Conservation of a Neutralization Epitope of Human T-cell Leukemia Virus Type 1 (HTLV-1) among Currently Endemic Clinical Isolates in Okinawa, Japan
Abstract
1. Introduction
2. Results
2.1. The Major Neutralizing Domain of gp46 is Conserved in Clinical Isolates of HTLV-1
2.2. hu-LAT-27 Inhibits In Vitro Infection with Clinical HTLV-1 Isolates
2.3. The LAT-27 Epitope is Conserved in HTLV-1-Infected Individuals
2.4. hu-LAT-27 Cross-Reacts with a Peptide Carrying a P192S Substitution in the LAT-27 Epitope
3. Discussion
4. Materials and Methods
4.1. Cells and Cell Culture
4.2. Antibodies and FCM
4.3. Genomic DNA Isolation and Sequencing
4.4. HTLV-1 Neutralization Assay
4.5. ELISA
4.6. Statistics Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gessain, A.; Cassar, O. Epidemiological Aspects and World Distribution of HTLV-1 Infection. Front. Microbiol. 2012, 3, 388. [Google Scholar] [CrossRef] [PubMed]
- Poiesz, B.J.; Ruscetti, F.W.; Reitz, M.S.; Kalyanaraman, V.S.; Gallo, R.C. Isolation of a new type C retrovirus (HTLV) in primary uncultured cells of a patient with Sézary T-cell leukaemia. Nature 1981, 294, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Hinuma, Y.; Nagata, K.; Hanaoka, M.; Nakai, M.; Matsumoto, T.; Kinoshita, K.I.; Shirakawa, S.; Miyoshi, I. Adult T-cell leukemia: Antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc. Natl. Acad. Sci. 1981, 78, 6476–6480. [Google Scholar] [CrossRef] [PubMed]
- Osame, M.; Usuku, K.; Izumo, S.; Ijichi, N.; Amitani, H.; Igata, A.; Matsumoto, M.; Tara, M. HTLV-I Associated Myelopathy, a New Clinical Entity. Lancet 1986, 327, 1031–1032. [Google Scholar] [CrossRef]
- Jacobson, S.; Raine, C.S.; Mingioli, E.S.; McFarlin, D.E. Isolation of an HTLV-1-like retrovirus from patients with tropical spastic paraparesis. Nature 1988, 331, 540–543. [Google Scholar] [CrossRef]
- Ishii, T.; Ishida, T.; Utsunomiya, A.; Inagaki, A.; Yano, H.; Komatsu, H.; Iida, S.; Imada, K.; Uchiyama, T.; Akinaga, S.; et al. Defucosylated Humanized Anti-CCR4 Monoclonal Antibody KW-0761 as a Novel Immunotherapeutic Agent for Adult T-cell Leukemia/Lymphoma. Clin. Cancer Res. 2010, 16, 1520–1531. [Google Scholar] [CrossRef]
- Scott, A.M.; Wolchok, J.D.; Old, L.J. Antibody therapy of cancer. Nat. Rev. Cancer 2012, 12, 278–287. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Drake, C.G.; Wollner, I.; Powderly, J.D.; Picus, J.; Sharfman, W.H.; Stankevich, E.; Pons, A.; Salay, T.M.; McMiller, T.L.; et al. Phase I Study of Single-Agent Anti–Programmed Death-1 (MDX-1106) in Refractory Solid Tumors: Safety, Clinical Activity, Pharmacodynamics, and Immunologic Correlates. J. Clin. Oncol. 2010, 28, 3167–3175. [Google Scholar] [CrossRef]
- Bar-On, Y.; Gruell, H.; Schoofs, T.; Pai, J.A.; Nogueira, L.; Butler, A.L.; Millard, K.; Lehmann, C.; Suárez, I.; Oliveira, T.Y.; et al. Safety and antiviral activity of combination HIV-1 broadly neutralizing antibodies in viremic individuals. Nat. Med. 2018, 24, 1701–1707. [Google Scholar] [CrossRef]
- Mendoza, P.; Gruell, H.; Nogueira, L.; Pai, J.A.; Butler, A.L.; Millard, K.; Lehmann, C.; Suárez, I.; Oliveira, T.Y.; Lorenzi, J.C.C.; et al. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature 2018, 561, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.S.; Lambert, S.; Bouttier, M.; Benit, L.; Ruscetti, F.W.; Hermine, O.; Pique, C. Molecular Aspects of HTLV-1 Entry: Functional Domains of the HTLV-1 Surface Subunit (SU) and Their Relationships to the Entry Receptors. Viruses 2011, 3, 794–810. [Google Scholar] [CrossRef] [PubMed]
- Manel, N.; Kim, F.J.; Kinet, S.; Taylor, N.; Sitbon, M.; Battini, J.-L. The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV. Cell 2003, 115, 449–459. [Google Scholar] [CrossRef]
- Ghez, D.; Lepelletier, Y.; Lambert, S.; Fourneau, J.-M.; Blot, V.; Janvier, S.; Arnulf, B.; Van Endert, P.M.; Heveker, N.; Pique, C.; et al. Neuropilin-1 Is Involved in Human T-Cell Lymphotropic Virus Type 1 Entry. J. Virol. 2006, 80, 6844–6854. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.S.; Petrow-Sadowski, C.; Bertolette, D.C.; Huang, Y.; Ruscetti, F.W. Heparan sulfate proteoglycans mediate attachment and entry of human T-cell leukemia virus type 1 virions into CD4+ T cells. J. Virol. 2005, 79, 12692–12702. [Google Scholar] [CrossRef] [PubMed]
- Palker, T.J.; Riggs, E.R.; E Spragion, D.; Muir, A.J.; Scearce, R.M.; Randall, R.R.; McAdams, M.W.; McKnight, A.; Clapham, P.R.; A Weiss, R. Mapping of homologous, amino-terminal neutralizing regions of human T-cell lymphotropic virus type I and II gp46 envelope glycoproteins. J. Virol. 1992, 66, 5879–5889. [Google Scholar] [CrossRef]
- Desgranges, C.; Souche, S.; Vernant, J.-C.; Smadja, D.; Vahlne, A.; Horal, P. Identification of Novel Neutralization-Inducing Regions of the Human T Cell Lymphotropic Virus Type I Envelope Glycoproteins with Human HTLV-I-Seropositive Sera. AIDS Res. Hum. Retroviruses 1994, 10, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Baba, E.; Nakamura, M.; Tanaka, Y.; Kuroki, M.; Itoyama, Y.; Nakano, S.; Niho, Y. Multiple neutralizing B-cell epitopes of human T-cell leukemia virus type 1 (HTLV-1) identified by human monoclonal antibodies. A basis for the design of an HTLV-1 peptide vaccine. J. Immunol. 1993, 151, 1013–1024. [Google Scholar]
- Kuroki, M.; Nakamura, M.; Itoyama, Y.; Tanaka, Y.; Shiraki, H.; Baba, E.; Esaki, T.; Tatsumoto, T.; Nagafuchi, S.; Nakano, S. Identification of new epitopes recognized by human monoclonal antibodies with neutralizing and antibody-dependent cellular cytotoxicity activities specific for human T cell leukemia virus type 1. J. Immunol. 1992, 149, 940–948. [Google Scholar]
- Tanaka, Y.; Zeng, L.; Shiraki, H.; Shida, H.; Tozawa, H. Identification of a neutralization epitope on the envelope gp46 antigen of human T cell leukemia virus type I and induction of neutralizing antibody by peptide immunization. J. Immunol. 1991, 147, 354–360. [Google Scholar]
- Fujii, H.; Shimizu, M.; Miyagi, T.; Kunihiro, M.; Tanaka, R.; Takahashi, Y.; Tanaka, Y. A Potential of an Anti-HTLV-I gp46 Neutralizing Monoclonal Antibody (LAT-27) for Passive Immunization against Both Horizontal and Mother-to-Child Vertical Infection with Human T Cell Leukemia Virus Type-I. Viruses 2016, 8, 41. [Google Scholar] [CrossRef]
- Okuma, K.; Tatsuo, H.; Yanagi, Y.; Inagaki, Y.; Nakamura, M.; Yamamoto, N.; Matsuura, Y. Analysis of the molecules involved in human T-cell leukaemia virus type 1 entry by a vesicular stomatitis virus pseudotype bearing its envelope glycoproteins. J. Gen. Virol. 2001, 82, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Takahashi, Y.; Tanaka, R.; Kodama, A.; Fujii, H.; Hasegawa, A.; Kannagi, M.; Ansari, A.A.; Saito, M. Elimination of Human T Cell Leukemia Virus Type-1-Infected Cells by Neutralizing and Antibody-Dependent Cellular Cytotoxicity-Inducing Antibodies Against Human T Cell Leukemia Virus Type-1 Envelope gp46. AIDS Res. Hum. Retroviruses 2014, 30, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.P.; Dube, S.; Spicer, T.P.; Kane, T.D.; Love, J.L.; Saksena, N.K.; Iannone, R.; Gibbs, C.J.; Yanagihara, R.; Dube, D.K. Sequence analysis of an immunogenic and neutralizing domain of the human T-cell lymphoma/leukemia virus type I gp46 surface membrane protein among various primate T-cell lymphoma/leukemia virus isolates including those from a patient with both HTLV-I-associated myelopathy and adult T-cell leukemia. Cancer Res. 1993, 53, 6067–6073. [Google Scholar] [PubMed]
- Blanchard, S.; Astier-Gin, T.; Moynet, D.; Edouard, E.; Guillemain, B. Different HTLV-I Neutralization Patterns among Sera of Patients Infected with Cosmopolitan HTLV-I. Virology 1998, 245, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Viana, M.D.N.D.S.D.A.; Nobre, A.F.S.; Costa, E.; Silva, I.C.; Pinheiro, B.T.; Pereira, C.C.C.; Ferreira, L.D.S.C.; De Almeida, D.S.; De Araújo, M.W.L.; Borges, M.D.S.; et al. Stability of the HTLV-1 glycoprotein 46 (gp46) gene in an endemic region of the Brazilian Amazon and the presence of a significant mutation (N93D) in symptomatic patients. Virol. J. 2018, 15, 80. [Google Scholar] [CrossRef] [PubMed]
- Seiki, M.; Hattori, S.; Hirayama, Y.; Yoshida, M. Human adult T-cell leukemia virus: Complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc. Natl. Acad. Sci. 1983, 80, 3618–3622. [Google Scholar] [CrossRef]
- Moriuchi, M.; Inoue, H.; Moriuchi, H. Reciprocal Interactions between Human T-Lymphotropic Virus Type 1 and Prostaglandins: Implications for Viral Transmission. J. Virol. 2001, 75, 192–198. [Google Scholar] [CrossRef]
- Hinuma, Y.; Gotoh, Y.; Sugamura, K.; Nagata, K.; Goto, T.; Nakai, M.; Kamada, N.; Matsumoto, T.; Kinoshita, K. A retrovirus associated with human adult T-cell leukemia: In vitro activation. Gan 1982, 73, 341–344. [Google Scholar] [PubMed]
- Umadome, H.; Uchiyama, T.; Hori, T.; Tamori, S.; Motoi, T.; Araki, K.; Uchino, H. Close association between interleukin 2 receptor mRNA expression and human T cell leukemia/lymphoma virus type I viral RNA expression in short-term cultured leukemic cells from adult T cell leukemia patients. J. Clin. Investig. 1988, 81, 52–61. [Google Scholar] [CrossRef]
- Kinpara, S.; Hasegawa, A.; Utsunomiya, A.; Nishitsuji, H.; Furukawa, H.; Masuda, T.; Kannagi, M. Stromal Cell-Mediated Suppression of Human T-Cell Leukemia Virus Type 1 Expression In Vitro and In Vivo by Type I Interferon. J. Virol. 2009, 83, 5101–5108. [Google Scholar] [CrossRef] [PubMed]
- Hanon, E.; Hall, S.; Taylor, G.P.; Saito, M.; Davis, R.; Tanaka, Y.; Usuku, K.; Osame, M.; Weber, J.N.; Bangham, C.R. Abundant tax protein expression in CD4+ T cells infected with human T-cell lymphotropic virus type I (HTLV-I) is prevented by cytotoxic T lymphocytes. Blood 2000, 95, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Sakihama, S.; Saito, M.; Kuba-Miyara, M.; Tomoyose, T.; Taira, N.; Miyagi, T.; Hayashi, M.; Kinjo, S.; Nakachi, S.; Tedokon, I.; et al. Human T-cell leukemia virus type I Tax genotype analysis in Okinawa, the southernmost and remotest islands of Japan: Different distributions compared with mainland Japan and the potential value for the prognosis of aggressive adult T-cell leukemia/lymphoma. Leuk. Res. 2017, 61, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Fukunaga, T.; Igarashi, T.; Yamashita, M.; Ido, E.; Funahashi, S.; Ishida, T.; Washio, K.; Ueda, S.; Hashimoto, K. Phylogenetic subtypes of human T-lymphotropic virus type I and their relations to the anthropological background. Proc. Natl. Acad. Sci. 1994, 91, 1124–1127. [Google Scholar] [CrossRef] [PubMed]
- Hahn, B.; Shaw, G.; Taylor, M.; Redfield, R.; Markham, P.; Salahuddin, S.; Wong-Staal, F.; Gallo, R.; Parks, E.; Parks, W. Genetic variation in HTLV-III/LAV over time in patients with AIDS or at risk for AIDS. Science 1986, 232, 1548–1553. [Google Scholar] [CrossRef] [PubMed]
- Mansky, L.M. In Vivo Analysis of Human T-Cell Leukemia Virus Type 1 Reverse Transcription Accuracy. J. Virol. 2000, 74, 9525–9531. [Google Scholar] [CrossRef] [PubMed]
- Kim, F.J.; Lavanya, M.; Gessain, A.; Gallego, S.; Battini, J.-L.; Sitbon, M.; Courgnaud, V. Intrahost variations in the envelope receptor-binding domain (RBD) of HTLV-1 and STLV-1 primary isolates. Retrovirology 2006, 3, 29. [Google Scholar] [CrossRef]
- Pique, C.; Tursz, T.; Dokhelar, M.C. Mutations introduced along the HTLV-I envelope gene result in a non-functional protein: A basis for envelope conservation? EMBO J. 1990, 9, 4243–4248. [Google Scholar] [CrossRef]
- Shimoyama, M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984-87). Br. J. Haematol. 1991, 79, 428–437. [Google Scholar] [CrossRef]
- Lee, B.; Tanaka, Y.; Tozawa, H. Monoclonal antibody defining Tax1 protein of human T-cell leukemia virus type-I. Tohoku J. Exp. Med. 1989, 157, 1–11. [Google Scholar] [CrossRef]
- Kunihiro, M.; Fujii, H.; Miyagi, T.; Takahashi, Y.; Tanaka, R.; Fukushima, T.; Ansari, A.A.; Tanaka, Y. Heat Shock Enhances the Expression of the Human T Cell Leukemia Virus Type-I (HTLV-I) Trans-Activator (Tax) Antigen in Human HTLV-I Infected Primary and Cultured T Cells. Viruses 2016, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Mizuguchi, M.; Takahashi, Y.; Fujii, H.; Tanaka, R.; Fukushima, T.; Tomoyose, T.; A Ansari, A.; Nakamura, M. Human T-cell leukemia virus type-I Tax induces the expression of CD83 on T cells. Retrovirology 2015, 12, 56. [Google Scholar] [CrossRef] [PubMed]
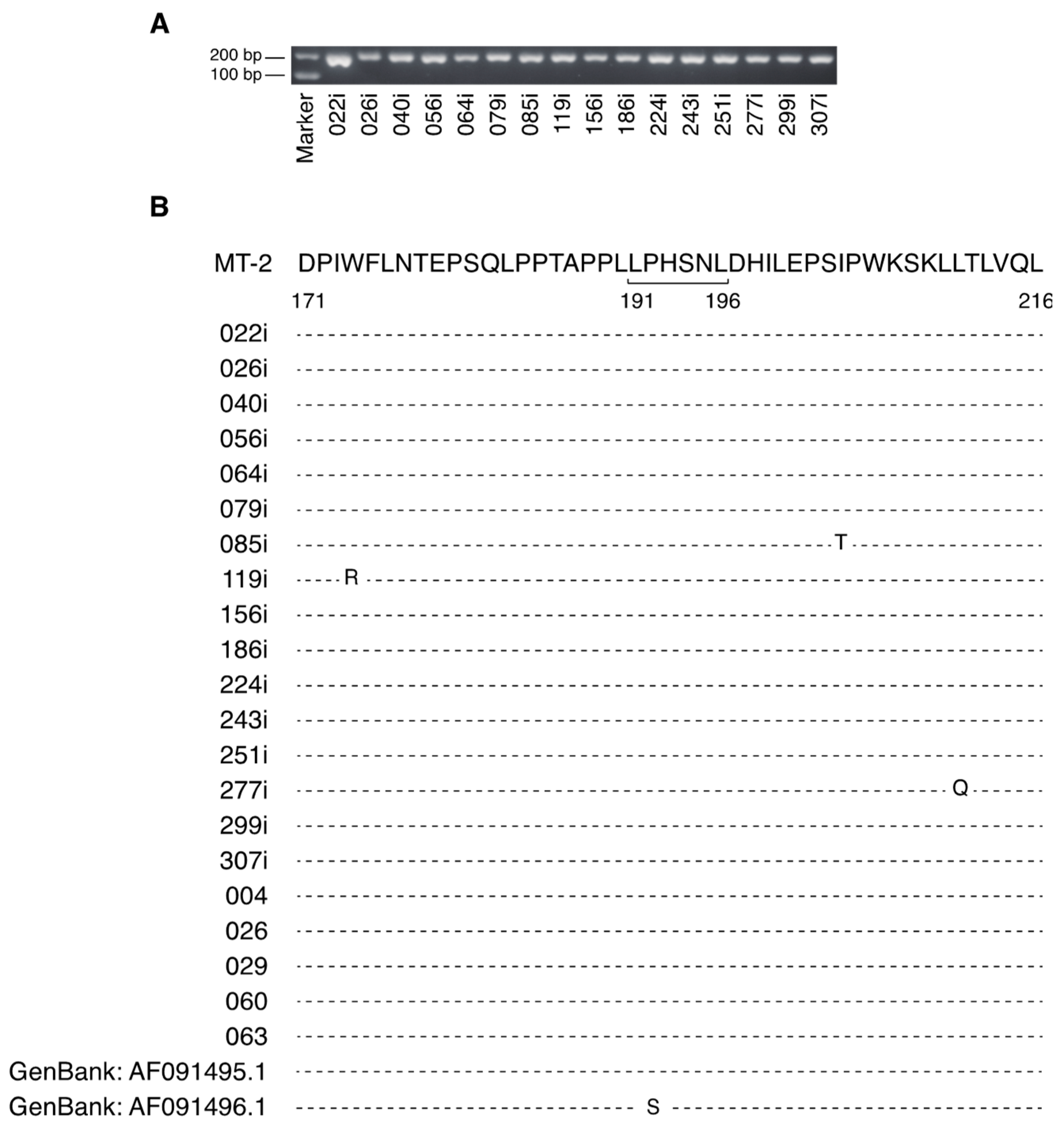
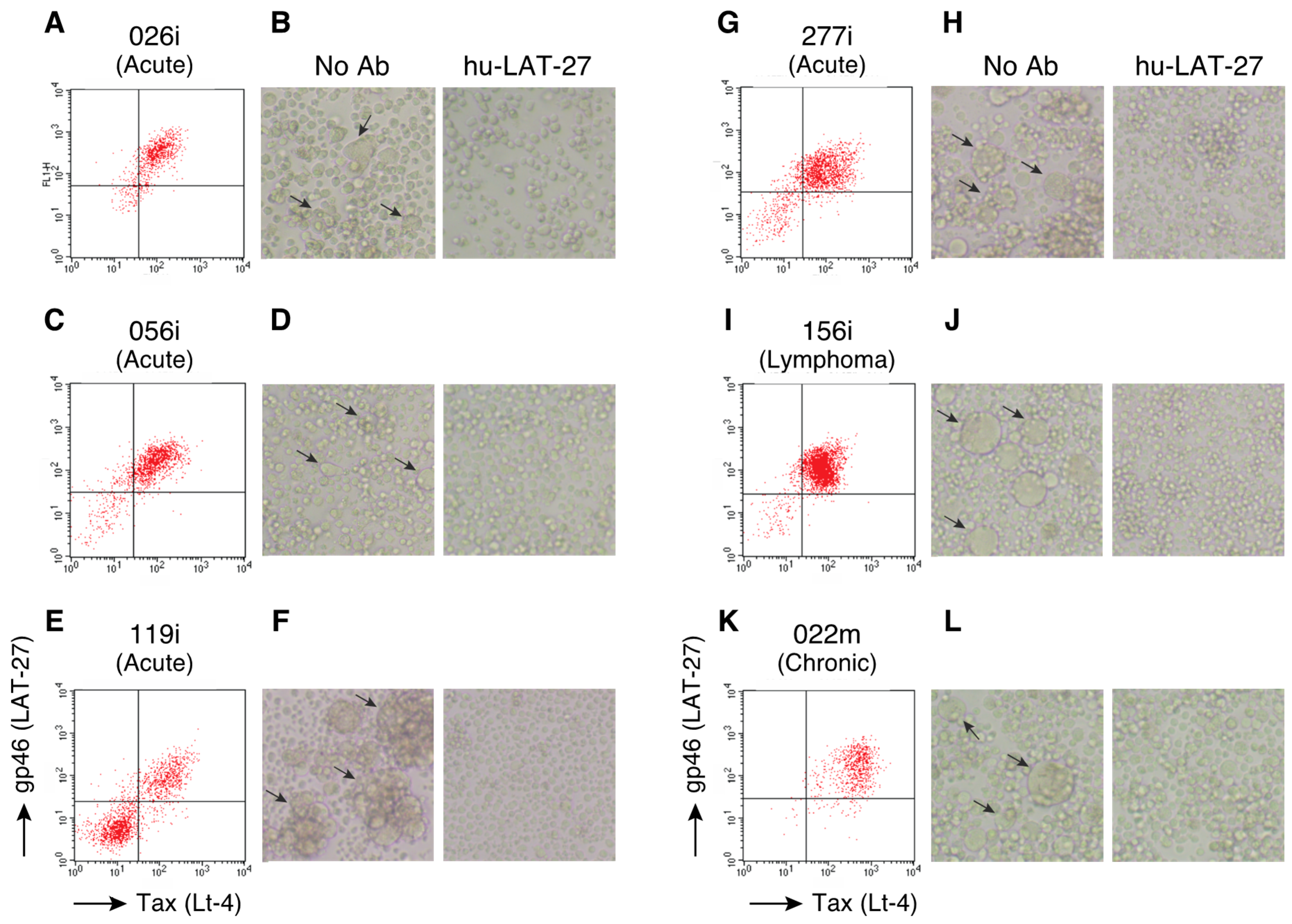
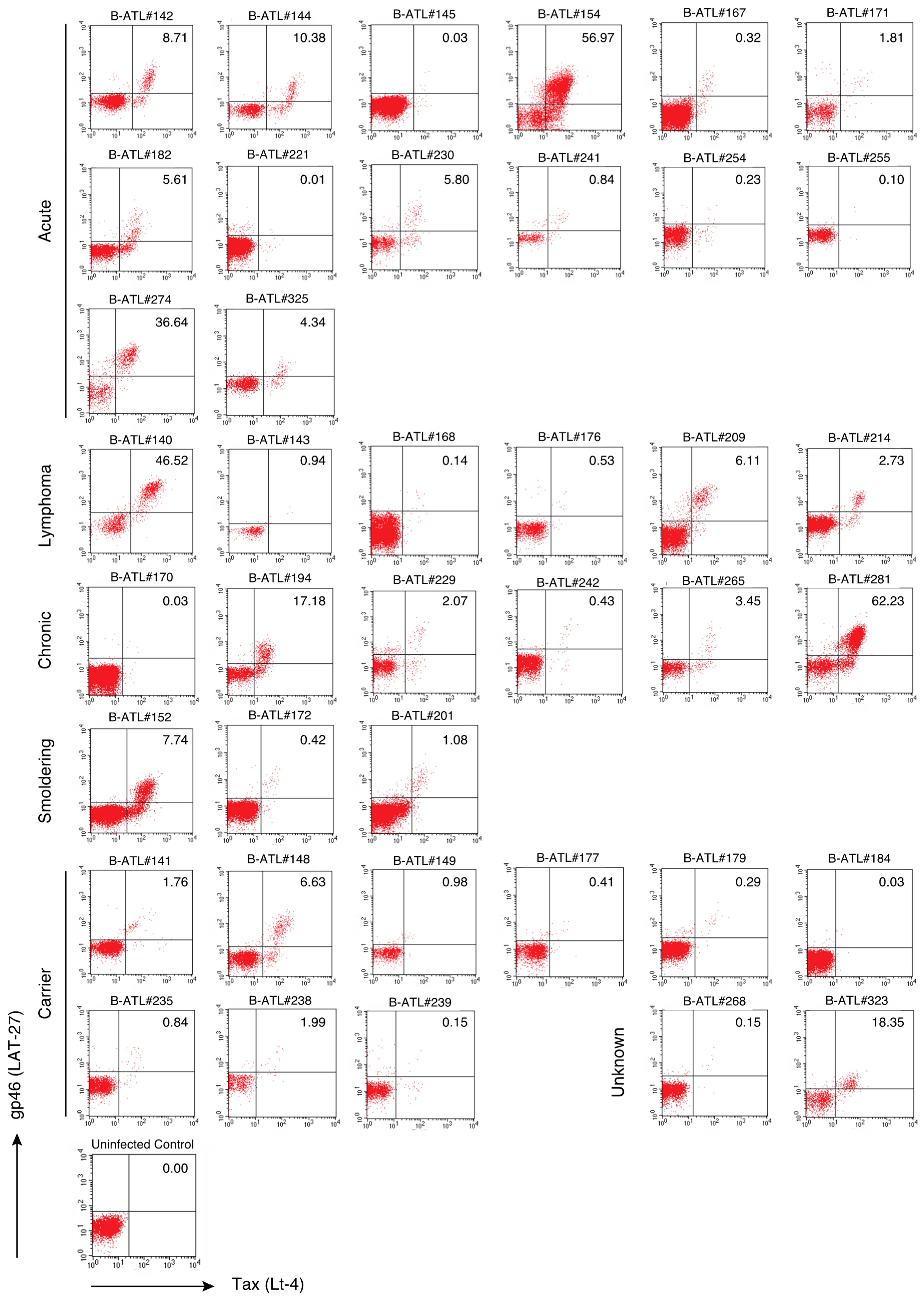
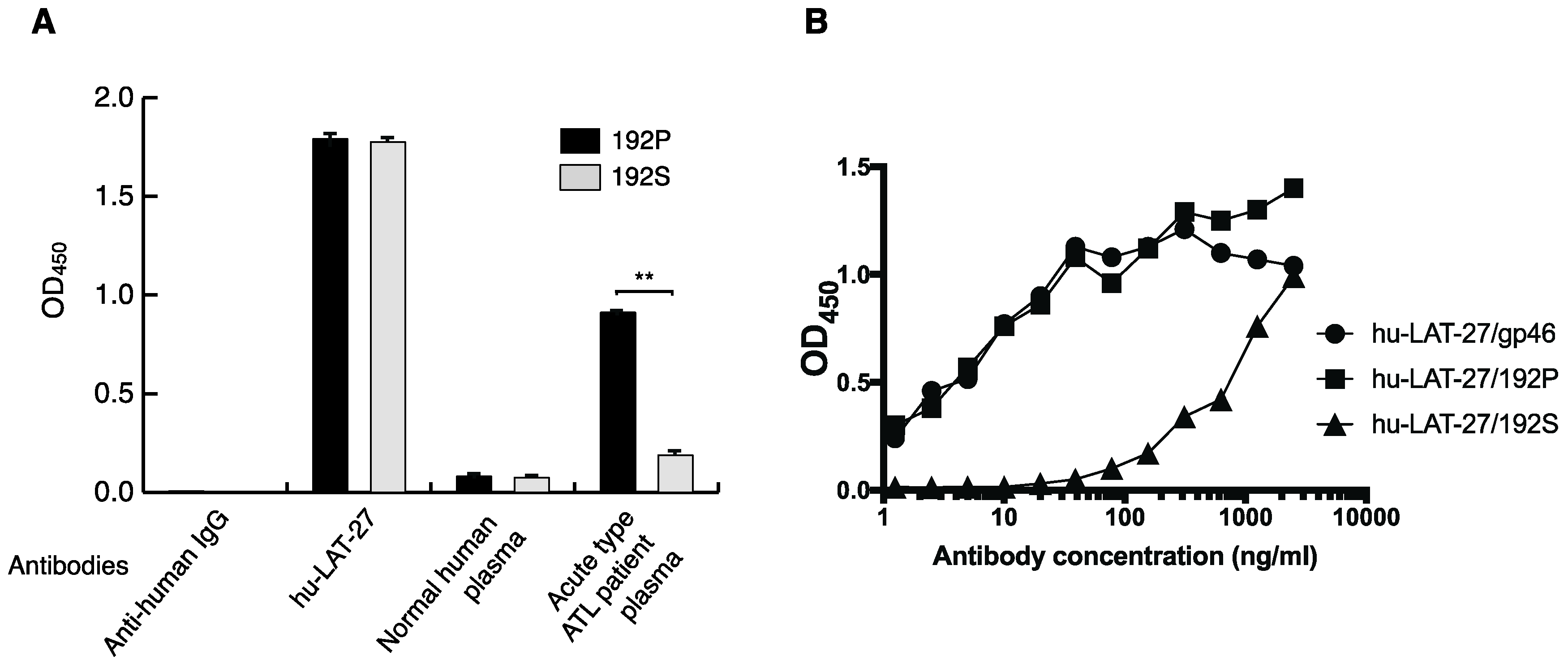
| Bank Number | Cell Line | Disease Status | Age | Sex | |
|---|---|---|---|---|---|
| 1 | B-ATL#022 | 022i | Chronic | 46 | M |
| 2 | B-ATL#026 | 026i | Acute | 67 | M |
| 3 | B-ATL#040 | 040i | Acute | 37 | M |
| 4 | B-ATL#056 | 056i | Acute | 63 | M |
| 5 | B-ATL#064 | 064i | Smoldering | 51 | F |
| 6 | B-ATL#079 | 079i | Chronic | 64 | M |
| 7 | B-ATL#085 | 085i | Acute | 53 | F |
| 8 | B-ATL#119 | 119i | Acute | 63 | F |
| 9 | B-ATL#156 | 156i | Lymphoma | 77 | M |
| 10 | B-ATL#186 | 186i | Chronic or Acute | 54 | F |
| 11 | B-ATL#224 | 224i | Acute | 80 | M |
| 12 | B-ATL#243 | 243i | Acute | 82 | M |
| 13 | B-ATL#251 | 251i | Acute | 64 | F |
| 14 | B-ATL#277 | 277i | Acute | 73 | F |
| 15 | B-ATL#299 | 299i | Acute | 75 | M |
| 16 | B-ATL#307 | 307i | Unknown | 64 | F |
| 17 | B-ATL#004 | Acute | 44 | M | |
| 18 | B-ATL#029 | Acute | 64 | F | |
| 19 | B-ATL#060 | Acute | 83 | F | |
| 20 | B-ATL#063 | Acute | 74 | F |
| Bank Number | Disease Status | Age | Sex | Bank Number | Disease Status | Age | Sex | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 | B-ATL#140 | Lymphoma | 86 | M | 21 | B-ATL#194 | Chronic | 73 | M |
| 2 | B-ATL#141 | Carrier | 67 | M | 22 | B-ATL#201 | Smoldering or Pre-ATL | 24 | M |
| 3 | B-ATL#142 | Acute | 67 | M | 23 | B-ATL#209 | Lymphoma | 66 | F |
| 4 | B-ATL#143 | Lymphoma | 43 | M | 24 | B-ATL#214 | Lymphoma | 68 | F |
| 5 | B-ATL#144 | Acute | 66 | M | 25 | B-ATL#221 | Acute | 72 | F |
| 6 | B-ATL#145 | Acute | 64 | F | 26 | B-ATL#229 | Chronic | 69 | M |
| 7 | B-ATL#148 | Carrier | 62 | F | 27 | B-ATL#230 | Acute | 83 | F |
| 8 | B-ATL#149 | Carrier | 66 | M | 28 | B-ATL#235 | Carrier or Pre-ATL | 67 | M |
| 9 | B-ATL#152 | Carrier or Smoldering | 63 | F | 29 | B-ATL#238 | Carrier | 65 | F |
| 10 | B-ATL#154 | Acute | 71 | M | 30 | B-ATL#239 | Carrier | 74 | F |
| 11 | B-ATL#167 | Acute | 70 | M | 31 | B-ATL#241 | Acute | 62 | M |
| 12 | B-ATL#168 | Lymphoma | 68 | M | 32 | B-ATL#242 | Chronic | 75 | F |
| 13 | B-ATL#170 | Chronic | 60 | M | 33 | B-ATL#254 | Acute | 82 | M |
| 14 | B-ATL#171 | Acute | 71 | F | 34 | B-ATL#255 | Acute | 82 | F |
| 15 | B-ATL#172 | Chronic or Smoldering | 74 | M | 35 | B-ATL#265 | Chronic | 61 | M |
| 16 | B-ATL#176 | Lymphoma | 64 | M | 36 | B-ATL#268 | Unknown | 79 | F |
| 17 | B-ATL#177 | Carrier | 56 | F | 37 | B-ATL#274 | Acute | 62 | M |
| 18 | B-ATL#179 | Carrier | 61 | F | 38 | B-ATL#281 | Chronic | 84 | F |
| 19 | B-ATL#182 | Acute | 66 | M | 39 | B-ATL#323 | Unknown | 77 | F |
| 20 | B-ATL#184 | Carrier | 38 | F | 40 | B-ATL#325 | Acute | 71 | M |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizuguchi, M.; Takahashi, Y.; Tanaka, R.; Fukushima, T.; Tanaka, Y. Conservation of a Neutralization Epitope of Human T-cell Leukemia Virus Type 1 (HTLV-1) among Currently Endemic Clinical Isolates in Okinawa, Japan. Pathogens 2020, 9, 82. https://doi.org/10.3390/pathogens9020082
Mizuguchi M, Takahashi Y, Tanaka R, Fukushima T, Tanaka Y. Conservation of a Neutralization Epitope of Human T-cell Leukemia Virus Type 1 (HTLV-1) among Currently Endemic Clinical Isolates in Okinawa, Japan. Pathogens. 2020; 9(2):82. https://doi.org/10.3390/pathogens9020082
Chicago/Turabian StyleMizuguchi, Mariko, Yoshiaki Takahashi, Reiko Tanaka, Takuya Fukushima, and Yuetsu Tanaka. 2020. "Conservation of a Neutralization Epitope of Human T-cell Leukemia Virus Type 1 (HTLV-1) among Currently Endemic Clinical Isolates in Okinawa, Japan" Pathogens 9, no. 2: 82. https://doi.org/10.3390/pathogens9020082
APA StyleMizuguchi, M., Takahashi, Y., Tanaka, R., Fukushima, T., & Tanaka, Y. (2020). Conservation of a Neutralization Epitope of Human T-cell Leukemia Virus Type 1 (HTLV-1) among Currently Endemic Clinical Isolates in Okinawa, Japan. Pathogens, 9(2), 82. https://doi.org/10.3390/pathogens9020082




