JP2 Genotype of Aggregatibacter actinomycetemcomitans in Caucasian Patients: A Presentation of Two Cases
Abstract
1. Introduction
2. Results
2.1. Case 1
2.2. Case 2
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tonetti, M. Etiology and pathogenesis. In Proceedings of the 1st European Workshop on Periodontology, Thurgau, Switzerland, 1–4 February 1993; Lang, N.P., Karring, T., Eds.; Quintessenz Verlags-GmbH: Berlin, Germany, 1993; pp. 54–89. [Google Scholar]
- Faveri, M.; Figueiredo, L.C.; Duarte, P.M.; Mestnik, M.J.; Mayer, M.P.; Feres, M. Microbiological profile of untreated subjects with localized aggressive periodontitis. J. Clin. Periodontol. 2009, 36, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Casagni, F.; Madianos, P.N. Can presence or absence of periodontal pathogens distinguish between subjects with chronic and aggressive periodontitis? A systematic review. J. Clin. Periodontol. 2002, 29 (Suppl. 3), 10–21. [Google Scholar] [CrossRef]
- Eick, S.; Nydegger, J.; Burgin, W.; Salvi, G.E.; Sculean, A.; Ramseier, C. Microbiological analysis and the outcomes of periodontal treatment with or without adjunctive systemic antibiotics-a retrospective study. Clin. Oral Investig. 2018, 22, 3031–3041. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, P.; Pormohammad, A.; Eslami, H.; Shokouhi, B.; Fakhrzadeh, V.; Kafil, H.S. Oral pathogenesis of Aggregatibacter actinomycetemcomitans. Microb. Pathog. 2017, 113, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A. Aggregatibacter actinomycetemcomitans leukotoxin: A powerful tool with capacity to cause imbalance in the host inflammatory response. Toxins 2011, 3, 242–259. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Shenker, B.J.; DiRienzo, J.M.; Malamud, D.; Taichman, N.S. Extraction and isolation of a leukotoxin from Actinobacillus actinomycetemcomitans with polymyxin B. Infect. Immun. 1984, 43, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, K.; Theilade, E.; Lally, E.T.; Demuth, D.R.; Kilian, M. Population structure of Actinobacillus actinomycetemcomitans: A framework for studies of disease-associated properties. Microbiology 1994, 140 Pt 8, 2049–2060. [Google Scholar] [CrossRef][Green Version]
- Haubek, D.; Poulsen, K.; Westergaard, J.; Dahlen, G.; Kilian, M. Highly toxic clone of Actinobacillus actinomycetemcomitans in geographically widespread cases of juvenile periodontitis in adolescents of African origin. J. Clin. Microbiol. 1996, 34, 1576–1578. [Google Scholar] [CrossRef]
- Haubek, D.; Dirienzo, J.M.; Tinoco, E.M.; Westergaard, J.; Lopez, N.J.; Chung, C.P.; Poulsen, K.; Kilian, M. Racial tropism of a highly toxic clone of Actinobacillus actinomycetemcomitans associated with juvenile periodontitis. J. Clin. Microbiol. 1997, 35, 3037–3042. [Google Scholar] [CrossRef]
- Haubek, D.; Ennibi, O.K.; Poulsen, K.; Poulsen, S.; Benzarti, N.; Kilian, M. Early-onset periodontitis in Morocco is associated with the highly leukotoxic clone of Actinobacillus actinomycetemcomitans. J. Dent. Res. 2001, 80, 1580–1583. [Google Scholar] [CrossRef]
- Haraszthy, V.I.; Hariharan, G.; Tinoco, E.M.; Cortelli, J.R.; Lally, E.T.; Davis, E.; Zambon, J.J. Evidence for the role of highly leukotoxic Actinobacillus actinomycetemcomitans in the pathogenesis of localized juvenile and other forms of early-onset periodontitis. J. Periodontol. 2000, 71, 912–922. [Google Scholar] [CrossRef]
- Brogan, J.M.; Lally, E.T.; Poulsen, K.; Kilian, M.; Demuth, D.R. Regulation of Actinobacillus actinomycetemcomitans leukotoxin expression: Analysis of the promoter regions of leukotoxic and minimally leukotoxic strains. Infect. Immun. 1994, 62, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Hritz, M.; Fisher, E.; Demuth, D.R. Differential regulation of the leukotoxin operon in highly leukotoxic and minimally leukotoxic strains of Actinobacillus actinomycetemcomitans. Infect. Immun. 1996, 64, 2724–2729. [Google Scholar] [CrossRef] [PubMed]
- Haubek, D. The highly leukotoxic JP2 clone of Aggregatibacter actinomycetemcomitans: Evolutionary aspects, epidemiology and etiological role in aggressive periodontitis. APMIS Suppl. 2010, 130, 1–53. [Google Scholar] [CrossRef]
- Takada, K.; Saito, M.; Tsuzukibashi, O.; Kawashima, Y.; Ishida, S.; Hirasawa, M. Characterization of a new serotype g isolate of Aggregatibacter actinomycetemcomitans. Mol. Oral Microbiol. 2010, 25, 200–206. [Google Scholar] [CrossRef]
- Brigido, J.A.; da Silveira, V.R.; Rego, R.O.; Nogueira, N.A. Serotypes of Aggregatibacter actinomycetemcomitans in relation to periodontal status and geographic origin of individuals-a review of the literature. Med. Oral Patol. Oral Cir. Bucal 2014, 19, e184. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, T.; Chen, W. Occurrence of Aggregatibacter actinomycetemcomitans serotypes in subgingival plaque from United States subjects. Mol. Oral Microbiol. 2010, 25, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.E.; Mendes, E.N.; Roque de Carvalho, M.A.; Nicoli, J.R.; Farias Lde, M.; Magalhaes, P.P. Actinobacillus actinomycetemcomitans serotype-specific genotypes and periodontal status in Brazilian subjects. Can. J. Microbiol. 2006, 52, 182–188. [Google Scholar] [CrossRef]
- Cortelli, J.R.; Aquino, D.R.; Cortelli, S.C.; Roman-Torres, C.V.; Franco, G.C.; Gomez, R.S.; Batista, L.H.; Costa, F.O. Aggregatibacter actinomycetemcomitans serotypes infections and periodontal conditions: A two-way assessment. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1311–1318. [Google Scholar] [CrossRef]
- Thiha, K.; Takeuchi, Y.; Umeda, M.; Huang, Y.; Ohnishi, M.; Ishikawa, I. Identification of periodontopathic bacteria in gingival tissue of Japanese periodontitis patients. Oral Microbiol. Immunol. 2007, 22, 201–207. [Google Scholar] [CrossRef]
- Haubek, D.; Johansson, A. Pathogenicity of the highly leukotoxic JP2 clone of Aggregatibacter actinomycetemcomitans and its geographic dissemination and role in aggressive periodontitis. J. Oral Microbiol. 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.S.; Frank, P.; Eickholz, P.; Eick, S.; Kim, C.K. Serotypes of Aggregatibacter actinomycetemcomitans in patients with different ethnic backgrounds. J. Periodontol. 2009, 80, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, H.; Cachovan, G.; Guentsch, A.; Eickholz, P.; Pfister, W.; Eick, S. Characterization of Aggregatibacter actinomycetemcomitans strains in periodontitis patients in Germany. Clin. Oral Investig. 2012, 16, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Claesson, R.; Hoglund-Aberg, C.; Haubek, D.; Johansson, A. Age-related prevalence and characteristics of Aggregatibacter actinomycetemcomitans in periodontitis patients living in Sweden. J. Oral Microbiol. 2017, 9, 1334504. [Google Scholar] [CrossRef]
- Armitage, G.C. Development of a classification system for periodontal diseases and conditions. Ann. Periodontol. 1999, 4, 1–6. [Google Scholar] [CrossRef]
- Eke, P.I.; Page, R.C.; Wei, L.; Thornton-Evans, G.; Genco, R.J. Update of the case definitions for population-based surveillance of periodontitis. J. Periodontol. 2012, 83, 1449–1454. [Google Scholar] [CrossRef]
- Page, R.C.; Eke, P.I. Case definitions for use in population-based surveillance of periodontitis. J. Periodontol. 2007, 78, 1387–1399. [Google Scholar] [CrossRef]
- O’Leary, T.J.; Drake, R.B.; Naylor, J.E. The plaque control record. J. Periodontol. 1972, 43, 38. [Google Scholar] [CrossRef]
- Diamanti-Kipioti, A.; Papapanou, P.N.; Moraitaki-Tsami, A.; Lindhe, J.; Mitsis, F. Comparative estimation of periodontal conditions by means of different index systems. J. Clin. Periodontol. 1993, 20, 656–661. [Google Scholar] [CrossRef]
- Claesson, R.; Lagervall, M.; Hoglund-Aberg, C.; Johansson, A.; Haubek, D. Detection of the highly leucotoxic JP2 clone of Aggregatibacter actinomycetemcomitans in members of a Caucasian family living in Sweden. J. Clin. Periodontol. 2011, 38, 115–121. [Google Scholar] [CrossRef]
- Albandar, J.M.; Buischi, Y.A.; Barbosa, M.F. Destructive forms of periodontal disease in adolescents. A 3-year longitudinal study. J. Periodontol. 1991, 62, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Haubek, D.; Ennibi, O.K.; Poulsen, K.; Vaeth, M.; Poulsen, S.; Kilian, M. Risk of aggressive periodontitis in adolescent carriers of the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans in Morocco: A prospective longitudinal cohort study. Lancet 2008, 371, 237–242. [Google Scholar] [CrossRef]
- Johansson, A.; Hanstrom, L.; Kalfas, S. Inhibition of Actinobacillus actinomycetemcomitans leukotoxicity by bacteria from the subgingival flora. Oral Microbiol. Immunol. 2000, 15, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Nguyen, K.A.; Potempa, J. Dichotomy of gingipains action as virulence factors: From cleaving substrates with the precision of a surgeon’s knife to a meat chopper-like brutal degradation of proteins. Periodontol 2000 2010, 54, 15–44. [Google Scholar] [CrossRef]
- Kato, S.; Kowashi, Y.; Demuth, D.R. Outer membrane-like vesicles secreted by Actinobacillus actinomycetemcomitans are enriched in leukotoxin. Microb. Pathog. 2002, 32, 1–13. [Google Scholar] [CrossRef]
- Baer, P.N. The case for periodontosis as a clinical entity. J. Periodontol. 1971, 42, 516–520. [Google Scholar] [CrossRef]
- Jensen, A.B.; Lund, M.; Norskov-Lauritsen, N.; Johansson, A.; Claesson, R.; Reinholdt, J.; Haubek, D. Differential cell lysis among periodontal strains of JP2 and Non-JP2 genotype of aggregatibacter actinomycetemcomitans serotype B is not reflected in dissimilar expression and production of leukotoxin. Pathogens 2019, 8, 211. [Google Scholar] [CrossRef]
- Pavicic, M.J.; van Winkelhoff, A.J.; Pavicic-Temming, Y.A.; de Graaff, J. Amoxycillin causes an enhanced uptake of metronidazole in Actinobacillus actinomycetemcomitans: A mechanism of synergy. J. Antimicrob. Chemother. 1994, 34, 1047–1050. [Google Scholar] [CrossRef]
- van Winkelhoff, A.J.; Rodenburg, J.P.; Goene, R.J.; Abbas, F.; Winkel, E.G.; de Graaff, J. Metronidazole plus amoxycillin in the treatment of Actinobacillus actinomycetemcomitans associated periodontitis. J. Clin. Periodontol. 1989, 16, 128–131. [Google Scholar] [CrossRef]
- Mombelli, A.; Cionca, N.; Almaghlouth, A.; Decaillet, F.; Courvoisier, D.S.; Giannopoulou, C. Are there specific benefits of amoxicillin plus metronidazole in Aggregatibacter actinomycetemcomitans-associated periodontitis? Double-masked, randomized clinical trial of efficacy and safety. J. Periodontol. 2013, 84, 715–724. [Google Scholar] [CrossRef]
- Cionca, N.; Giannopoulou, C.; Ugolotti, G.; Mombelli, A. Microbiologic testing and outcomes of full-mouth scaling and root planing with or without amoxicillin/metronidazole in chronic periodontitis. J. Periodontol. 2010, 81, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Cortelli, S.C.; Costa, F.O.; Kawai, T.; Aquino, D.R.; Franco, G.C.; Ohara, K.; Roman-Torres, C.V.; Cortelli, J.R. Diminished treatment response of periodontally diseased patients infected with the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans. J. Clin. Microbiol. 2009, 47, 2018–2025. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Kachlany, S.C.; Fine, D.H.; Figurski, D.H. Purification of secreted leukotoxin (LtxA) from Actinobacillus actinomycetemcomitans. Protein. Expr. Purif. 2002, 25, 465–471. [Google Scholar] [CrossRef]
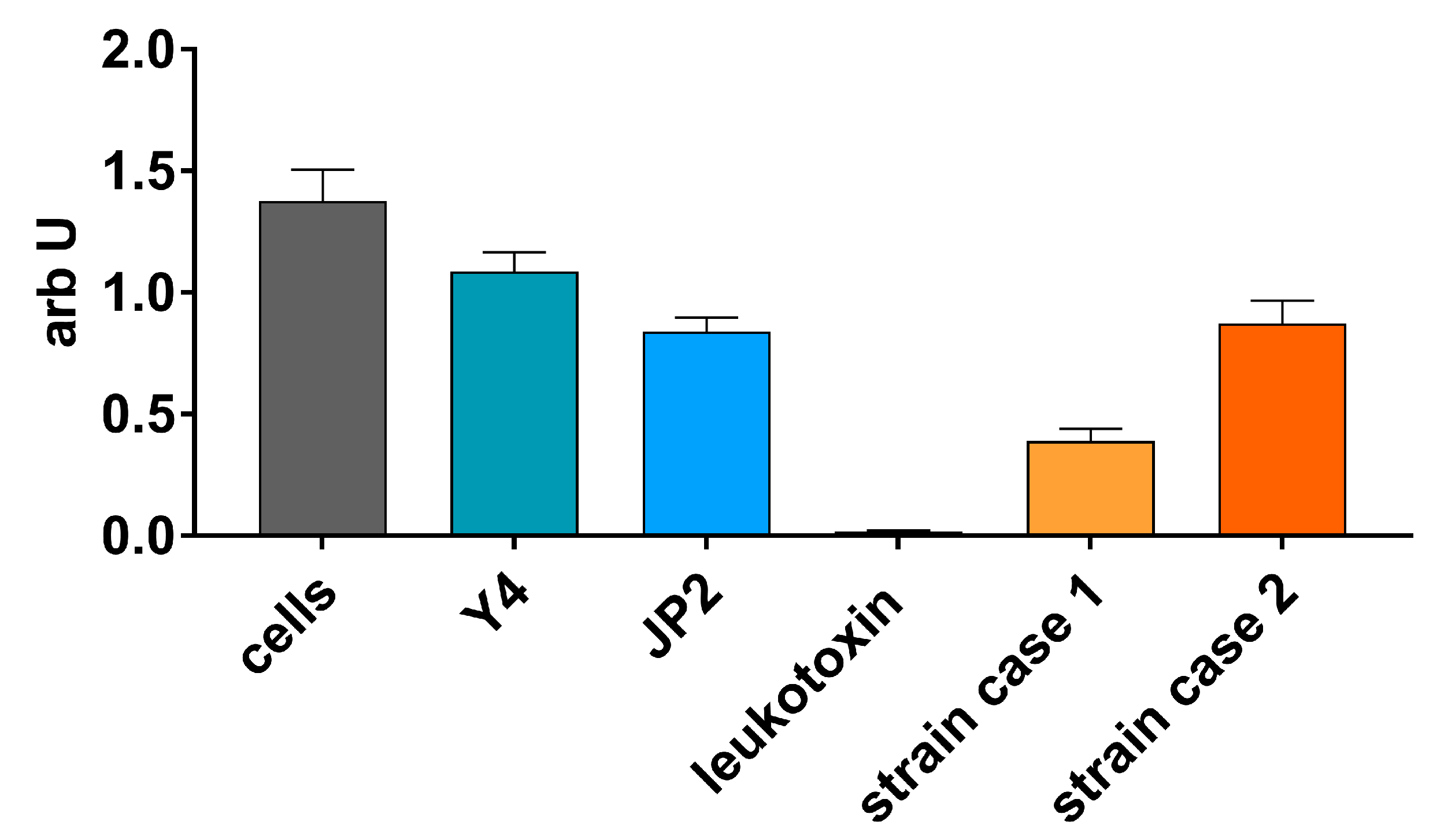
| Patient 1 | Patient 2 | |
|---|---|---|
| Age (years) | 50 | 55 |
| Gender | f | f |
| Mean probing depth in mm | 2.5 | 4.36 |
| Number of sites ≥ 5 mm PD | 8 | 43 |
| Mean attachment loss in mm | 2 | 5 |
| Bleeding on probing in % | 47 | 31 |
| Plaque index in % | 38 | 75 |
| Number of teeth | 26 | 29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stähli, A.; Sculean, A.; Eick, S. JP2 Genotype of Aggregatibacter actinomycetemcomitans in Caucasian Patients: A Presentation of Two Cases. Pathogens 2020, 9, 178. https://doi.org/10.3390/pathogens9030178
Stähli A, Sculean A, Eick S. JP2 Genotype of Aggregatibacter actinomycetemcomitans in Caucasian Patients: A Presentation of Two Cases. Pathogens. 2020; 9(3):178. https://doi.org/10.3390/pathogens9030178
Chicago/Turabian StyleStähli, Alexandra, Anton Sculean, and Sigrun Eick. 2020. "JP2 Genotype of Aggregatibacter actinomycetemcomitans in Caucasian Patients: A Presentation of Two Cases" Pathogens 9, no. 3: 178. https://doi.org/10.3390/pathogens9030178
APA StyleStähli, A., Sculean, A., & Eick, S. (2020). JP2 Genotype of Aggregatibacter actinomycetemcomitans in Caucasian Patients: A Presentation of Two Cases. Pathogens, 9(3), 178. https://doi.org/10.3390/pathogens9030178






