complex of complement induce endothelial interleukin-8 and monocyte chemoattractant protein-1 through nuclear factor-kappa B activation. Am. J. Pathol. 1997, 150, 2019–2031. [Google Scholar] [PubMed]
- Reiter, Y.; Ciobotariu, A.; Fishelson, Z. Sublytic complement attack protects tumor cells from lytic doses of antibody and complement. Eur. J. Immunol. 1992, 22, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Wang, L.; Zhao, C.; Qian, B.; Yao, C.; He, F.; Zhu, Y.; Cai, M.; Li, M.; Zhao, D.; et al. Sublytic C5b-9 induces proliferation of glomerular mesangial cells via ERK5/MZF1/RGC-32 axis activated by FBXO28-TRAF6 complex. J. Cell. Mol. Med. 2019, 23, 5654–5671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Shan, K.; Wang, L.; Qiu, W.; Lu, Y.; Zhao, D.; Zhu, G.; He, F.; Wang, Y. Sublytic C5b-9 induces IL-6 and TGF-beta1 production by glomerular mesangial cells in rat Thy-1 nephritis through p300-mediated C/EBPbeta acetylation. FASEB J. 2014, 28, 1511–1525. [Google Scholar] [CrossRef] [PubMed]
- Eglite, S.; Pluss, K.; Dahinden, C.A. Requirements for C5a receptor-mediated IL-4 and IL-13 production and leukotriene C4 generation in human basophils. J. Immunol. 2000, 165, 2183–2189. [Google Scholar] [CrossRef] [PubMed]
- Erdei, A.; Kerekes, K.; Pecht, I. Role of C3a and C5a in the activation of mast cells. Exp. Clin. Immunogenet. 1997, 14, 16–18. [Google Scholar]
- Hung, S.L.; Srinivasan, S.; Friedman, H.M.; Eisenberg, R.J.; Cohen, G.H. Structural basis of C3b binding by glycoprotein C of herpes simplex virus. J. Virol. 1992, 66, 4013–4027. [Google Scholar] [CrossRef]
- Isaacs, S.N.; Kotwal, G.J.; Moss, B. Vaccinia virus complement-control protein prevents antibody-dependent complement-enhanced neutralization of infectivity and contributes to virulence. Proc. Natl. Acad. Sci. USA 1992, 89, 628–632. [Google Scholar] [CrossRef]
- Kapadia, S.B.; Molina, H.; van Berkel, V.; Speck, S.H.; Virgin, H.W.T. Murine gammaherpesvirus 68 encodes a functional regulator of complement activation. J. Virol. 1999, 73, 7658–7670. [Google Scholar] [CrossRef]
- Albrecht, J.C.; Fleckenstein, B. New member of the multigene family of complement control proteins in herpesvirus saimiri. J. Virol. 1992, 66, 3937–3940. [Google Scholar] [CrossRef] [PubMed]
- Spiller, O.B.; Blackbourn, D.J.; Mark, L.; Proctor, D.G.; Blom, A.M. Functional activity of the complement regulator encoded by Kaposi’s sarcoma-associated herpesvirus. J. Biol. Chem. 2003, 278, 9283–9289. [Google Scholar] [CrossRef] [PubMed]
- Mullick, J.; Bernet, J.; Singh, A.K.; Lambris, J.D.; Sahu, A. Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) open reading frame 4 protein (kaposica) is a functional homolog of complement control proteins. J. Virol. 2003, 77, 3878–3881. [Google Scholar] [CrossRef] [PubMed]
- Mark, L.; Spiller, O.B.; Villoutreix, B.O.; Blom, A.M. Kaposi’s sarcoma-associated herpes virus complement control protein: KCP-complement inhibition and more. Mol. Immunol. 2007, 44, 11–22. [Google Scholar] [CrossRef]
- Spiller, O.B.; Robinson, M.; O’Donnell, E.; Milligan, S.; Morgan, B.P.; Davison, A.J.; Blackbourn, D.J. Complement regulation by Kaposi’s sarcoma-associated herpesvirus ORF4 protein. J. Virol. 2003, 77, 592–599. [Google Scholar] [CrossRef]
- Mark, L.; Lee, W.H.; Spiller, O.B.; Proctor, D.; Blackbourn, D.J.; Villoutreix, B.O.; Blom, A.M. The Kaposi’s sarcoma-associated herpesvirus complement control protein mimics human molecular mechanisms for inhibition of the complement system. J. Biol. Chem. 2004, 279, 45093–45101. [Google Scholar] [CrossRef]
- Spiller, O.B.; Mark, L.; Blue, C.E.; Proctor, D.G.; Aitken, J.A.; Blom, A.M.; Blackbourn, D.J. Dissecting the regions of virion-associated Kaposi’s sarcoma-associated herpesvirus complement control protein required for complement regulation and cell binding. J. Virol. 2006, 80, 4068–4078. [Google Scholar] [CrossRef]
- Okroj, M.; Spiller, O.B.; Korodi, Z.; Tedeschi, R.; Dillner, J.; Blom, A.M. Antibodies against Kaposi sarcoma-associated herpes virus (KSHV) complement control protein (KCP) in infected individuals. Vaccine 2007, 25, 8102–8109. [Google Scholar] [CrossRef]
- Jeon, H.; Yoo, S.M.; Choi, H.S.; Mun, J.Y.; Kang, H.G.; Lee, J.; Park, J.; Gao, S.J.; Lee, M.S. Extracellular vesicles from KSHV-infected endothelial cells activate the complement system. Oncotarget 2017, 8, 99841–99860. [Google Scholar] [CrossRef]
- Yoo, S.M.; Jeon, H.; Lee, S.; Lee, M.S. Susceptibility of KSHV-Infected PEL Cell Lines to the Human Complement System. J. Microbiol. Biotechnol. 2016, 26, 618–626. [Google Scholar] [CrossRef]
- Ganem, D. KSHV and the pathogenesis of Kaposi sarcoma: Listening to human biology and medicine. J. Clin. Investig. 2010, 120, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Aneja, K.K.; Yuan, Y. Reactivation and Lytic Replication of Kaposi’s Sarcoma-Associated Herpesvirus: An Update. Front. Microbiol. 2017, 8, 613. [Google Scholar] [CrossRef] [PubMed]
- Moutabarrik, A.; Nakanishi, I.; Namiki, M.; Hara, T.; Matsumoto, M.; Ishibashi, M.; Okuyama, A.; Zaid, D.; Seya, T. Cytokine-mediated regulation of the surface expression of complement regulatory proteins, CD46(MCP), CD55(DAF), and CD59 on human vascular endothelial cells. Lymphokine Cytokine Res. 1993, 12, 167–172. [Google Scholar] [PubMed]
- Qiao, P.; Dang, E.L.; Fang, H.; Zhang, J.Y.; Li, B.; Shen, S.X.; Luo, Y.X.; Lei, J.; Shao, S.; Qiao, H.J.; et al. Decreased expression levels of complement regulator CD55 contribute to the development of bullous pemphigoid. Oncotarget 2018, 9, 35517–35527. [Google Scholar] [CrossRef][Green Version]
- Gao, S.J.; Deng, J.H.; Zhou, F.C. Productive lytic replication of a recombinant Kaposi’s sarcoma-associated herpesvirus in efficient primary infection of primary human endothelial cells. J. Virol. 2003, 77, 9738–9749. [Google Scholar] [CrossRef]
- Trouw, L.A.; Blom, A.M.; Gasque, P. Role of complement and complement regulators in the removal of apoptotic cells. Mol. Immunol. 2008, 45, 1199–1207. [Google Scholar] [CrossRef]
- Cortes, C.; Ferreira, V.P.; Pangburn, M.K. Native properdin binds to Chlamydia pneumoniae and promotes complement activation. Infect. Immun. 2011, 79, 724–731. [Google Scholar] [CrossRef]
- Spitzer, D.; Mitchell, L.M.; Atkinson, J.P.; Hourcade, D.E. Properdin can initiate complement activation by binding specific target surfaces and providing a platform for de novo convertase assembly. J. Immunol. 2007, 179, 2600–2608. [Google Scholar] [CrossRef]
- Bongrazio, M.; Pries, A.R.; Zakrzewicz, A. The endothelium as physiological source of properdin: Role of wall shear stress. Mol. Immunol. 2003, 39, 669–675. [Google Scholar] [CrossRef]
- Johnson, E.; Hetland, G. Human umbilical vein endothelial cells synthesize functional C3, C5, C6, C8 and C9 in vitro. Scand. J. Immunol. 1991, 33, 667–671. [Google Scholar] [CrossRef]
- Narkhede, M.; Arora, S.; Ujjani, C. Primary effusion lymphoma: Current perspectives. OncoTargets Ther. 2018, 11, 3747–3754. [Google Scholar] [CrossRef] [PubMed]
- Budzko, D.B.; Lachmann, P.J.; McConnell, I. Activation of the alternative complement pathway by lymphoblastoid cell lines derived from patients with Burkitt’s lymphoma and infectious mononucleosis. Cell Immunol. 1976, 22, 98–109. [Google Scholar] [CrossRef]
- Caudwell, V.; Porteu, F.; Calender, A.; Pangburn, M.K.; Halbwachs-Mecarelli, L. Complement alternative pathway activation and control on membranes of human lymphoid B cell lines. Eur. J. Immunol. 1990, 20, 2643–2650. [Google Scholar] [CrossRef] [PubMed]
- Mack, A.A.; Sugden, B. EBV is necessary for proliferation of dually infected primary effusion lymphoma cells. Cancer Res. 2008, 68, 6963–6968. [Google Scholar] [CrossRef] [PubMed]
- Ricklin, D.; Reis, E.S.; Lambris, J.D. Complement in disease: A defence system turning offensive. Nat. Rev. Nephrol. 2016, 12, 383–401. [Google Scholar] [CrossRef]
- Afshar-Kharghan, V. The role of the complement system in cancer. J. Clin. Investig. 2017, 127, 780–789. [Google Scholar] [CrossRef]
- Tan, L.X.; Toops, K.A.; Lakkaraju, A. Protective responses to sublytic complement in the retinal pigment epithelium. Proc. Natl. Acad. Sci. USA 2016, 113, 8789–8794. [Google Scholar] [CrossRef]
- Triantafilou, K.; Hughes, T.R.; Triantafilou, M.; Morgan, B.P. The complement membrane attack complex triggers intracellular Ca2+ fluxes leading to NLRP3 inflammasome activation. J. Cell. Sci. 2013, 126, 2903–2913. [Google Scholar] [CrossRef]
- Jeon, H.; Han, S.R.; Lee, S.; Park, S.J.; Kim, J.H.; Yoo, S.M.; Lee, M.S. Activation of the complement system in an osteosarcoma cell line promotes angiogenesis through enhanced production of growth factors. Sci. Rep. 2018, 8, 5415. [Google Scholar] [CrossRef]
- Jeon, H.; Lee, J.S.; Yoo, S.; Lee, M.S. Quantification of complement system activation by measuring C5b-9 cell surface deposition using a cell-ELISA technique. J. Immunol. Methods 2014, 415, 57–62. [Google Scholar] [CrossRef]
- Morgan, B.P.; Boyd, C.; Bubeck, D. Molecular cell biology of complement membrane attack. Semin. Cell Dev. Biol. 2017, 72, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Gao, H. New insights for C5a and C5a receptors in sepsis. Front. Immunol. 2012, 3, 368. [Google Scholar] [CrossRef] [PubMed]
- Strey, C.W.; Markiewski, M.; Mastellos, D.; Tudoran, R.; Spruce, L.A.; Greenbaum, L.E.; Lambris, J.D. The proinflammatory mediators C3a and C5a are essential for liver regeneration. J. Exp. Med. 2003, 198, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, R.; Yamaoka, K.; Sawamukai, N.; Shimajiri, S.; Oshita, K.; Yukawa, S.; Tokunaga, M.; Iwata, S.; Saito, K.; Chiba, K.; et al. C5a promotes migration, proliferation, and vessel formation in endothelial cells. Inflamm. Res. 2010, 59, 659–666. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, S.-M.; Lee, M.-S. Kaposi’s Sarcoma-Associated Herpesvirus and Host Interaction by the Complement System. Pathogens 2020, 9, 260. https://doi.org/10.3390/pathogens9040260
Yoo S-M, Lee M-S. Kaposi’s Sarcoma-Associated Herpesvirus and Host Interaction by the Complement System. Pathogens. 2020; 9(4):260. https://doi.org/10.3390/pathogens9040260
Chicago/Turabian StyleYoo, Seung-Min, and Myung-Shin Lee. 2020. "Kaposi’s Sarcoma-Associated Herpesvirus and Host Interaction by the Complement System" Pathogens 9, no. 4: 260. https://doi.org/10.3390/pathogens9040260
APA StyleYoo, S.-M., & Lee, M.-S. (2020). Kaposi’s Sarcoma-Associated Herpesvirus and Host Interaction by the Complement System. Pathogens, 9(4), 260. https://doi.org/10.3390/pathogens9040260




