Response of Human Neutrophil Granulocytes to the Hyphae of the Emerging Fungal Pathogen Curvularia lunata
Abstract
:1. Introduction
2. Results
2.1. Activation of the Neutrophils by the C. lunata Hyphae
2.2. Generation of Hydrogen Peroxide (H2O2)
2.3. Detection of Extracellular Melanin
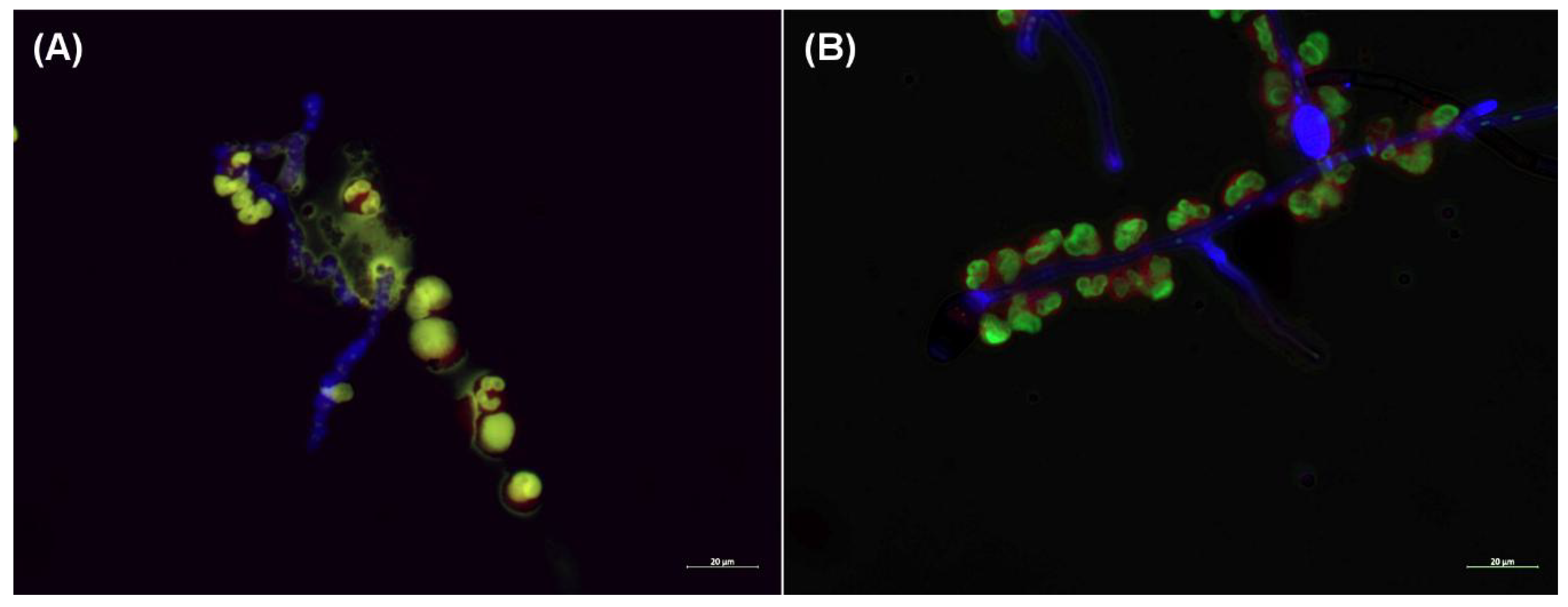
2.4. NET Formation in the Presence of the Hyphae
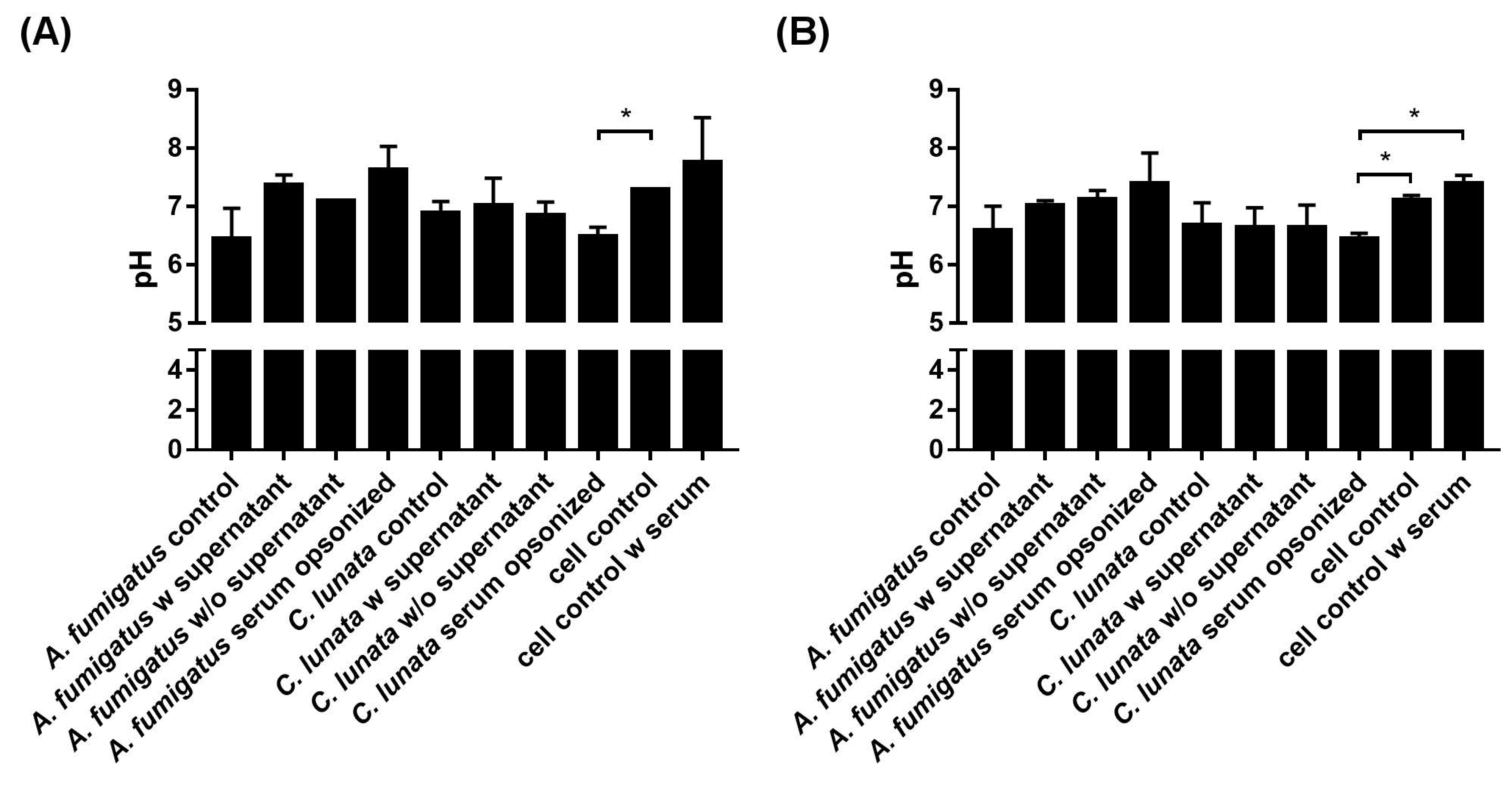
2.5. Extracellular pH of the Neutrophil–Fungus Interaction
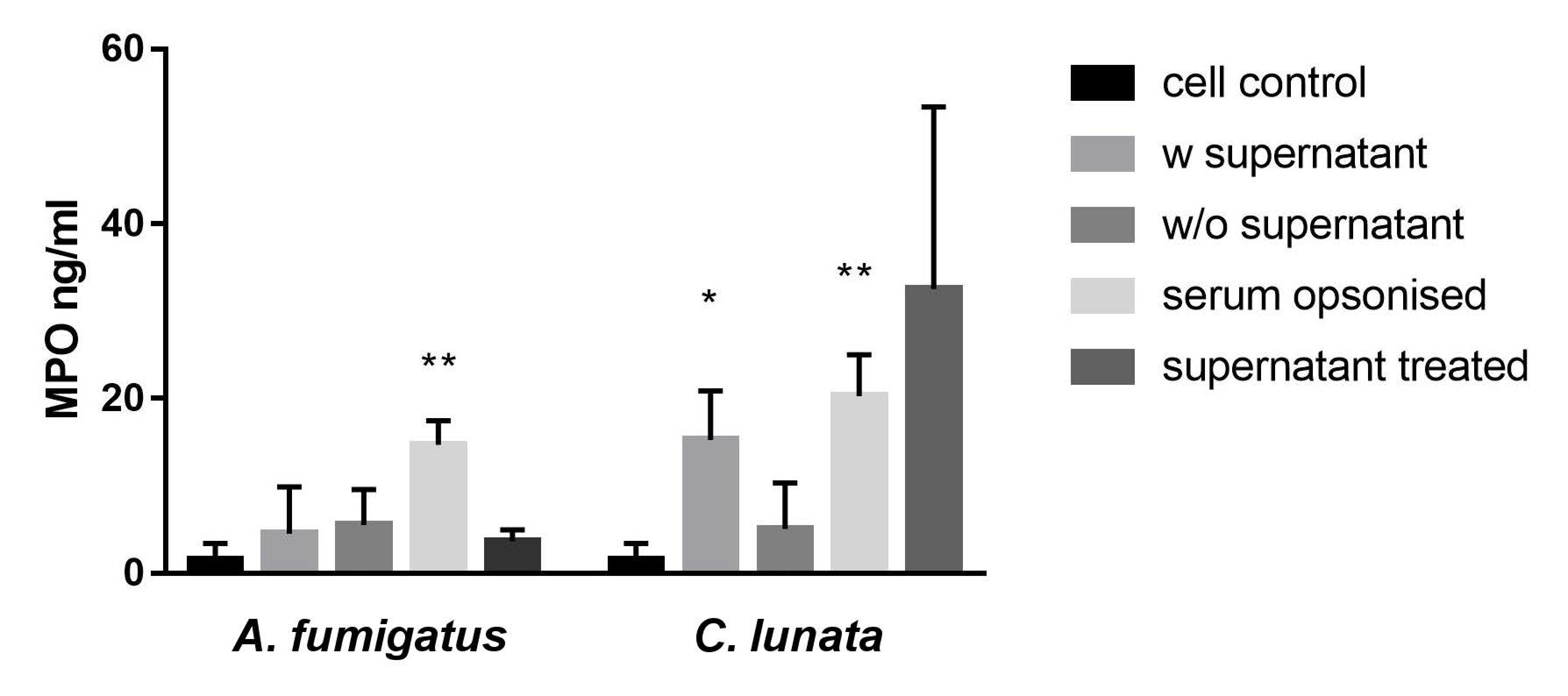
2.6. Killing Efficiency of the Neutrophil Granulocytes
2.7. Viability of the Neutrophils in the Presence of the Fungal Hyphae
3. Discussion
4. Materials and Methods
4.1. Fungal Strains, Culture Conditions, and Inoculum Preparation
4.2. Isolation of Human Neutrophil Granulocytes and Serum
4.3. Interaction of the Neutrophils and the Hyphae
4.4. Detection of Neutrophil Activation
4.5. Measurement of the H2O2 Release of Neutrophils
4.6. Detection of Extracellular Melanin Release
4.7. Visualization of NET Formation
4.8. Measurement of Chromosome Decondensation
4.9. Measurement of Extracellular pH
4.10. Viability Assay for the Hyphae
4.11. Neutrophil Viability Assay
4.12. Statistical Analysis
4.13. Ethics Statement
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Perfect, J.R.; Alexander, B.D.; Schell, W.A. Phaeohyphomycoses (Brown-Black Moulds). In Essentials of Clinical Mycology, 2nd ed.; Kauffman, C.A., Pappas, P.G., Sobel, J.D., Dismukes, W.E., Eds.; Springer: New York, NY, USA, 2011; pp. 305–317. [Google Scholar]
- Paredes, K.; Capilla, J.; Sutton, D.A.; Mayayo, E.; Fothergill, A.W.; Guarro, J. Experimental treatment of Curvularia infection. Diagn. Microbiol. Infect. Dis. 2014, 79, 428–431. [Google Scholar] [CrossRef]
- Krizsan, K.; Toth, E.; Nagy, L.G.; Galgoczy, L.; Manikandan, P.; Chandrasekaran, M.; Kadaikunnan, S.; Alharbi, N.S.; Vágvölgyi, C.; Papp, T. Molecular identification and antifungal susceptibility of Curvularia australiensis, C. hawaiiensis and C. spicifera isolated from human eye infections. Mycoses 2015, 58, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Revankar, S.G.; Baddley, J.W.; Chen, S.C.; Kauffman, C.A.; Slavin, M.; Vazquez, J.A.; Seas, C.; Morris, M.I.; Nguyen, M.H.; Shoham, S.; et al. A mycoses study group international prospective study of phaeohyphomycosis: An analysis of 99 proven/probable cases. Open Forum. Infect. Dis. 2017, 4, ofx200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, I.D.; Makkar, A.; Malik, A.; Khan, S.; Mehdi, I.; Arif, S.; Aden, D.; Somayaji, P.; Roomi, K. Curvularia keratomycosis after cataract surgery. J. Arch. Milit. Med. 2017, 5, e57331. [Google Scholar]
- Rasheeduddin, M.; Visalakshi, P. Cutaneous phaeohyphomycosis of foot web by Curvularia lunata. Glob. J. Med. Clin. Case Rep. 2017, 4, 074–075. [Google Scholar] [CrossRef] [Green Version]
- Vineetha, M.; Palakkal, S.; Sobhanakumari, K.; Celine, M.I.; Letha, V. Verrucous onychomycosis caused by Curvularia in a patient with congenital pterygium. Indian J. Dermatol. 2016, 61, 701. [Google Scholar] [CrossRef] [PubMed]
- Krizsán, K.; Papp, T.; Manikandan, P.; Shobana, S.; Chandrasekaran, M.; Vágvölgyi, C.; Kredics, L. Clinical importance of the genus Curvularia. In Medical Mycology: Current Trends and Future Prospects; Razzaghi-Abyaneh, M., Shams-Ghahfarokhi, M., Rai, M., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 147–204. [Google Scholar]
- Cavanna, C.; Seminari, E.; Pusateri, A.; Mangione, F.; Lallitto, F.; Esposto, M.C.; Pagella, F. Allergic fungal rhinosinusitis due to Curvularia lunata. New Microbiol. 2014, 37, 241–245. [Google Scholar]
- Skovrlj, B.; Haghighi, M.; Smethurst, M.E.; Caridi, J.; Bederson, J.B. Curvularia abscess of the brainstem. World Neurosurg. 2014, 82, e9–e241. [Google Scholar] [CrossRef]
- Revankar, S.G.; Patterson, J.E.; Sutton, D.A.; Pullen, R.; Rinaldi, M.G. Disseminated phaeohyphomycosis: Review of an emerging mycosis. Clin. Infect. Dis. 2002, 34, 467–476. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, H.; Sano, A.; Aragane, N.; Fukuoka, M.; Tanaka, M.; Kawaura, F.; Fukuno, Y.; Matsuishi, E.; Hayashi, S. Disseminated infection by Bipolaris spicifera in an immunocompetent subject. Med. Mycol. 2008, 46, 361–365. [Google Scholar] [CrossRef] [Green Version]
- Balla, A.; Pierson, J.; Hugh, J.; Wojewoda, C.; Gibson, P.; Greene, L. Disseminated cutaneous Curvularia infection in an immunocompromised host; diagnostic challenges and experience with voriconazole. J. Cutan. Pathol. 2016, 43, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Beckett, A.R.; Kahn, S.A.; Seay, R.; Lintner, A.C. Invasive Curvularia infections in burn patients: A case series. Surg. Infect. Case Rep. 2017, 2, 76–79. [Google Scholar] [CrossRef] [Green Version]
- Shrivastava, A.; Tadepalli, K.; Goel, G.; Gupta, K.; Gupta, P.K. Melanized fungus as an epidural abscess: A diagnostic and therapeutic challenge. Med. Mycol. Case Rep. 2017, 16, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Tóth, E.J.; Boros, E.; Hoffmann, A.; Szebenyi, C.; Homa, M.; Nagy, G.; Vágvölgyi, C.; Nagy, I.; Papp, T. Interaction of THP-1 monocytes with conidia and hyphae of different Curvularia strains. Front. Immunol. 2017, 8, 1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gazendam, R.P.; van Hamme, J.L.; Tool, A.T.; Hoogenboezem, M.; van den Berg, J.M.; Prins, J.M.; Vitkov, L.; van de Veerdonk, F.L.; van den Berg, T.K.; Roos, D.; et al. Human neutrophils use different mechanisms to kill Aspergillus fumigatus conidia and hyphae: Evidence from Phagocyte Defects. J. Immunol. 2016, 196, 1272–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brakhage, A.A.; Bruns, S.; Thywissen, A.; Zipfel, P.F.; Behnsen, J. Interaction of phagocytes with filamentous fungi. Curr. Opin. Microbiol. 2010, 13, 409–415. [Google Scholar] [CrossRef]
- LeibundGut-Landmann, S.; Wuthrich, M.; Hohl, T.M. Immunity to fungi. Curr. Opin. Immunol. 2012, 24, 449–458. [Google Scholar] [CrossRef] [Green Version]
- Babior, B.M.; Lambeth, J.D.; Nauseef, W. The neutrophil NADPH oxidase. Arch. Biochem. Biophys. 2002, 397, 342–344. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Braem, S.G.; Rooijakkers, S.H.; van Kessel, K.P.; de Cock, H.; Wosten, H.A.; van Strijp, J.A.; Haas, P.J.A. Effective neutrophil phagocytosis of Aspergillus fumigatus is mediated by classical pathway complement activation. J. Innate Immun. 2015, 7, 364–374. [Google Scholar] [CrossRef]
- Feldmesser, M. Role of neutrophils in invasive aspergillosis. Infect. Immun. 2006, 74, 6514–6516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Ami, R.; Lewis, R.E.; Raad, I.I.; Kontoyiannis, D.P. Phaeohyphomycosis in a tertiary care cancer center. Clin. Infect. Dis. 2009, 48, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Morton, C.O.; Bouzani, M.; Loeffler, J.; Rogers, T.R. Direct interaction studies between Aspergillus fumigatus and human immune cells; what have we learned about pathogenicity and host immunity? Front. Microbiol. 2012, 3, 413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, S.D.; Malachowa, N.; DeLeo, F.R. Influence of microbes on neutrophil life and death. Front. Cell Infect. Microbiol. 2017, 7, 159. [Google Scholar] [CrossRef] [Green Version]
- Dagenais, T.R.; Keller, N.P. Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clin. Microbiol. Rev. 2009, 22, 447–465. [Google Scholar] [CrossRef] [Green Version]
- Bianchi, M.; Hakkim, A.; Brinkmann, V.; Siler, U.; Seger, R.A.; Zychlinsky, A.; Reichenbach, J. Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood 2009, 114, 2619–2622. [Google Scholar] [CrossRef] [Green Version]
- Aratani, Y. Role of myeloperoxidase in the host defense against fungal infection. Nihon Ishinkin Gakkai Zasshi 2006, 47, 195–199. [Google Scholar] [CrossRef] [Green Version]
- Klebanoff, S.J.; Kettle, A.J.; Rosen, H.; Winterbourn, C.C.; Nauseef, W.M. Myeloperoxidase: A front-line defender against phagocytosed microorganisms. J. Leukoc. Biol. 2013, 93, 185–198. [Google Scholar] [CrossRef] [Green Version]
- Revankar, S.G.; Sutton, D.A. Melanized fungi in human disease. Clin. Microbiol. Rev. 2010, 23, 884–928. [Google Scholar] [CrossRef] [Green Version]
- de Cassia, R.G.R.; Pombeiro-Sponchiado, S.R. Antioxidant activity of the melanin pigment extracted from Aspergillus nidulans. Biol. Pharm. Bull. 2005, 28, 1129–1131. [Google Scholar]
- Warnatsch, A.; Tsourouktsoglou, T.D.; Branzk, N.; Wang, Q.; Reincke, S.; Herbst, S.; Gutierrez, M.; Papayannopoulos, V. Reactive oxygen species localization programs inflammation to clear microbes of different size. Immunity 2017, 46, 421–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, D.; Mollnau, H.; Eiserich, J.P.; Freeman, B.A.; Daiber, A.; Gehling, U.M.; Brümmer, J.; Rudolph, V.; Münzel, T.; Heitzer, T.; et al. Myeloperoxidase mediates neutrophil activation by association with CD11b/CD18 integrins. Proc. Natl. Acad. Sci. USA 2005, 102, 431–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, M.W.; Patarroyo, M.; Oberg, F.; Siegbahn, A.; Nilsson, K. Myeloperoxidase mediates cell adhesion via the alpha M beta 2 integrin (Mac-1; CD11b/CD18). J. Cell Sci. 1997, 110, 1133–1139. [Google Scholar] [PubMed]
- Lefkowitz, D.L.; Roberts, E.; Grattendick, K.; Schwab, C.; Stuart, R.; Lincoln, J.; Allen, R.C.; Moguilevsky, N.; Bollen, A.; Lefkowitz, S.S. The endothelium and cytokine secretion: The role of peroxidases as immunoregulators. Cell Immunol. 2000, 202, 23–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Nishinaka, Y.; Arai, T.; Adachi, S.; Takaori-Kondo, A.; Yamashita, K. Singlet oxygen is essential for neutrophil extracellular trap formation. Biochem. Biophys. Res. Commun. 2011, 413, 75–79. [Google Scholar] [CrossRef] [Green Version]
- Palmer, L.J.; Cooper, P.R.; Ling, M.R.; Wright, H.J.; Huissoon, A.; Chapple, I.L. Hypochlorous acid regulates neutrophil extracellular trap release in humans. Clin. Exp. Immunol. 2012, 167, 261–268. [Google Scholar] [CrossRef]
- Tripathi, J.K.; Sharma, A.; Sukumaran, P.; Sun, Y.; Mishra, B.B.; Singh, B.B.; Sharma, J. Oxidant sensor cation channel TRPM2 regulates neutrophil extracellular trap formation and protects against pneumoseptic bacterial infection. FASEB J. 2018, 32, 6848–6859. [Google Scholar] [CrossRef]
- Behnen, M.; Moller, S.; Brozek, A.; Klinger, M.; Laskay, T. Extracellular acidification inhibits the ROS-dependent formation of neutrophil extracellular traps. Front. Immunol. 2017, 8, 184. [Google Scholar] [CrossRef] [Green Version]
- Clark, R.A.; Nauseef, W.M. Isolation and functional analysis of neutrophils. Curr. Protoc. Immunol. 2001, 7. [Google Scholar] [CrossRef]
- Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef]
- Conn, H.J. Biological Stains, 8th ed.; Williams and Wilkins: Baltimore, MD, USA, 1969; p. 498. [Google Scholar]
- Clark, G. Staining Procedures, 3rd ed.; Williams and Wilkins: Baltimore, MD, USA, 1973; p. 418. [Google Scholar]
- Suzuki, K.; Ota, H.; Sasagawa, S.; Sakatani, T.; Fujikura, T. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal. Biochem. 1983, 132, 345–352. [Google Scholar] [CrossRef]
- Goncalves, R.C.; Lisboa, H.C.; Pombeiro-Sponchiado, S.R. Characterization of melanin pigment produced by Aspergillus nidulans. World J. Microbiol. Biotechnol. 2012, 28, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.S.; Bardoel, B.W.; Harbort, C.J.; Zychlinsky, A. Induction and quantification of neutrophil extracellular traps. Methods Mol. Biol. 2014, 1124, 307–318. [Google Scholar] [PubMed]
- Levitz, S.M.; Diamond, R.D. A rapid colorimetric assay of fungal viability with the tetrazolium salt MTT. J. Infect. Dis. 1985, 152, 938–945. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tóth, E.J.; Varga, M.; Takó, M.; Homa, M.; Jáger, O.; Hermesz, E.; Orvos, H.; Nagy, G.; Vágvölgyi, C.; Papp, T. Response of Human Neutrophil Granulocytes to the Hyphae of the Emerging Fungal Pathogen Curvularia lunata. Pathogens 2020, 9, 235. https://doi.org/10.3390/pathogens9030235
Tóth EJ, Varga M, Takó M, Homa M, Jáger O, Hermesz E, Orvos H, Nagy G, Vágvölgyi C, Papp T. Response of Human Neutrophil Granulocytes to the Hyphae of the Emerging Fungal Pathogen Curvularia lunata. Pathogens. 2020; 9(3):235. https://doi.org/10.3390/pathogens9030235
Chicago/Turabian StyleTóth, Eszter Judit, Mónika Varga, Miklós Takó, Mónika Homa, Olivér Jáger, Edit Hermesz, Hajnalka Orvos, Gábor Nagy, Csaba Vágvölgyi, and Tamás Papp. 2020. "Response of Human Neutrophil Granulocytes to the Hyphae of the Emerging Fungal Pathogen Curvularia lunata" Pathogens 9, no. 3: 235. https://doi.org/10.3390/pathogens9030235
APA StyleTóth, E. J., Varga, M., Takó, M., Homa, M., Jáger, O., Hermesz, E., Orvos, H., Nagy, G., Vágvölgyi, C., & Papp, T. (2020). Response of Human Neutrophil Granulocytes to the Hyphae of the Emerging Fungal Pathogen Curvularia lunata. Pathogens, 9(3), 235. https://doi.org/10.3390/pathogens9030235






