The Carrier Conundrum; A Review of Recent Advances and Persistent Gaps Regarding the Carrier State of Foot-and-Mouth Disease Virus
Abstract
1. Introduction
2. Overview of FMDV Pathogenesis
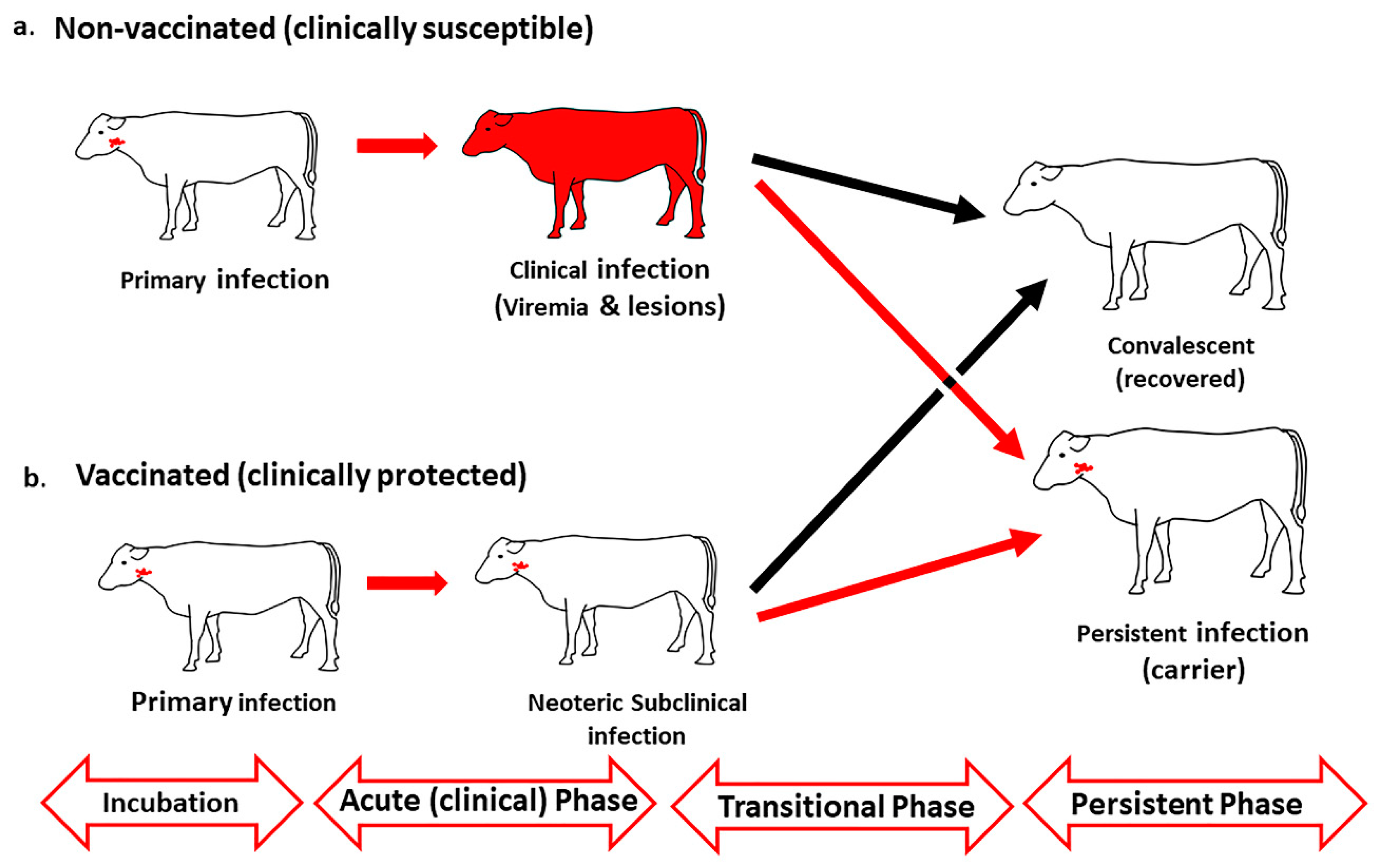
2.1. FMDV Infection and Definitions of Disease Stages
2.2. Temporo-Anatomical Progression of FMDV Infection
3. The FMDV Carrier State
3.1. Early Studies and Methods for Identifying FMDV Carriers
3.2. Anatomic Localization of Persistent FMDV in Cattle
3.3. Anatomic Localization of Persistent FMDV in Asian and African Buffalo
3.4. FMDV Persistence in Small Ruminants
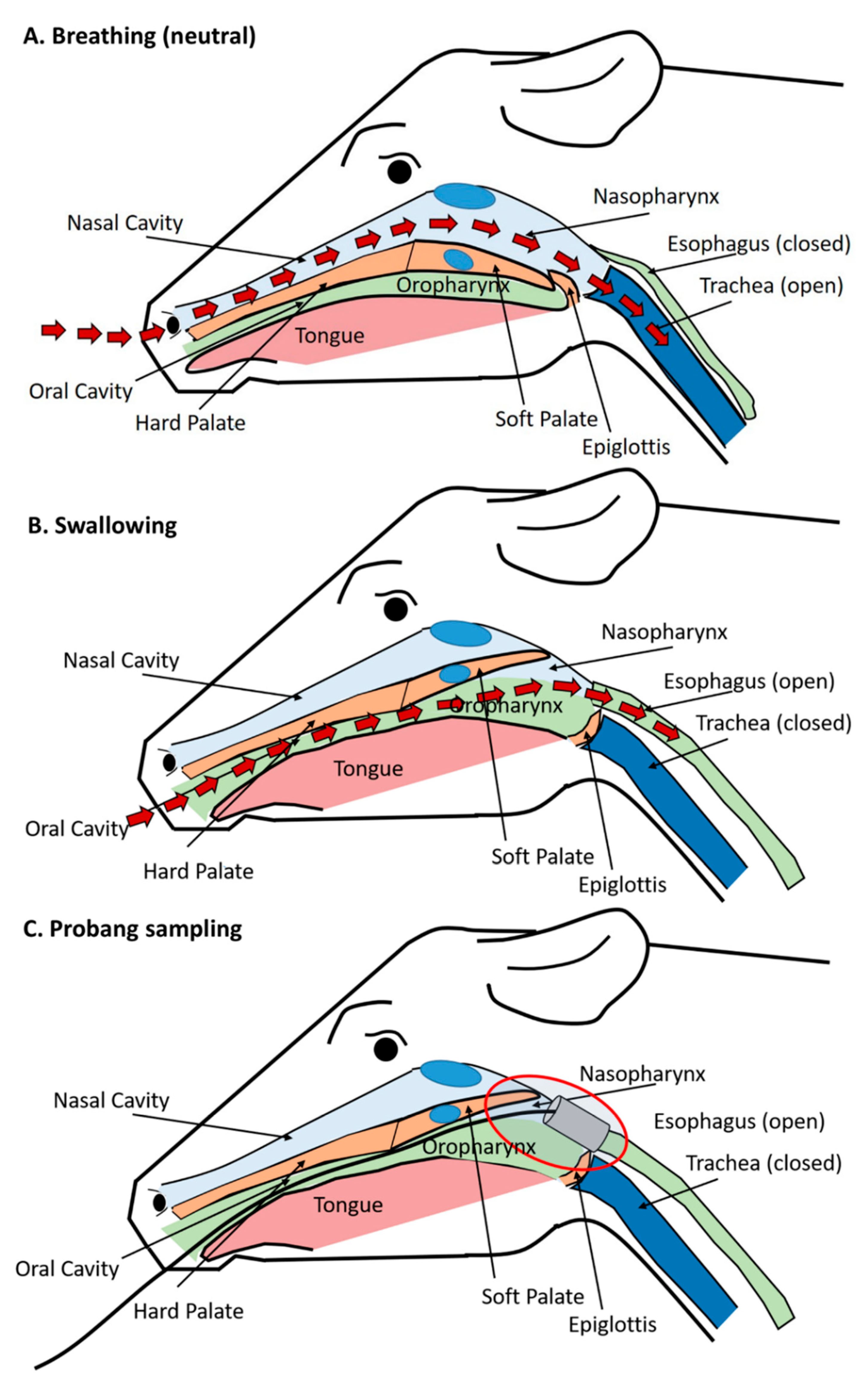
3.5. Anatomic and Physiologic Considerations of Detection of FMDV in Probang Samples
4. Host Responses as Related to the Carrier Phase
4.1. Acute Phase Proteins and Early Anti-Viral Response
4.2. Humoral Response
4.3. Duration of Immunity
4.4. Cellular Response
4.5. Transcriptomics
4.5.1. In Vivo
4.5.2. In Vitro
5. FMDV Genomics during Persistent Infection
6. Epidemiological Aspects of the FMDV Carrier State
6.1. Duration of the FMDV Carrier State
6.2. Transmission from FMDV Carriers
6.3. Epidemiological Concerns of Neoteric Versus Persistent Infection
6.4. FMDV Persistence in Wildlife
7. FMD Vaccines and FMDV Persistence
7.1. FMD Outbreak Control by Vaccination
7.2. The Discrepancy between Clinical Protection and Protection against FMDV Persistence
7.3. Current OIE-Guidelines for Vaccine Trials Do Not Consider Carrier Prevention
8. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grubman, M.J.; Baxt, B. Foot-and-mouth disease. Clin. Microbiol. Rev. 2004, 17, 465–493. [Google Scholar] [CrossRef] [PubMed]
- Knight-Jones, T.J.; Rushton, J. The economic impacts of foot and mouth disease-what are they, how big are they and where do they occur? Prev. Vet. Med. 2013, 112, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. The Progressive Control Pathway for Foot and Mouth Disease Control (PCP-FMD) Principles, Stage Descriptions and Standards; FAO: Rome, Italy, 2018. [Google Scholar]
- Knight-Jones, T.J.; McLaws, M.; Rushton, J. Foot-and-Mouth Disease Impact on Smallholders-What Do We Know, What Don’t We Know and How Can We Find Out More? Transbound Emerg. Dis. 2016, 12507. [Google Scholar] [CrossRef] [PubMed]
- Knowles, N.J.; Samuel, A.R. Molecular epidemiology of foot-and-mouth disease virus. Virus Res. 2003, 91, 65–80. [Google Scholar] [CrossRef]
- Brito, B.P.; Rodriguez, L.L.; Hammond, J.M.; Pinto, J.; Perez, A.M. Review of the global distribution of foot-and-mouth disease virus from 2007 to 2014. Transbound Emerg. Dis. 2017, 64, 316–332. [Google Scholar] [CrossRef]
- Arzt, J.; Baxt, B.; Grubman, M.J.; Jackson, T.; Juleff, N.; Rhyan, J.; Rieder, E.; Waters, R.; Rodriguez, L.L. The pathogenesis of foot-and-mouth disease II: Viral pathways in swine, small ruminants, and wildlife; myotropism, chronic syndromes, and molecular virus-host interactions. Transbound Emerg. Dis. 2011, 58, 305–326. [Google Scholar] [CrossRef]
- Arzt, J.; Juleff, N.; Zhang, Z.; Rodriguez, L.L. The pathogenesis of foot-and-mouth disease I: Viral pathways in cattle. Transbound Emerg. Dis. 2011, 58, 291–304. [Google Scholar] [CrossRef]
- Sellers, R.F. Quantitative aspects of the spread of foot and mouth disease. Vet. Bull. 1971, 41, 431–439. [Google Scholar]
- Yang, P.C.; Chu, R.M.; Chung, W.B.; Sung, H.T. Epidemiological characteristics and financial costs of the 1997 foot-and-mouth disease epidemic in Taiwan. Vet. Rec. 1999, 145, 731–734. [Google Scholar] [CrossRef]
- Dunn, C.S.; Donaldson, A.I. Natural adaption to pigs of a Taiwanese isolate of foot-and-mouth disease virus. Vet. Rec. 1997, 141, 174–175. [Google Scholar] [CrossRef]
- Stenfeldt, C.; Arzt, J.; Pacheco, J.M.; Gladue, D.P.; Smoliga, G.R.; Silva, E.B.; Rodriguez, L.L.; Borca, M.V. A partial deletion within foot-and-mouth disease virus non-structural protein 3A causes clinical attenuation in cattle but does not prevent subclinical infection. Virology 2018, 516, 115–126. [Google Scholar] [CrossRef]
- Pacheco, J.M.; Gladue, D.P.; Holinka, L.G.; Arzt, J.; Bishop, E.; Smoliga, G.; Pauszek, S.J.; Bracht, A.J.; O’Donnell, V.; Fernandez-Sainz, I.; et al. A partial deletion in non-structural protein 3A can attenuate foot-and-mouth disease virus in cattle. Virology 2013, 446, 260–267. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gloster, J.; Blackall, R.M.; Sellers, R.F.; Donaldson, A.I. Forecasting the airborne spread of foot-and-mouth disease. Vet. Rec. 1981, 108, 370–374. [Google Scholar] [CrossRef]
- Arzt, J.; Branan, M.A.; Delgado, A.H.; Yadav, S.; Moreno-Torres, K.I.; Tildesley, M.J.; Stenfeldt, C. Quantitative impacts of incubation phase transmission of foot-and-mouth disease virus. Sci. Rep. 2019, 9, 2707. [Google Scholar] [CrossRef] [PubMed]
- Charleston, B.; Bankowski, B.M.; Gubbins, S.; Chase-Topping, M.E.; Schley, D.; Howey, R.; Barnett, P.V.; Gibson, D.; Juleff, N.D.; Woolhouse, M.E. Relationship between clinical signs and transmission of an infectious disease and the implications for control. Science 2011, 332, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Stenfeldt, C.; Branan, M.A.; Moreno-Torres, K.I.; Holmstrom, L.K.; Delgado, A.H.; Arzt, J. Parameterization of the Durations of Phases of Foot-And-Mouth Disease in Cattle. Front. Vet. Sci. 2019, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Mardones, F.; Perez, A.; Sanchez, J.; Alkhamis, M.; Carpenter, T. Parameterization of the duration of infection stages of serotype O foot-and-mouth disease virus: An analytical review and meta-analysis with application to simulation models. Vet. Res. 2010, 41, 45. [Google Scholar] [CrossRef]
- Thomson, G.R.; Vosloo, W.; Esterhuysen, J.J.; Bengis, R.G. Maintenance of foot and mouth disease viruses in buffalo (Syncerus caffer Sparrman, 1779) in southern Africa. Rev. Sci. Tech. 1992, 11, 1097–1107. [Google Scholar] [CrossRef]
- Maddur, M.S.; Gajendragad, M.R.; Gopalakrishna, S.; Singh, N. Comparative study of experimental Foot-and-Mouth Disease in cattle (Bos indicus) and buffaloes (Bubalis bubalus). Vet. Res. Commun. 2008, 32, 481–489. [Google Scholar] [CrossRef][Green Version]
- Maddur, M.S.; Kishore, S.; Gopalakrishna, S.; Singh, N.; Suryanarayana, V.V.; Gajendragad, M.R. Immune response and viral persistence in Indian buffaloes (Bubalus bubalis) infected with foot-and-mouth disease virus serotype Asia 1. Clin. Vaccine Immunol. 2009, 16, 1832–1836. [Google Scholar] [CrossRef]
- Huang, C.C.; Lin, Y.L.; Huang, T.S.; Tu, W.J.; Lee, S.H.; Jong, M.H.; Lin, S.Y. Molecular characterization of foot-and-mouth disease virus isolated from ruminants in Taiwan in 1999–2000. Vet. Microbiol. 2001, 81, 193–205. [Google Scholar] [CrossRef]
- Kitching, R.P.; Hughes, G.J. Clinical variation in foot and mouth disease: Sheep and goats. Rev. Sci. Tech. 2002, 21, 505–512. [Google Scholar] [CrossRef]
- Anderson, E.C.; Doughty, W.J.; Anderson, J. The role of sheep and goats in the epizootiology of foot-and-mouth disease in Kenya. J. Hyg. (Lond.) 1976, 76, 395–402. [Google Scholar] [CrossRef]
- Stenfeldt, C.; Pacheco, J.M.; Singanallur, N.B.; Ferreira, H.C.; Vosloo, W.; Rodriguez, L.L.; Arzt, J. Clinical and virological dynamics of a serotype O 2010 South East Asia lineage foot-and-mouth disease virus in sheep using natural and simulated natural inoculation and exposure systems. Vet. Microbiol. 2015, 178, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Horsington, J.; Nfon, C.; Gonzales, J.L.; Singanallur, N.; Bittner, H.; Vosloo, W. Protection in sheep against heterologous challenge with serotype Asia-1 foot-and-mouth disease virus using high potency vaccine. Vaccine 2018, 36, 6095–6102. [Google Scholar] [CrossRef] [PubMed]
- Burrows, R. The persistence of foot-and mouth disease virus in sheep. J. Hyg. 1968, 66, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Alexandersen, S.; Zhang, Z.; Reid, S.M.; Hutchings, G.H.; Donaldson, A.I. Quantities of infectious virus and viral RNA recovered from sheep and cattle experimentally infected with foot-and-mouth disease virus O UK 2001. J. Gen. Virol. 2002, 83, 1915–1923. [Google Scholar] [CrossRef]
- Stenfeldt, C.; Pacheco, J.M.; Singanallur, N.B.; Vosloo, W.; Rodriguez, L.L.; Arzt, J. Virulence beneath the fleece; a tale of foot-and-mouth disease virus pathogenesis in sheep. PLoS ONE 2019, 14, e0227061. [Google Scholar] [CrossRef]
- Fukai, K.; Yamada, M.; Morioka, K.; Ohashi, S.; Yoshida, K.; Kitano, R.; Yamazoe, R.; Kanno, T. Dose-dependent responses of pigs infected with foot-and-mouth disease virus O/JPN/2010 by the intranasal and intraoral routes. Arch. Virol. 2015, 160, 129–139. [Google Scholar] [CrossRef]
- Murphy, C.; Bashiruddin, J.B.; Quan, M.; Zhang, Z.; Alexandersen, S. Foot-and-mouth disease viral loads in pigs in the early, acute stage of disease. Vet. Rec. 2010, 166, 10–14. [Google Scholar] [CrossRef]
- Stenfeldt, C.; Pacheco, J.M.; Rodriguez, L.L.; Arzt, J. Infection dynamics of foot-and-mouth disease virus in pigs using two novel simulated-natural inoculation methods. Res. Vet. Sci. 2014, 96, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Fukai, K.; Morioka, K.; Nishi, T.; Yamazoe, R.; Kitano, R.; Shimada, N.; Yoshida, K.; Kanno, T.; Sakamoto, K.; et al. Early pathogenesis of the foot-and-mouth disease virus O/JPN/2010 in experimentally infected pigs. J. Vet. Med. Sci. Jpn. Soc. Vet. Sci. 2018, 80, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Stenfeldt, C.; Pacheco, J.M.; Smoliga, G.R.; Bishop, E.; Pauszek, S.J.; Hartwig, E.J.; Rodriguez, L.L.; Arzt, J. Detection of Foot-and-mouth Disease Virus RNA and Capsid Protein in Lymphoid Tissues of Convalescent Pigs Does Not Indicate Existence of a Carrier State. Transbound Emerg. Dis. 2016, 63, 152–164. [Google Scholar] [CrossRef]
- Stenfeldt, C.; Pacheco, J.M.; Borca, M.V.; Rodriguez, L.L.; Arzt, J. Morphologic and phenotypic characteristics of myocarditis in two pigs infected by foot-and mouth disease virus strains of serotypes O or A. Acta Vet. Scand. 2014, 56, 42. [Google Scholar] [CrossRef]
- Gulbahar, M.Y.; Davis, W.C.; Guvenc, T.; Yarim, M.; Parlak, U.; Kabak, Y.B. Myocarditis associated with foot-and-mouth disease virus type O in lambs. Vet. Pathol. 2007, 44, 589–599. [Google Scholar] [CrossRef]
- Ryan, E.; Horsington, J.; Durand, S.; Brooks, H.; Alexandersen, S.; Brownlie, J.; Zhang, Z. Foot-and-mouth disease virus infection in young lambs: Pathogenesis and tissue tropism. Vet. Microbiol. 2008, 127, 258–274. [Google Scholar] [CrossRef]
- Farooq, U.; Ahmed, Z.; Naeem, K.; Bertram, M.; Brito, B.; Stenfeldt, C.; Pauszek, S.J.; LaRocco, M.; Rodriguez, L.; Arzt, J. Characterization of naturally occurring, new and persistent subclinical foot-and-mouth disease virus infection in vaccinated Asian buffalo in Islamabad Capital Territory, Pakistan. Transbound Emerg. Dis. 2018. [Google Scholar] [CrossRef]
- Stenfeldt, C.; Eschbaumer, M.; Rekant, S.I.; Pacheco, J.M.; Smoliga, G.R.; Hartwig, E.J.; Rodriguez, L.L.; Arzt, J. The foot-and-mouth disease carrier state divergence in cattle. J. Virol. 2016, 90, 6344–6364. [Google Scholar] [CrossRef]
- Stenfeldt, C.; Lohse, L.; Belsham, G.J. The comparative utility of oral swabs and probang samples for detection of foot-and-mouth disease virus infection in cattle and pigs. Vet. Microbiol. 2013, 162, 330–337. [Google Scholar] [CrossRef]
- Parthiban, A.B.; Mahapatra, M.; Gubbins, S.; Parida, S. Virus excretion from foot-and-mouth disease virus carrier cattle and their potential role in causing new outbreaks. PLoS ONE 2015, 10, e0128815. [Google Scholar] [CrossRef]
- Arzt, J.; Pacheco, J.M.; Rodriguez, L.L. The early pathogenesis of foot-and-mouth disease in cattle after aerosol inoculation: Identification of the nasopharynx as the primary site of infection. Vet. Pathol. 2010, 47, 1048–1063. [Google Scholar] [CrossRef] [PubMed]
- Stenfeldt, C.; Eschbaumer, M.; Pacheco, J.M.; Rekant, S.I.; Rodriguez, L.L.; Arzt, J. Pathogenesis of primary foot-and-mouth disease virus infection in the nasopharynx of vaccinated and non-vaccinated cattle. PLoS ONE 2015, 10, e0143666. [Google Scholar] [CrossRef]
- Stenfeldt, C.; Hartwig, E.J.; Smoliga, G.R.; Palinski, R.; Silva, E.B.; Bertram, M.R.; Fish, I.H.; Pauszek, S.J.; Arzt, J. Contact Challenge of Cattle with Foot-and-Mouth Disease Virus Validates the Role of the Nasopharyngeal Epithelium as the Site of Primary and Persistent Infection. mSphere 2018, 3. [Google Scholar] [CrossRef]
- Burrows, R.; Mann, J.A.; Garland, A.J.; Greig, A.; Goodridge, D. The pathogenesis of natural and simulated natural foot-and-mouth disease infection in cattle. J. Comp. Pathol. 1981, 91, 599–609. [Google Scholar] [CrossRef]
- Brown, C.C.; Meyer, R.F.; Olander, H.J.; House, C.; Mebus, C.A. A pathogenesis study of foot-and-mouth disease in cattle, using in situ hybridization. Can. J. Vet. Res. 1992, 56, 189–193. [Google Scholar]
- Stenfeldt, C.; Pacheco, J.M.; Rodriguez, L.L.; Arzt, J. Early events in the pathogenesis of foot-and-mouth disease in pigs; identification of oropharyngeal tonsils as sites of primary and sustained viral replication. PLoS ONE 2014, 9, e106859. [Google Scholar] [CrossRef]
- Palmer, M.V.; Thacker, T.C.; Waters, W.R. Histology, immunohistochemistry and ultrastructure of the bovine palatine tonsil with special emphasis on reticular epithelium. Vet. Immunol. Immunopathol. 2009, 127, 277–285. [Google Scholar] [CrossRef]
- Alexandersen, S.; Mowat, N. Foot-and-mouth disease: Host range and pathogenesis. Curr. Top Microbiol. Immunol. 2005, 288, 9–42. [Google Scholar]
- Monaghan, P.; Simpson, J.; Murphy, C.; Durand, S.; Quan, M.; Alexandersen, S. Use of confocal immunofluorescence microscopy to localize viral nonstructural proteins and potential sites of replication in pigs experimentally infected with foot-and-mouth disease virus. J. Virol. 2005, 79, 6410–6418. [Google Scholar] [CrossRef]
- Arzt, J.; Gregg, D.A.; Clavijo, A.; Rodriguez, L.L. Optimization of immunohistochemical and fluorescent antibody techniques for localization of Foot-and-mouth disease virus in animal tissues. J. Vet. Diagn Investig. 2009, 21, 779–792. [Google Scholar] [CrossRef]
- Eschbaumer, M.; Stenfeldt, C.; Rekant, S.I.; Pacheco, J.M.; Hartwig, E.J.; Smoliga, G.R.; Kenney, M.A.; Golde, W.T.; Rodriguez, L.L.; Arzt, J. Systemic immune response and virus persistence after foot-and-mouth disease virus infection of naive cattle and cattle vaccinated with a homologous adenovirus-vectored vaccine. BMC Vet. Res. 2016, 12, 205. [Google Scholar] [CrossRef]
- Stenfeldt, C.; Heegaard, P.M.; Stockmarr, A.; Tjornehoj, K.; Belsham, G.J. Analysis of the acute phase responses of Serum Amyloid A, Haptoglobin and Type 1 Interferon in cattle experimentally infected with foot-and-mouth disease virus serotype O. Vet. Res. 2011, 42, 66. [Google Scholar] [CrossRef]
- Pacheco, J.M.; Butler, J.E.; Jew, J.; Ferman, G.S.; Zhu, J.; Golde, W.T. IgA antibody response of swine to foot-and-mouth disease virus infection and vaccination. Clin. Vaccine Immunol. 2010, 17, 550–558. [Google Scholar] [CrossRef][Green Version]
- Salt, J.S.; Mulcahy, G.; Kitching, R.P. Isotype-specific antibody responses to foot-and-mouth disease virus in sera and secretions of “carrier’ and “non-carrier’ cattle. Epidemiol. Infect 1996, 117, 349–360. [Google Scholar] [CrossRef]
- Zhang, Z.; Alexandersen, S. Quantitative analysis of foot-and-mouth disease virus RNA loads in bovine tissues: Implications for the site of viral persistence. J. Gen. Virol. 2004, 85, 2567–2575. [Google Scholar] [CrossRef]
- Alexandersen, S.; Zhang, Z.; Donaldson, A.I. Aspects of the persistence of foot-and-mouth disease virus in animals-the carrier problem. Microbes. Infect. 2002, 4, 1099–1110. [Google Scholar] [CrossRef]
- Moonen, P.; Schrijver, R. Carriers of foot-and-mouth disease virus: A review. Vet. Q. 2000, 22, 193–197. [Google Scholar] [CrossRef]
- Van Bekkum, J.G.; Frenkel, H.S.; Frederiks, H.H.J.; Frenkel, S. Observations on the carrier state of cattle exposed to foot-and-mouth disease virus. Tijdschr. Diergeneeskd 1959, 84, 1159–1164. [Google Scholar]
- Sutmoller, P.; Gaggero, A. Foot-and mouth diseases carriers. Vet. Rec. 1965, 77, 968–969. [Google Scholar] [CrossRef]
- Sutmoller, P.; McVicar, J.W.; Cottral, G.E. The epizootiological importance of foot-and-mouth disease carriers. I. Experimentally produced foot-and-mouth disease carriers in susceptible and immune cattle. Arch. Gesamte Virusforsch 1968, 23, 227–235. [Google Scholar] [CrossRef]
- Hedger, R.S.; Condy, J.B.; Golding, S. Infection of some species of African wildlife with FMDV. J. Comp. Pathol. 1972, 82, 455–461. [Google Scholar] [CrossRef]
- Hedger, R.S. Observations on the carrier state and related antibody titres during an outbreak of foot-and-mouth disease. J. Hyg. (Lond.) 1970, 68, 53–60. [Google Scholar] [CrossRef][Green Version]
- Maree, F.; de Klerk-Lorist, L.M.; Gubbins, S.; Zhang, F.; Seago, J.; Perez-Martin, E.; Reid, L.; Scott, K.; van Schalkwyk, L.; Bengis, R.; et al. Differential Persistence of Foot-and-Mouth Disease Virus in African Buffalo Is Related to Virus Virulence. J. Virol. 2016, 90, 5132–5140. [Google Scholar] [CrossRef]
- World Organisation for Animal Health. Infection with foot-and-mouth disease virus. In Terrestrial Animal Health Code; OIE: Paris, France, 2017. [Google Scholar]
- Stenfeldt, C.; Belsham, G.J. Detection of foot-and-mouth disease virus RNA in pharyngeal epithelium biopsy samples obtained from infected cattle: Investigation of possible sites of virus replication and persistence. Vet. Microbiol. 2012, 154, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Arzt, J.; Fish, I.; Pauszek, S.J.; Johnson, S.L.; Chain, P.S.; Rai, D.K.; Rieder, E.; Goldberg, T.L.; Rodriguez, L.L.; Stenfeldt, C. The evolution of a super-swarm of foot-and-mouth disease virus in cattle. PLoS ONE 2019, 14, e0210847. [Google Scholar] [CrossRef]
- Burrows, R. Studies on the carrier state of cattle exposed to foot-and-mouth disease virus. J. Hyg. 1966, 64, 81–90. [Google Scholar] [CrossRef]
- Salt, J.S. Persistence of foot-and-mouth disease. In Foot and Mouth Disease: Current Perspectives; Horizon Bioscience: Oxford, UK, 2004; pp. 103–143. [Google Scholar]
- Pacheco, J.M.; Smoliga, G.R.; O’Donnell, V.; Brito, B.P.; Stenfeldt, C.; Rodriguez, L.L.; Arzt, J. Persistent foot-and-mouth disease virus infection in the nasopharynx of cattle; tissue-specific distribution and local cytokine expression. PLoS ONE 2015, 10, e0125698. [Google Scholar] [CrossRef]
- Prato Murphy, M.L.; Forsyth, M.A.; Belsham, G.J.; Salt, J.S. Localization of foot-and-mouth disease virus RNA by in situ hybridization within bovine tissues. Virus Res. 1999, 62, 67–76. [Google Scholar] [CrossRef]
- Zhang, Z.D.; Kitching, R.P. The localization of persistent foot and mouth disease virus in the epithelial cells of the soft palate and pharynx. J. Comp. Pathol. 2001, 124, 89–94. [Google Scholar] [CrossRef]
- Juleff, N.; Windsor, M.; Reid, E.; Seago, J.; Zhang, Z.; Monaghan, P.; Morrison, I.W.; Charleston, B. Foot-and-mouth disease virus persists in the light zone of germinal centres. PLoS ONE 2008, 3, e3434. [Google Scholar] [CrossRef]
- Keele, B.F.; Tazi, L.; Gartner, S.; Liu, Y.; Burgon, T.B.; Estes, J.D.; Thacker, T.C.; Crandall, K.A.; McArthur, J.C.; Burton, G.F. Characterization of the follicular dendritic cell reservoir of human immunodeficiency virus type 1. J. Virol. 2008, 82, 5548–5561. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, R. (Directorate for foot-and-Mouth Disease. Bhubaneswar, Odisha, India), Personal Communication. 2018. [Google Scholar]
- de Carvalho Ferreira, H.C.; Pauszek, S.J.; Ludi, A.; Huston, C.L.; Pacheco, J.M.; Le, V.T.; Nguyen, P.T.; Bui, H.H.; Nguyen, T.D.; Nguyen, T.; et al. An Integrative Analysis of Foot-and-Mouth Disease Virus Carriers in Vietnam Achieved Through Targeted Surveillance and Molecular Epidemiology. Transbound Emerg. Dis. 2017, 64, 547–563. [Google Scholar] [CrossRef] [PubMed]
- McVicar, J.W.; Sutmoller, P. Sheep and goats as foot-and-mouth disease carriers. Proc. Mtg. U. S. Livest. San. Assoc. 1969, 72, 400–406. [Google Scholar]
- Singanallur, N.B.; Pacheco, J.M.; Arzt, J.; Stenfeldt, C.; Fosgate, G.T.; Rodriguez, L.; Vosloo, W. Efficacy of a high potency O1 Manisa monovalent vaccine against heterologous challenge with foot-and-mouth disease virus of O/SEA/Mya-98 lineage in sheep. Antivir. Res. 2017, 145, 114–122. [Google Scholar] [CrossRef]
- Sutmoller, P.; Cottral, G.E. Improved techniques for the detection of foot-and-mouth disease virus in carrier cattle. Arch. Gesamte Virusforsch 1967, 21, 170–177. [Google Scholar] [CrossRef]
- Arzt, J.; Belsham, G.J.; Lohse, L.; Botner, A.; Stenfeldt, C. Transmission of Foot-and-Mouth Disease from Persistently Infected Carrier Cattle to Naive Cattle via Transfer of Oropharyngeal Fluid. mSphere 2018. [Google Scholar] [CrossRef]
- Brown, F.; Cartwright, B. Purification of the virus of foot-and-mouth disease by fluorocarbon treatment and its dissociation from neutralizing antibody. J. Immunol. 1960, 85, 309–313. [Google Scholar]
- Windsor, M.A.; Carr, B.V.; Bankowski, B.; Gibson, D.; Reid, E.; Hamblin, P.; Gubbins, S.; Juleff, N.; Charleston, B. Cattle remain immunocompetent during the acute phase of foot-and-mouth disease virus infection. Vet. Res. 2011, 42, 108. [Google Scholar] [CrossRef]
- Arzt, J.; Pacheco, J.M.; Smoliga, G.R.; Tucker, M.T.; Bishop, E.; Pauszek, S.J.; Hartwig, E.J.; de los Santos, T.; Rodriguez, L.L. Foot-and-mouth disease virus virulence in cattle is co-determined by viral replication dynamics and route of infection. Virology 2014, 452-453, 12–22. [Google Scholar] [CrossRef]
- Alsemgeest, S.P.; Kalsbeek, H.C.; Wensing, T.; Koeman, J.P.; van Ederen, A.M.; Gruys, E. Concentrations of serum amyloid-A (SAA) and haptoglobin (HP) as parameters of inflammatory diseases in cattle. Vet. Q. 1994, 16, 21–23. [Google Scholar] [CrossRef]
- Arredouani, M.; Matthijs, P.; Van Hoeyveld, E.; Kasran, A.; Baumann, H.; Ceuppens, J.L.; Stevens, E. Haptoglobin directly affects T cells and suppresses T helper cell type 2 cytokine release. Immunology 2003, 108, 144–151. [Google Scholar] [CrossRef]
- Arredouani, M.; Matthys, P.; Kasran, A.; Baumann, H.; Ceuppen, J.L. Haptoglobin and the Th1/Th2 balance: Hints from in vitro and in vivo studies. Redox Rep. 2001, 6, 369–371. [Google Scholar] [CrossRef]
- Stenfeldt, C.; Eschbaumer, M.; Smoliga, G.R.; Rodriguez, L.L.; Zhu, J.; Arzt, J. Clearance of a persistent picornavirus infection is associated with enhanced pro-apoptotic and cellular immune responses. Sci. Rep. 2017, 7, 17800. [Google Scholar] [CrossRef] [PubMed]
- Juleff, N.; Windsor, M.; Lefevre, E.A.; Gubbins, S.; Hamblin, P.; Reid, E.; McLaughlin, K.; Beverley, P.C.; Morrison, I.W.; Charleston, B. Foot-and-mouth disease virus can induce a specific and rapid CD4+ T-cell-independent neutralizing and isotype class-switched antibody response in naive cattle. J. Virol. 2009, 83, 3626–3636. [Google Scholar] [CrossRef] [PubMed]
- Parida, S.; Anderson, J.; Cox, S.J.; Barnett, P.V.; Paton, D.J. Secretory IgA as an indicator of oro-pharyngeal foot-and-mouth disease virus replication and as a tool for post vaccination surveillance. Vaccine 2006, 24, 1107–1116. [Google Scholar] [CrossRef]
- Maddur, M.S.; Gajendragad, M.R.; Kishore, S.; Chockalingam, A.K.; Suryanarayana, V.V.; Gopalakrishna, S.; Singh, N. Enhanced mucosal immune response in cattle persistently infected with foot-and-mouth disease virus. Vet. Immunol. Immunopathol. 2008, 125, 337–343. [Google Scholar] [CrossRef]
- Cunliffe, H.R. Observations on the Duration of Immunity in Cattle after Experimental Infection with Foot-and-Mouth Disease Virus. Cornell Vet. 1964, 54, 501–510. [Google Scholar] [PubMed]
- Patch, J.R.; Kenney, M.; Pacheco, J.M.; Grubman, M.J.; Golde, W.T. Characterization of cytotoxic T lymphocyte function after foot-and-mouth disease virus infection and vaccination. Viral. Immunol. 2013, 26, 239–249. [Google Scholar] [CrossRef]
- Bautista, E.M.; Ferman, G.S.; Golde, W.T. Induction of lymphopenia and inhibition of T cell function during acute infection of swine with foot and mouth disease virus (FMDV). Vet. Immunol. Immunopathol. 2003, 92, 61–73. [Google Scholar] [CrossRef]
- Nfon, C.K.; Toka, F.N.; Kenney, M.; Pacheco, J.M.; Golde, W.T. Loss of plasmacytoid dendritic cell function coincides with lymphopenia and viremia during foot-and-mouth disease virus infection. Viral. Immunol. 2010, 23, 29–41. [Google Scholar] [CrossRef]
- Toka, F.N.; Nfon, C.; Dawson, H.; Golde, W.T. Natural killer cell dysfunction during acute infection with foot-and-mouth disease virus. Clin. Vaccine Immunol. 2009, 16, 1738–1749. [Google Scholar] [CrossRef]
- Pega, J.; Bucafusco, D.; Di Giacomo, S.; Schammas, J.M.; Malacari, D.; Capozzo, A.V.; Arzt, J.; Perez-Beascoechea, C.; Maradei, E.; Rodriguez, L.L.; et al. Early adaptive immune responses in the respiratory tract of foot-and-mouth disease virus-infected cattle. J. Virol. 2013, 87, 2489–2495. [Google Scholar] [CrossRef]
- Toka, F.N.; Golde, W.T. Cell mediated innate responses of cattle and swine are diverse during foot-and-mouth disease virus (FMDV) infection: A unique landscape of innate immunity. Immunol. Lett. 2013, 152, 135–143. [Google Scholar] [CrossRef]
- Stenfeldt, C.; Heegaard, P.M.; Stockmarr, A.; Belsham, G.J. Modulation of cytokine mRNA expression in pharyngeal epithelial samples obtained from cattle Infected with foot-and-mouth disease virus. J. Comp. Pathol. 2012, 146, 243–252. [Google Scholar] [CrossRef]
- Zhang, Z.; Bashiruddin, J.B.; Doel, C.; Horsington, J.; Durand, S.; Alexandersen, S. Cytokine and Toll-like receptor mRNAs in the nasal-associated lymphoid tissues of cattle during foot-and-mouth disease virus infection. J. Comp. Pathol. 2006, 134, 56–62. [Google Scholar] [CrossRef]
- Eschbaumer, M.; Stenfeldt, C.; Smoliga, G.R.; Pacheco, J.M.; Rodriguez, L.L.; Li, R.W.; Zhu, J.; Arzt, J. Transcriptomic Analysis of Persistent Infection with Foot-and-Mouth Disease Virus in Cattle Suggests Impairment of Apoptosis and Cell-Mediated Immunity in the Nasopharynx. PLoS ONE 2016, 11, e0162750. [Google Scholar] [CrossRef]
- Huang, X.; Li, Y.; Fang, H.; Zheng, C. Establishment of persistent infection with foot-and-mouth disease virus in BHK-21 cells. Virol. J. 2011, 8, 169. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Huang, X.; Zheng, C. Global transcriptional analysis of model of persistent FMDV infection reveals critical role of host cells in persistence. Vet. Microbiol. 2013, 162, 321–329. [Google Scholar] [CrossRef]
- Fang, H.; Yuan, B.; Han, L.; Xin, X.; Wang, H.; Yu, F.; Zheng, C.; Shen, C. Single-cell analysis reveals the relevance of foot-and-mouth disease virus persistence to emopamil-binding protein gene expression in host cells. Arch. Virol. 2017, 162, 3791–3802. [Google Scholar] [CrossRef]
- Pfaff, F.; Hagglund, S.; Zoli, M.; Blaise-Boisseau, S.; Laloy, E.; Koethe, S.; Zuhlke, D.; Riedel, K.; Zientara, S.; Bakkali-Kassimi, L.; et al. Proteogenomics Uncovers Critical Elements of Host Response in Bovine Soft Palate Epithelial Cells Following In Vitro Infection with Foot-And-Mouth Disease Virus. Viruses 2019, 11, 53. [Google Scholar] [CrossRef]
- O’Donnell, V.; Pacheco, J.M.; Larocco, M.; Gladue, D.P.; Pauszek, S.J.; Smoliga, G.; Krug, P.W.; Baxt, B.; Borca, M.V.; Rodriguez, L. Virus-host interactions in persistently FMDV-infected cells derived from bovine pharynx. Virology 2014, 468–470, 185–196. [Google Scholar] [CrossRef]
- Hagglund, S.; Laloy, E.; Naslund, K.; Pfaff, F.; Eschbaumer, M.; Romey, A.; Relmy, A.; Rikberg, A.; Svensson, A.; Huet, H.; et al. Model of persistent foot-and-mouth disease virus infection in multilayered cells derived from bovine dorsal soft palate. Transbound Emerg. Dis. 2019, 13332. [Google Scholar] [CrossRef]
- Ramirez-Carvajal, L.; Pauszek, S.J.; Ahmed, Z.; Farooq, U.; Naeem, K.; Shabman, R.S.; Stockwell, T.B.; Rodriguez, L.L. Genetic stability of foot-and-mouth disease virus during long-term infections in natural hosts. PLoS ONE 2018, 13, e0190977. [Google Scholar] [CrossRef]
- Parthiban, A.R.; Mahapatra, M.; Parida, S. Complete Genome Sequences of Serotype O Foot-and-Mouth Disease Viruses Recovered from Experimental Persistently Infected Cattle. Genome Announc. 2015, 3. [Google Scholar] [CrossRef]
- Bertram, M.R.; Vu, L.T.; Pauszek, S.J.; Brito, B.P.; Hartwig, E.J.; Smoliga, G.R.; Hoang, B.H.; Phuong, N.T.; Stenfeldt, C.; Fish, I.H.; et al. Lack of Transmission of Foot-and-Mouth Disease Virus From Persistently Infected Cattle to Naive Cattle Under Field Conditions in Vietnam. Front Vet. Sci. 2018, 5, 174. [Google Scholar] [CrossRef]
- Horsington, J.; Zhang, Z. Consistent change in the B-C loop of VP2 observed in foot-and-mouth disease virus from persistently infected cattle: Implications for association with persistence. Virus Res. 2007, 125, 114–118. [Google Scholar] [CrossRef]
- Pauszek, S.J.; Eschbaumer, M.; Brito, B.; de Carvalho Ferreira, H.C.; Vu, L.T.; Phuong, N.T.; Hoang, B.H.; Tho, N.D.; Dong, P.V.; Minh, P.Q.; et al. Site-specific substitution (Q172R) in the VP1 protein of FMDV isolates collected from asymptomatic carrier ruminants in Vietnam. Virol. Rep. 2016, 6, 90–96. [Google Scholar] [CrossRef]
- Cortey, M.; Ferretti, L.; Perez-Martin, E.; Zhang, F.; de Klerk-Lorist, L.M.; Scott, K.; Freimanis, G.; Seago, J.; Ribeca, P.; van Schalkwyk, L.; et al. Persistent Infection of African Buffalo (Syncerus caffer) with Foot-and-Mouth Disease Virus: Limited Viral Evolution and No Evidence of Antibody Neutralization Escape. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Fish, I.; Stenfeldt, C.; Palinski, R.M.; Pauszek, S.J.; Arzt, J. Into the deep (sequence) of the foot-and-mouth disease virus gene pool: Bottlenecks and adaptation during infection in naïve and vaccinated cattle. Pathogens 2019, 9, 208. [Google Scholar] [CrossRef]
- Biswal, J.K.; Ranjan, R.; Subramaniam, S.; Mohapatra, J.K.; Patidar, S.; Sharma, M.K.; Bertram, M.R.; Brito, B.; Rodriguez, L.L.; Pattnaik, B.; et al. Genetic and antigenic variation of foot-and-mouth disease virus during persistent infection in naturally infected cattle and Asian buffalo in India. PLoS ONE 2019, 14, e0214832. [Google Scholar] [CrossRef]
- Hayer, S.S.; Ranjan, R.; Biswal, J.K.; Subramaniam, S.; Mohapatra, J.K.; Sharma, G.K.; Rout, M.; Dash, B.B.; Das, B.; Prusty, B.R.; et al. Quantitative characteristics of the foot-and-mouth disease carrier state under natural conditions in India. Transbound Emerg. Dis. 2018, 65, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Bertram, M.R.; Delgado, A.; Pauszek, S.J.; Smoliga, G.R.; Brito, B.; Stenfeldt, C.; Hartwig, E.J.; Jumbo, S.D.; Abdoulmoumini, M.; Oliva Marie, A.A.; et al. Effect of vaccination on cattle subclinically infected with foot-and-mouth disease virus in Cameroon. Prev. Vet. Med. 2018, 155, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ilott, M.C.; Salt, J.S.; Gaskell, R.M.; Kitching, R.P. Dexamethasone inhibits virus production and the secretory IgA response in oesophageal-pharyngeal fluid in cattle persistently infected with foot-and-mouth disease virus. Epidemiol. Infect. 1997, 118, 181–187. [Google Scholar] [CrossRef]
- Cox, S.J.; Voyce, C.; Parida, S.; Reid, S.M.; Hamblin, P.A.; Paton, D.J.; Barnett, P.V. Protection against direct-contact challenge following emergency FMD vaccination of cattle and the effect on virus excretion from the oropharynx. Vaccine 2005, 23, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Bronsvoort, B.M.; Anderson, D.M.; Corteyn, A.; Hamblin, P.; Kitching, R.P.; Nfon, C.; Tanya, V.N.; Morgan, K.L. Geographical and age-stratified distributions of foot-and-mouth disease virus-seropositive and probang-positive cattle herds in the Adamawa province of Cameroon. Vet. Rec. 2006, 159, 299–308. [Google Scholar] [CrossRef]
- Hedger, R.S. The isolation and characterization of foot-and-mouth disease virus from clinically normal herds of cattle in Botswana. J. Hyg. (Lond.) 1968, 66, 27–36. [Google Scholar] [CrossRef]
- Straver, P.J.; Bool, P.H.; Claessens, A.M.; van Bekkum, J.G. Some properties of carrier strains of foot-and-mouth disease virus. Arch. Gesamte Virusforsch 1970, 29, 113–126. [Google Scholar] [CrossRef]
- Tenzin, D.A.; Vernooij, H.; Bouma, A.; Stegeman, A. Rate of foot-and-mouth disease virus transmission by carriers quantified from experimental data. Risk Anal. 2008, 28, 303–309. [Google Scholar] [CrossRef]
- Bronsvoort, B.M.; Handel, I.G.; Nfon, C.K.; Sorensen, K.J.; Malirat, V.; Bergmann, I.; Tanya, V.N.; Morgan, K.L. Redefining the “carrier” state for foot-and-mouth disease from the dynamics of virus persistence in endemically affected cattle populations. Sci. Rep. 2016, 6, 29059. [Google Scholar] [CrossRef]
- Bertram, M.; Yadav, S.; Stenfeldt, C.; Delgado, A.; Arzt, J. Extinction Dynamics of the Foot-and-Mouth Disease Virus Carrier State Under Natural Conditions. Front. Vet. Sci. 2020. (under review). [Google Scholar]
- Geale, D.W.; Barnett, P.V.; Clarke, G.W.; Davis, J.; Kasari, T.R. A review of OIE country status recovery using vaccinate-to-live versus vaccinate-to-die foot-and-mouth disease response policies II: Waiting periods after emergency vaccination in FMD free countries. Transbound Emerg. Dis. 2015, 62, 388–406. [Google Scholar] [CrossRef] [PubMed]
- Sutmoller, P.; McVicar, J.W.; Cottral, G.E. Foot-and-mouth Disease virus carrier studies at the Plum Island Animal Disease Laboratory. In European Commission for the Control of Foot-and-Mouth Disease Standing Technical Commitee, Working paper no. 11; European Commission: Brussels, Belgium, 1967. [Google Scholar]
- Dawe, P.S.; Flanagan, F.O.; Madekurozwa, R.L.; Sorensen, K.J.; Anderson, E.C.; Foggin, C.M.; Ferris, N.P.; Knowles, N.J. Natural transmission of foot-and-mouth-disease virus from African buffalo (Syncerus Caffer) to cattle in a wildlife area of Zimbabwe. Vet. Rec. 1994, 134, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Dawe, P.S.; Sorensen, K.; Ferris, N.P.; Barnett, I.T.R.; Armstrong, R.M.; Knowles, N.J. Experimental transmission of foot-and-mouth-disease virus from carrier African buffalo (Syncerus Caffer) to cattle in Zimbabwe. Vet. Rec. 1994, 134, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Omondi, G.; Alkhamis, M.A.; Obanda, V.; Gakuya, F.; Sangula, A.; Pauszek, S.; Perez, A.; Ngulu, S.; van Aardt, R.; Arzt, J.; et al. Phylogeographic and cross-species transmission dynamics of SAT1 and SAT2 Foot-and-Mouth Disease Virus in Eastern Africa. Mol. Ecol. 2019. [Google Scholar] [CrossRef]
- Klein, J.; Hussain, M.; Ahmad, M.; Afzal, M.; Alexandersen, S. Epidemiology of foot-and-mouth disease in Landhi Dairy Colony, Pakistan, the world largest Buffalo colony. Virol. J. 2008, 5, 53. [Google Scholar] [CrossRef]
- Jamal, S.M.; Ferrari, G.; Hussain, M.; Nawroz, A.H.; Aslami, A.A.; Khan, E.; Murvatulloev, S.; Ahmed, S.; Belsham, G.J. Detection and genetic characterization of foot-and-mouth disease viruses in samples from clinically healthy animals in endemic settings. Transbound Emerg. Dis. 2012, 59, 429–440. [Google Scholar] [CrossRef]
- Sutmoller, P.; Casas, O.R. Unapparent foot and mouth disease infection (sub-clinical infections and carriers): Implications for control. Rev. Sci. Tech. 2002, 21, 519–529. [Google Scholar] [CrossRef]
- Weaver, G.V.; Domenech, J.; Thiermann, A.R.; Karesh, W.B. Foot and mouth disease: A look from the wild side. J. Wildl. Dis. 2013, 49, 759–785. [Google Scholar] [CrossRef]
- Brown, V.R.; Bevins, S.N. Potential role of wildlife in the USA in the event of a foot-and-mouth disease virus incursion. Vet. Rec. 2019, 184, 741. [Google Scholar] [CrossRef]
- Olitsky, P.K.; Schoening, H.W.; Traum, J. Report of the Foot-and-Mouth Disease Commission of the United States Department of Agriculture; U.S. Government Printing Office: Washington, DC, USA, 1928.
- Breithaupt, A.; Depner, K.; Haas, B.; Alexandrov, T.; Polihronova, L.; Georgiev, G.; Meyer-Gerbaulet, H.; Beer, M. Experimental infection of wild boar and domestic pigs with a foot and mouth disease virus strain detected in the southeast of Bulgaria in December of 2010. Vet. Microbiol. 2012, 159, 33–39. [Google Scholar] [CrossRef]
- Mohamed, F.; Swafford, S.; Petrowski, H.; Bracht, A.; Schmit, B.; Fabian, A.; Pacheco, J.M.; Hartwig, E.; Berninger, M.; Carrillo, C.; et al. Foot-and-mouth disease in feral swine: Susceptibility and transmission. Transbound Emerg. Dis. 2011, 58, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Kittelberger, R.; Nfon, C.; Swekla, K.; Zhang, Z.; Hole, K.; Bittner, H.; Salo, T.; Goolia, M.; Embury-Hyatt, C.; Bueno, R.; et al. Foot-and-Mouth Disease in Red Deer-Experimental Infection and Test Methods Performance. Transbound Emerg. Dis. 2017, 64, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Moniwa, M.; Embury-Hyatt, C.; Zhang, Z.; Hole, K.; Clavijo, A.; Copps, J.; Alexandersen, S. Experimental foot-and-mouth disease virus infection in white tailed deer. J. Comp. Pathol. 2012, 147, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Rhyan, J.; McCollum, M.; Gidlewski, T.; Shalev, M.; Ward, G.; Donahue, B.; Arzt, J.; Stenfeldt, C.; Mohamed, F.; Nol, P.; et al. Foot-and-Mouth Disease in a Small Sample of Experimentally Infected Pronghorn (Antilocapra Americana). J. Wildl. Dis. 2016, 52, 862–873. [Google Scholar] [CrossRef]
- Rhyan, J.; Deng, M.; Wang, H.; Ward, G.; Gidlewski, T.; McCollum, M.; Metwally, S.; McKenna, T.; Wainwright, S.; Ramirez, A.; et al. Foot-and-mouth disease in North American bison (Bison bison) and elk (Cervus elaphus nelsoni): Susceptibility, intra- and interspecies transmission, clinical signs, and lesions. J. Wildl. Dis. 2008, 44, 269–279. [Google Scholar] [CrossRef]
- Keet, D.F.; Hunter, P.; Bengis, R.G.; Bastos, A.; Thomson, G.R. The 1992 foot-and-mouth disease epizootic in the Kruger National Park. J. S. Afr. Vet. Assoc. 1996, 67, 83–87. [Google Scholar]
- Correa Melo, E.; Lopez, A. Control of foot and mouth disease: The experience of the Americas. Rev. Sci. Tech. 2002, 21, 695–698. [Google Scholar]
- Saraiva, V. Vaccines and foot-and-mouth disease eradication in South America. Dev. Biol. (Basel) 2003, 114, 67–77. [Google Scholar]
- Sutmoller, P.; Barteling, S.S.; Olascoaga, R.C.; Sumption, K.J. Control and eradication of foot-and-mouth disease. Virus Res. 2003, 91, 101–144. [Google Scholar] [CrossRef]
- World Organisation for Animal Healt. Terresterrial Animal Health Code, Chapter 8.8 (Infection with Foot and Mouth Disease Virus). Available online: http://www.oie.int/fileadmin/Home/eng/Health_standards/tahc/current/chapitre_fmd.pdf (accessed on 14 July 2016).
- Garland, A.J.; de Clercq, K. Cattle, sheep and pigs vaccinated against foot and mouth disease: Does trade in these animals and their products present a risk of transmitting the disease? Rev. Sci. Tech. 2011, 30, 189–206. [Google Scholar] [CrossRef]
- World Organisation for Animal Health. Manual of Diagnistic Tests and Vaccines for Terrestrial Animals 2019. In 3.8.1 Foot-and-Mouth Disease; OIE: Paris, France, 2018; pp. 433–464. [Google Scholar]
- Robiolo, B.; La Torre, J.; Maradei, E.; Beascoechea, C.P.; Perez, A.; Seki, C.; Smitsaart, E.; Fondevila, N.; Palma, E.; Goris, N.; et al. Confidence in indirect assessment of foot-and-mouth disease vaccine potency and vaccine matching carried out by liquid phase ELISA and virus neutralization tests. Vaccine 2010, 28, 6235–6241. [Google Scholar] [CrossRef] [PubMed]
- Willems, T.; De Vleeschauwer, A.; Perez-Filgueira, M.; Li, Y.; Ludi, A.; Lefebvre, D.; Wilsden, G.; Statham, B.; Haas, B.; Mattion, N.; et al. FMD vaccine matching: Inter laboratory study for improved understanding of r1 values. J. Virol. Methods 2019, 276, 113786. [Google Scholar] [CrossRef] [PubMed]
- Jouneau, L.; Lefebvre, D.J.; Costa, F.; Romey, A.; Blaise-Boisseau, S.; Relmy, A.; Jaszczyszyn, Y.; Dard-Dascot, C.; Dejean, S.; Versille, N.; et al. The antibody response induced FMDV vaccines in sheep correlates with early transcriptomic responses in blood. NPJ Vaccines 2020, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.J.; Voyce, C.; Parida, S.; Reid, S.M.; Hamblin, P.A.; Hutchings, G.; Paton, D.J.; Barnett, P.V. Effect of emergency FMD vaccine antigen payload on protection, sub-clinical infection and persistence following direct contact challenge of cattle. Vaccine 2006, 24, 3184–3190. [Google Scholar] [CrossRef] [PubMed]
- Boklund, A.; Halasa, T.; Christiansen, L.E.; Enoe, C. Comparing control strategies against foot-and-mouth disease: Will vaccination be cost-effective in Denmark? Prev. Vet. Med. 2013, 111, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Jeong, W.; Han, J.H.; Choi, J.; Kang, Y.M.; Kim, Y.S.; Park, H.S.; Carpenter, T.E. Financial Impact of Foot-and-mouth disease outbreaks on pig farms in the Republic of Korea, 2014/2015. Prev. Vet. Med. 2018, 149, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Tark, D.; Lee, K.N.; Chun, J.E.; Lee, H.S.; Ko, Y.J.; Kye, S.J.; Kim, Y.J.; Oem, J.K.; Ryoo, S.; et al. Control of type O foot-and-mouth disease by vaccination in Korea, 2014-2015. J. Vet. Sci. 2018, 19, 271–279. [Google Scholar] [CrossRef] [PubMed]
- WRLFMD. Annual report 2016. In OIE/FAO Foot-and-Mouth Disease Reference Laboratory Network; King, D.J., Henstock, M., Eds.; WRLFMD: Pirbright, UK, 2016. [Google Scholar]
- WRLFMD. Annual report 2017. In OIE/FAO Foot-and-Mouth Disease Reference Laboratory Network; King, D.J., di Nardo, A., Henstock, M., Eds.; WRLFMD: Pirbright, UK, 2017. [Google Scholar]
- WRLFMD. Annual report 2018. In OIE/FAO Foot-and-Mouth Disease Reference Laboratory Network; King, D.J., di Nardo, A., Henstock, M., Eds.; WRLFMD: Pirbright, UK, 2018. [Google Scholar]
- Rawdon, T.G.; Garner, M.G.; Sanson, R.L.; Stevenson, M.A.; Cook, C.; Birch, C.; Roche, S.E.; Patyk, K.A.; Forde-Folle, K.N.; Dube, C.; et al. Evaluating vaccination strategies to control foot-and-mouth disease: A country comparison study. Epidemiol. Infect. 2018, 146, 1138–1150. [Google Scholar] [CrossRef]
- Miller, M.; Liu, L.; Shwiff, S.; Shwiff, S. Macroeconomic impact of foot-and-mouth disease vaccination strategies for an outbreak in the Midwestern United States: A computable general equilibrium. Transbound Emerg. Dis. 2019, 66, 156–165. [Google Scholar] [CrossRef]
- Barratt, A.S.; Rich, K.M.; Eze, J.I.; Porphyre, T.; Gunn, G.J.; Stott, A.W. Framework for Estimating Indirect Costs in Animal Health Using Time Series Analysis. Front. Vet. Sci. 2019, 6, 190. [Google Scholar] [CrossRef]
- Lanyon, S.R.; Hill, F.I.; Reichel, M.P.; Brownlie, J. Bovine viral diarrhoea: Pathogenesis and diagnosis. Vet. J. (Lond. Engl. 1997) 2014, 199, 201–209. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stenfeldt, C.; Arzt, J. The Carrier Conundrum; A Review of Recent Advances and Persistent Gaps Regarding the Carrier State of Foot-and-Mouth Disease Virus. Pathogens 2020, 9, 167. https://doi.org/10.3390/pathogens9030167
Stenfeldt C, Arzt J. The Carrier Conundrum; A Review of Recent Advances and Persistent Gaps Regarding the Carrier State of Foot-and-Mouth Disease Virus. Pathogens. 2020; 9(3):167. https://doi.org/10.3390/pathogens9030167
Chicago/Turabian StyleStenfeldt, Carolina, and Jonathan Arzt. 2020. "The Carrier Conundrum; A Review of Recent Advances and Persistent Gaps Regarding the Carrier State of Foot-and-Mouth Disease Virus" Pathogens 9, no. 3: 167. https://doi.org/10.3390/pathogens9030167
APA StyleStenfeldt, C., & Arzt, J. (2020). The Carrier Conundrum; A Review of Recent Advances and Persistent Gaps Regarding the Carrier State of Foot-and-Mouth Disease Virus. Pathogens, 9(3), 167. https://doi.org/10.3390/pathogens9030167