Bartonella Associated Cutaneous Lesions (BACL) in People with Neuropsychiatric Symptoms
Abstract
1. Introduction
2. Results
2.1. Study Design and Subject Enrollment
2.2. Reported Neuropsychiatric Symptoms
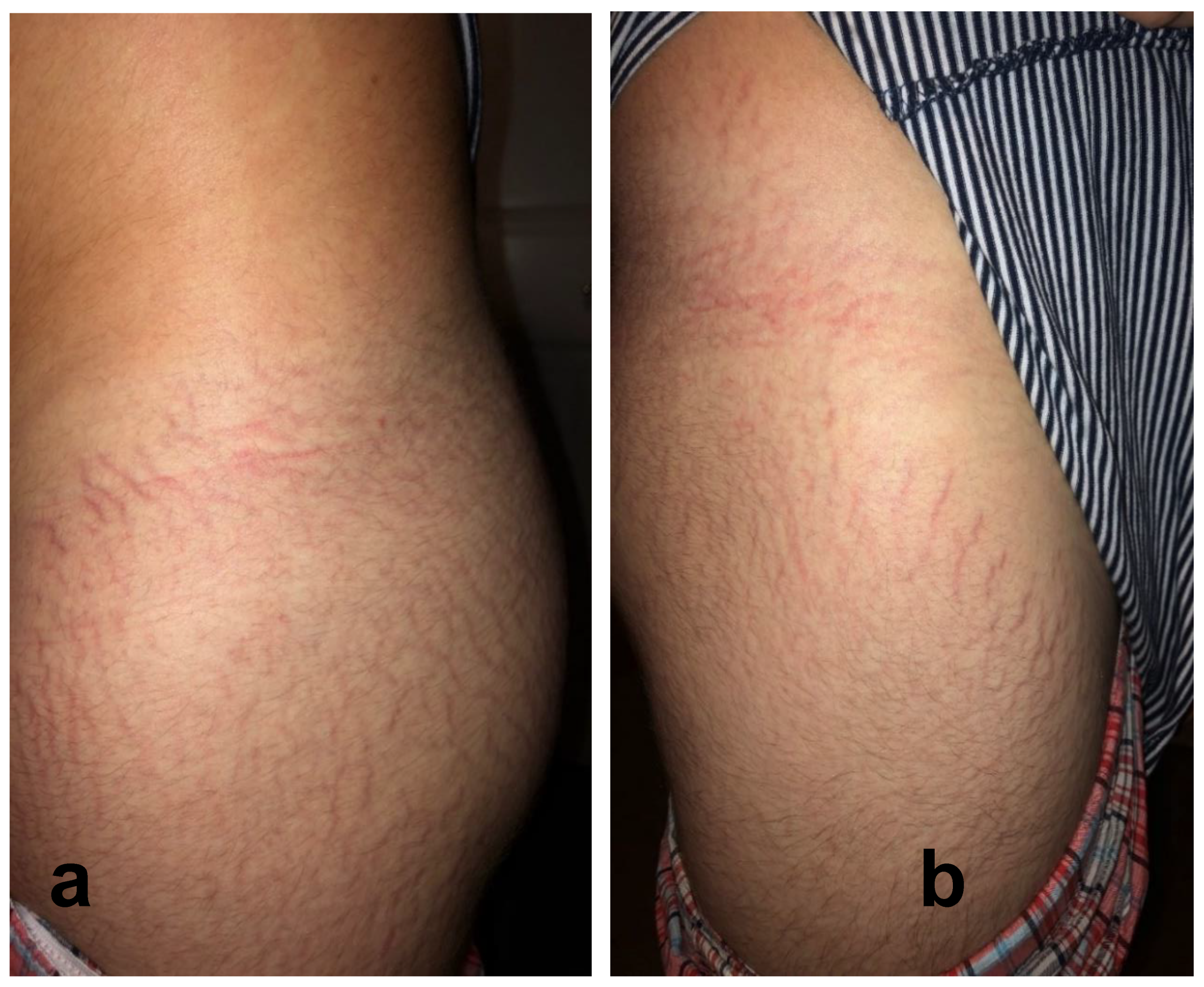
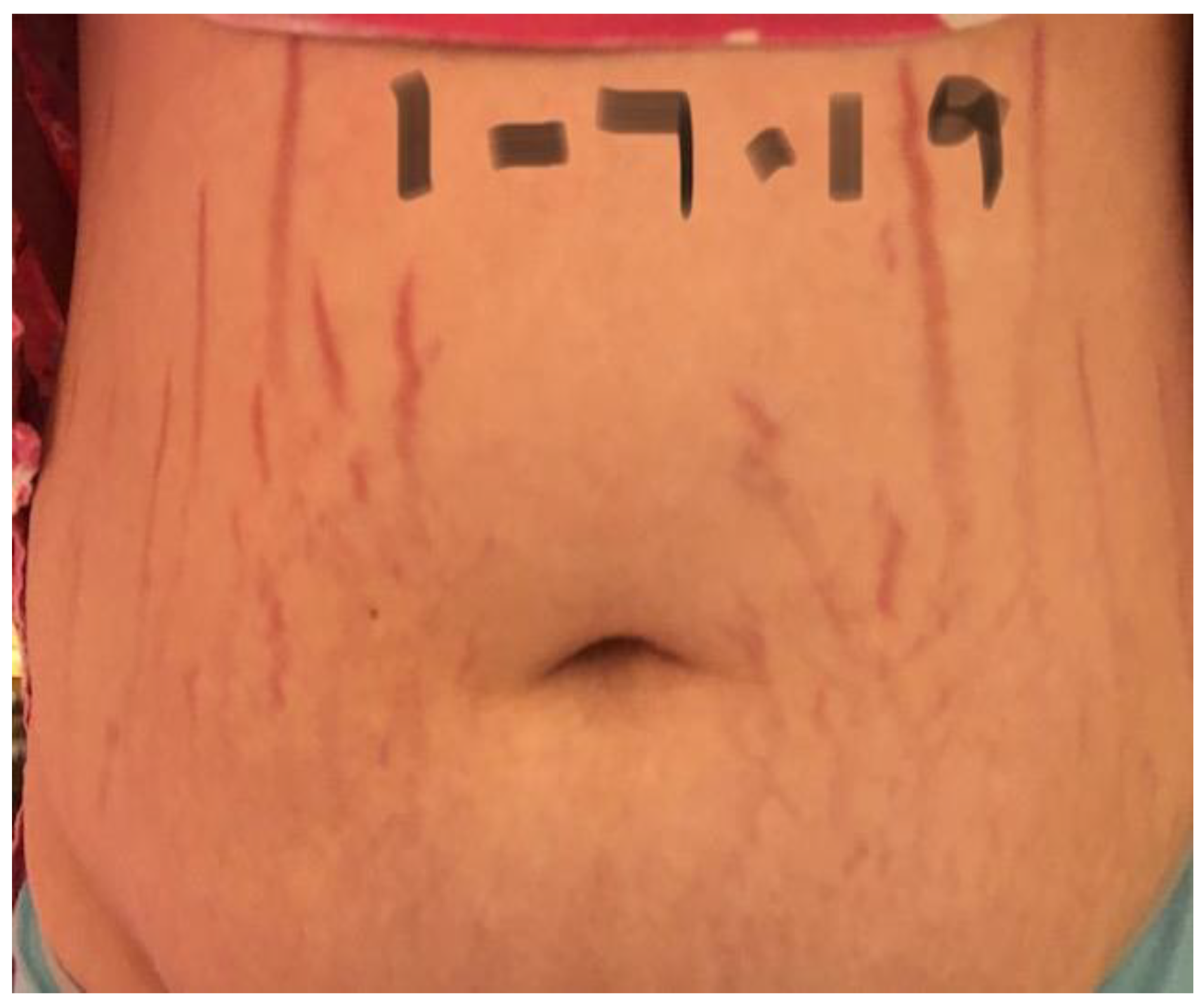
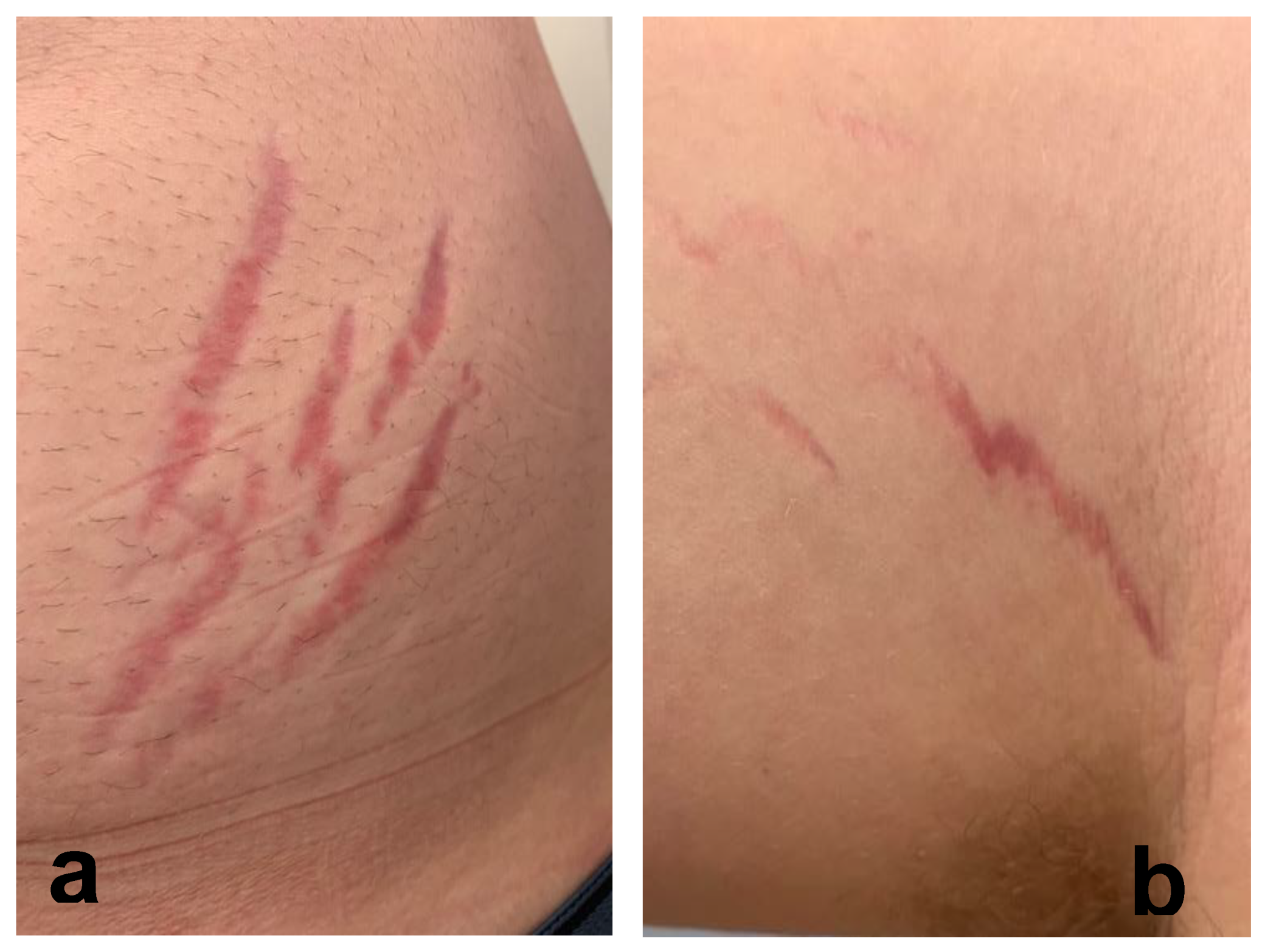
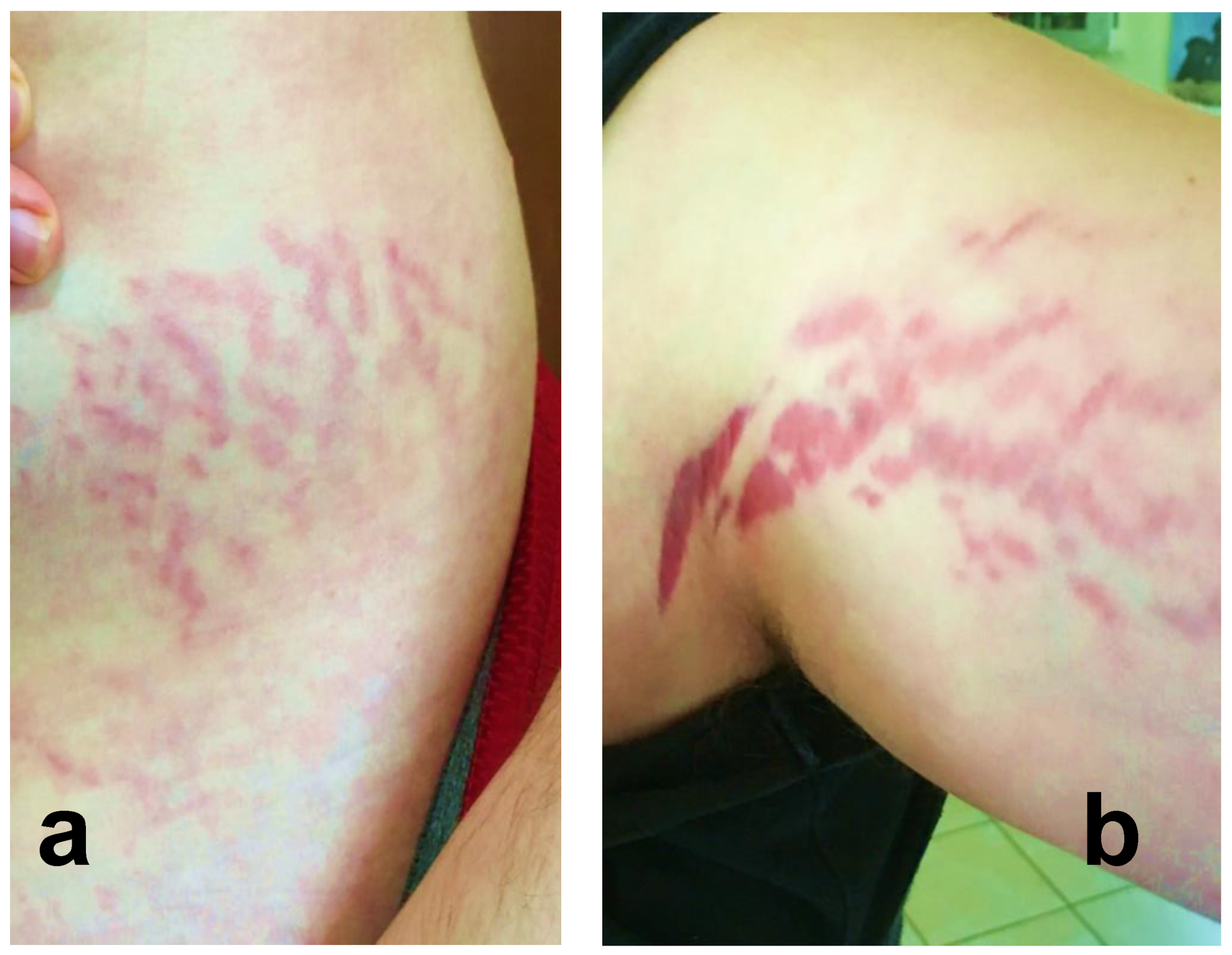
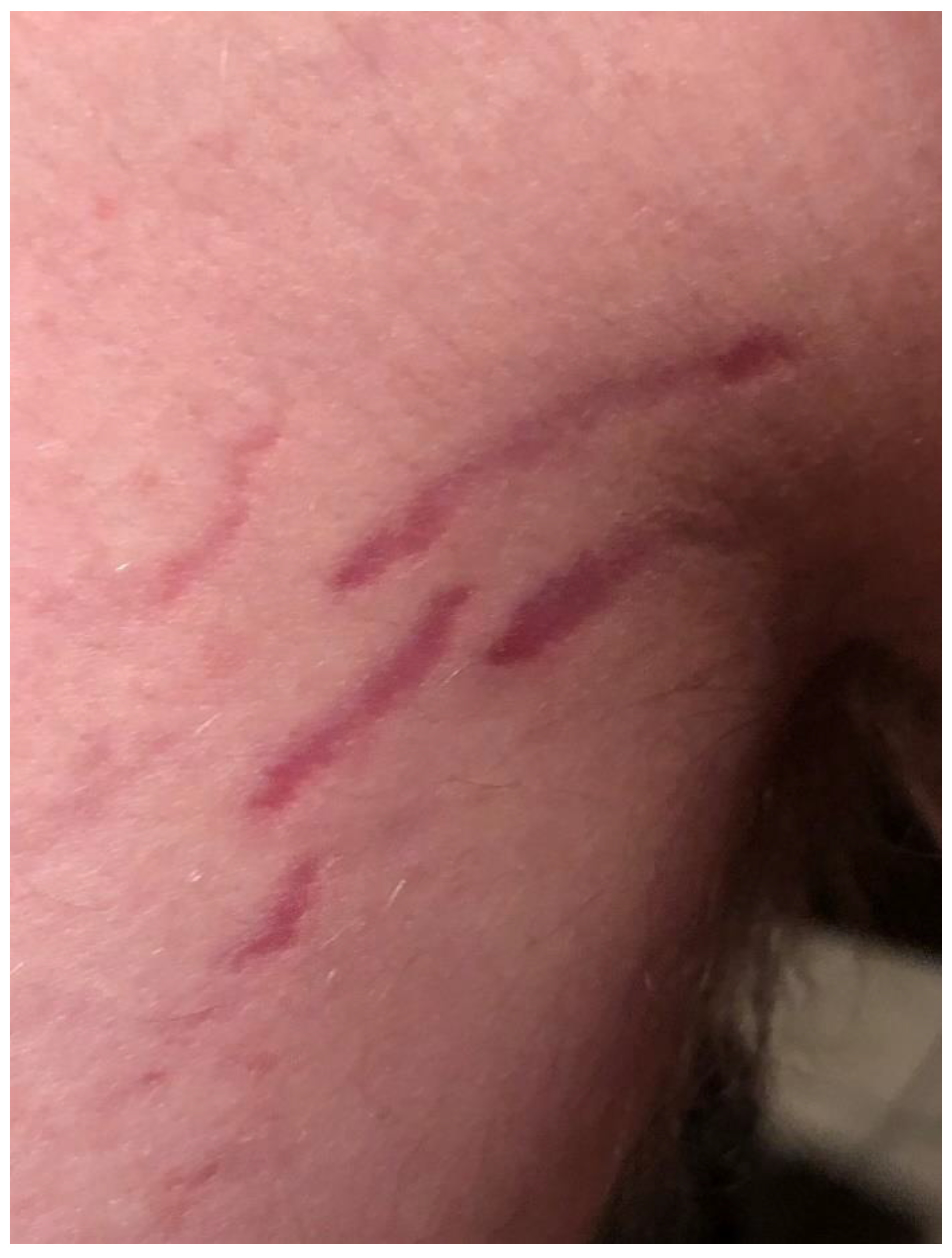
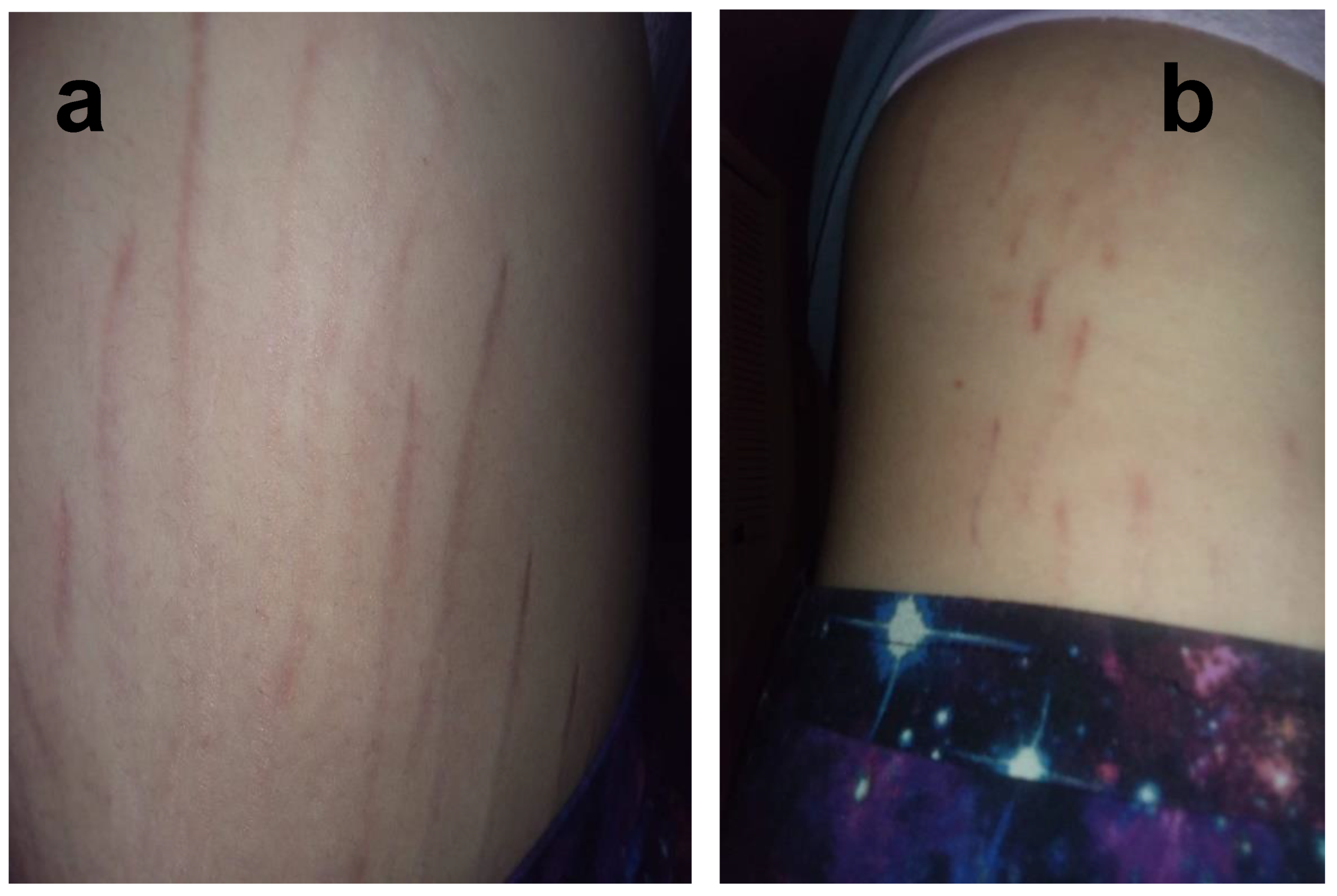
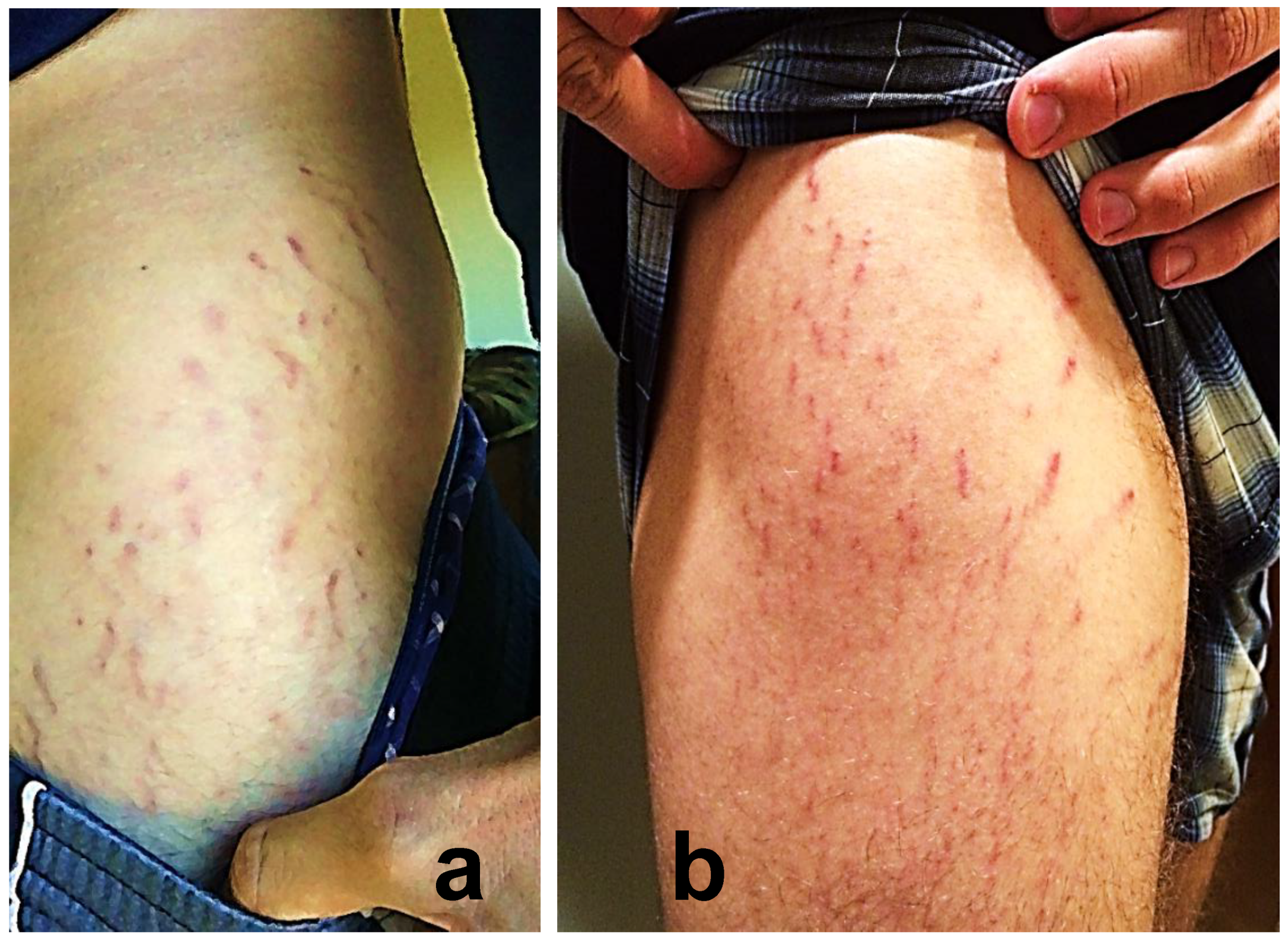
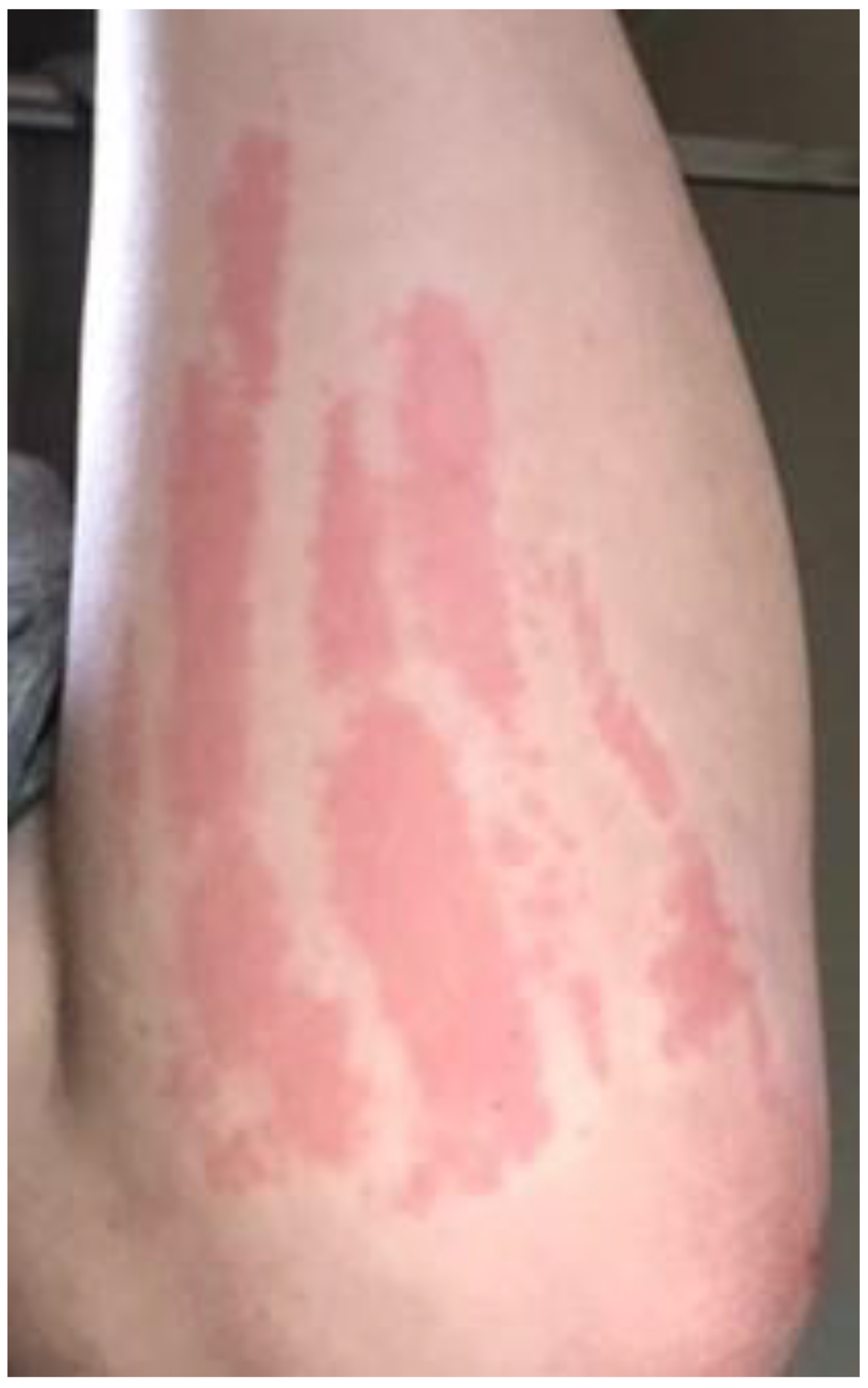
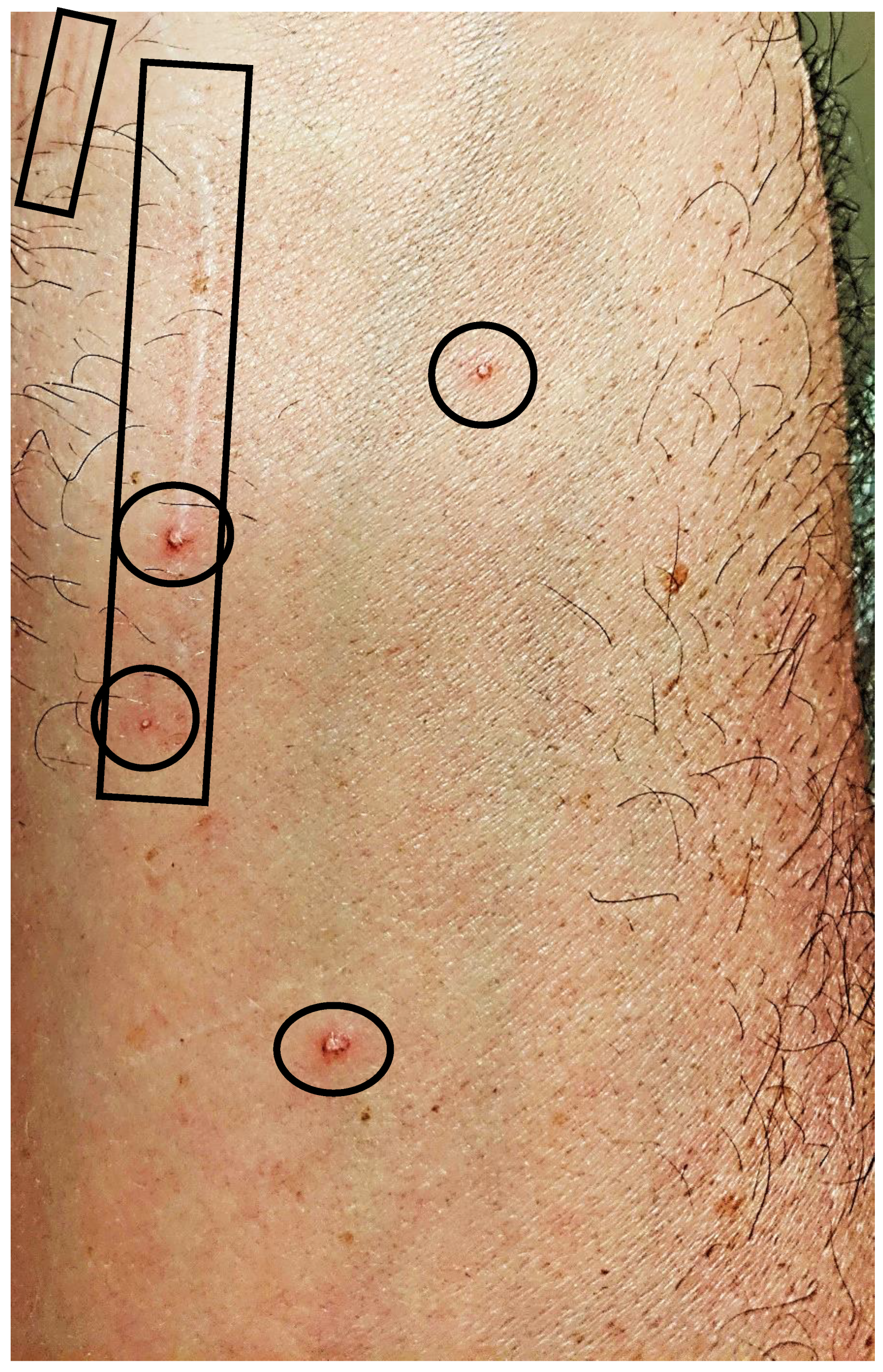
2.3. Reported Cutaneous Lesions
2.4. Bartonella spp. Seroprevalence and Blood Stream Infection
2.5. ddPCR Results on Direct Blood Testing versus BAPGM Enrichment Blood Culture
2.6. Animal and Vector Exposures
3. Discussion
4. Materials and Methods
4.1. Study Enrollment
4.2. Questionnaire Data and Blood Specimen Collection
4.3. Bartonella IFA Serological Testing
4.4. Bartonella Enrichment Blood Culture/PCR Testing
4.5. Statistics
4.6. Ethics Approval and Consent to Participate
Author Contributions
Funding
Conflicts of Interest
Availability of Data and Materials
References
- Regier, Y.; Rourke, F.O.; Kempf, V.A. Bartonella spp.—A chance to establish one health concepts in veterinary and human medicine. Parasit Vectors 2016, 9, 261. [Google Scholar] [CrossRef]
- Cheslock, M.A.; Embers, M.E. Human bartonellosis: An underappreciated public health problem? Trop. Med. Infect. Dis. 2019, 19, 2. [Google Scholar] [CrossRef]
- Landau, M.; Kletter, Y.; Avidor, B.; Ephrat, G.; Ephros, M.; Brenner, S.; Giladi, M. Unusual eruption as a presenting symptom of cat scratch disease. J. Am. Acad. Dermatol. 1999, 41, 833–836. [Google Scholar] [CrossRef]
- Álvarez-Fernández, A.; Breitschwerdt, E.B.; Solano-Gallego, L. Bartonella infections in cats and dogs including zoonotic aspects. Parasit Vectors 2018, 11, 624. [Google Scholar] [CrossRef] [PubMed]
- Fried, M.D. Abstract Bartonella henselae is associated with heartburn, abdominal pain, skin rash, mesenteric adenitis, gastritis and duodenitis. In Proceedings of the North American Society of Pediatric Gastroenterology, Hepatology, and Nutrition Annual Meeting, San Antonio, TX, USA, 24–27 October 2002. [Google Scholar]
- Schaller, J.L. The Diagnosis Treatment and Prevention of Bartonella; Hope Academic Press: Tampa, FL, USA, 2008. [Google Scholar]
- Maggi, R.G.; Ericson, M.; Mascarelli, P.E.; Bradley, J.M.; Breitschwerdt, E.B. Bartonella henselae bacteremia in a mother and son exposed to ticks in The Netherlands. Parasites Vectors 2013, 6, 101. [Google Scholar] [CrossRef] [PubMed]
- Breitschwerdt, E.B.; Greenberg, R.; Maggi, R.G.; Lewis, A.; Bradley, J.M. Bartonella henselae infection in a boy with Pediatric Acute-onset Neuropsychiatric Syndrome (PANS). J. Nerv. Syst. Dis. 2019, 11, 1–8. [Google Scholar]
- Azar, M.; Breitschwerdt, E.; Bemis, L.; Greenberg, R.; Mozayeni, R.; Dencklau, J.; Ericson, M. Imaging analysis of Bartonella species in the skin using single-photon and multi-photon (second harmonic generation) laser scanning microscopy. Clin. Case Rep. 2020, 1–7. [Google Scholar] [CrossRef]
- Boozalis, E.; Grossberg, A.L.; Puttgen, K.B.; Heath, C.R.; Cohen, B.A. Demographic characteristics of teenage boys with horizontal striae distensae of the lower back. Pediatr. Dermatol. 2018, 35, 59–63. [Google Scholar] [CrossRef]
- Breitschwerdt, E.B.; Maggi, R.G.; Quach, C.; Bradley, J.M. Bartonella spp. bloodstream infection in a canadian family. Vector Borne Zoonotic Dis. 2019, 19, 234–241. [Google Scholar] [CrossRef]
- Maggi, R.G.; Richardson, T.; Breitschwerdt, E.B.; Miller, J.C. Development and validation of a droplet digital PCR assay for the detection and quantification of Bartonella species in human clinical samples. J. Microbiol. Methods 2020, 176, e106022. [Google Scholar] [CrossRef]
- Maritsi, D.N.; Zarganis, D.; Metaxa, Z.; Papaioannou, G.; Vartzelis, G. Bartonella henselae infection: An uncommon mimicker of autoimmune disease. Case Rep. Pediatr. 2013, 2013, 726826. [Google Scholar] [CrossRef] [PubMed]
- Im, J.H.; Kwon, H.Y.; Baek, J.; Durey, A.; Lee, S.M.; Park, Y.K.; Kang, J.S.; Chung, M.H.; Lee, J.S. Serologic study of Bartonella henselae in patients with acute undifferentiated febrile illness in Korea. Vector Borne Zoonotic Dis. 2018, 18, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Safdar, A.; McEvoy, P.L.; Burns, R.G.; Perfect, J.R. Clinical microbiological case: Severe relapsing septal panniculitis in a healthy man from the southeastern USA. Clin. Microbiol. Infect. 2002, 8, 801–802. [Google Scholar] [CrossRef] [PubMed]
- Schmoor, P.; Darie, H.; Maccari, F.; Gros, P.; Millet, P. Cutaneous vasculitis disclosing cat-scratch disease. Ann. Dermatol. Venereol. 1998, 125, 894–896. [Google Scholar] [PubMed]
- Cozzani, E.; Cinotti, E.; Ameri, P.; Sofia, A.; Murialdo, G.; Parodi, A. Onset of cutaneous vasculitis and exacerbation of IgA nephropathy after Bartonella henselae infection. Clin. Dermatol. 2011, 37, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Lashnits, E.; Correa, M.; Hegarty, B.C.; Birkenheuer, A.; Breitschwerdt, E.B. Bartonella seroepidemiology in dogs from North America, 2008–2014. J. Vet. Intern. Med. 2018, 32, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Lashnits, E.W.; Dawson, D.E.; Breitschwerdt, E.; Lanzas, C. Ecological and socioeconomic factors associated with Bartonella henselae exposure in dogs tested for vector-borne diseases in North Carolina. Vector Borne Zoonotic Dis. 2019, 19, 582–595. [Google Scholar] [CrossRef]
- Breitschwerdt, E.B.; Linder, K.L.; Day, M.J.; Maggi, R.G.; Chomel, B.B.; Kempf, V.A.J. Koch’s postulates and the pathogenesis of comparative infectious disease causation associated with Bartonella species. J. Comp. Pathol. 2013, 1, 1–11. [Google Scholar] [CrossRef]
- Rossi, M.A.; Balakrishnan, N.; Linder, K.E.; Messa, J.B.; Breitschwerdt, E.B. Concurrent Bartonella henselae infection in a dog with panniculitis and owner with ulcerated nodular skin lesions. Vet. Dermatol. 2015, 26, 60–63. [Google Scholar] [CrossRef]
- Southern, B.L.; Neupane, P.; Ericson, M.E.; Dencklau, J.C.; Linder, K.E.; Bradley, J.M.; McKeon, G.P.; Long, C.T.; Breitschwerdt, E.B. Bartonella henselae in a dog with ear tip vasculitis. Vet. Dermatol. 2018, 29, 537-e180. [Google Scholar] [CrossRef]
- Breitschwerdt, E.B. Bartonellosis: One health perspectives for an emerging infectious disease. ILAR J. 2014, 55, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Mosbacher, M.E.; Klotz, S.; Klotz, J.; Pinnas, J.L. Bartonella henselae and the potential for arthropod vector borne transmission. Vector Borne Zoonotic Dis. 2011, 11, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Wormser, G.P.; Brady, K.C.; Cho, M.S.; Scavarda, C.A.; McKenna, D. Efficacy of a 14-day course of amoxicillin for patients with erythema migrans. Diagn. Microbiol. Infect. Dis. 2019, 94, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Muzumdar, S.; Rothe, M.J.; Grant-Kels, J.M. The rash with maculopapules and fever in adults. Clin. Dermatol. 2019, 37, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Maraspin, V.; Mrvič, T.; Ružić-Sabljić, E.; Jurčić, V.; Strle, F. Acrodermatitis chronica atrophicans in children: Report on two cases and review of the literature. Ticks Tick Borne Dis. 2019, 10, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Maggi, R.G.; Mascarelli, P.E.; Pultorak, E.L.; Hegarty, B.C.; Bradley, J.M.; Mozayeni, R.B.; Breitschwerdt, E.B. Bartonella spp. bacteremia in high-risk immunocompetent patients. Diagn. Microbiol. Infect. Dis. 2011, 71, 430–437. [Google Scholar] [CrossRef]
- Diniz, P.P.; Velho, P.E.; Pitassi, L.H.; Drummond, M.R.; Lania, B.G.; Barjas-Castro, M.L.; Sowy, S.; Breitschwerdt, E.B.; Scorpio, D.G. Risk factors for Bartonella species infection in blood donors from Southeast Brazil. PLoS Negl. Trop. Dis. 2016, 10, e0004509. [Google Scholar] [CrossRef]
- Oteo, J.A.; Maggi, R.; Portillo, A.; Bradley, J.; García-Álvarez, L.; San-Martín, M.; Roura, X.; Breitschwerdt, E. Prevalence of Bartonella spp. by culture, PCR and serology, in veterinary personnel from Spain. Parasit Vectors 2017, 10, 553. [Google Scholar] [CrossRef]
- Vaca, D.J.; Thibau, A.; Schutz, M.; Kraiczy, P.; Happonen, L.; Malmstrom, J.; Kempf, V.A.J. Interaction with the host: The role of fibronectin and extracellular matrix proteins in the adhesion of Gram-negative bacteria. Med. Microbiol. Immunol. 2020, 209, 277–299. [Google Scholar] [CrossRef]
- Da Silva, E.Z.M.; Jamur, M.C.; Oliver, C. Mast cell function: A new vision of an old cell. J. Histochem. Cytochem. 2014, 62, 698–738. [Google Scholar] [CrossRef]
- Pultorak, E.L.; Maggi, R.G.; Mascarelli, P.E.; Breitschwerdt, E.B. Serial testing from a three-day collection period using the BAPGM platform may enhance the sensitivity of Bartonella spp. detection in bacteremic human patients. J. Clin. Microbiol 2013, 57, 1673–1677. [Google Scholar] [CrossRef] [PubMed]
- Lantos, P.M.; Maggi, R.G.; Ferguson, B.; Varkey, J.; Park, L.P.; Breitschwerdt, E.B.; Woods, C.W. Detection of Bartonella species in the blood of veterinarians and veterinary technicians: A newly recognized occupational hazard. Vector Borne Zoonotic Dis. 2015, 14, 563–570. [Google Scholar] [CrossRef] [PubMed]
| Neuropsychological Symptoms | Participants Reporting | Non-Neuropsychological Symptoms | Participants Reporting |
|---|---|---|---|
| Sleep Disorders ^ | 27 | Fatigue ^ | 26 |
| Mental Confusion ^ | 24 | Ocular Involvement | 20 |
| Irritability/Rage ^ | 23 | Eye Pain ^ | 15 |
| Anxiety/Panic Attacks ^ | 22 | Blurred Vision ^ | 15 |
| Depression ^ | 21 | Light Sensitivity * | 4 |
| Headache/Migraine ^ | 21 | Floaters/Flashes * | 2 |
| Tremors ^ | 12 | Joint/Back/Neck Pain ^ | 20 |
| Hallucinations ^ | 7 | GI Issues * | 17 |
| Institutionalized (Psych) * | 2 | Bowel Dysfunction ^ | 11 |
| Postural Orthostatic Tachycardia (POTS) * | 5 | Diarrhea ^ | 8 |
| Seizures ^ | 4 | Abdominal Pain * | 3 |
| Schizophrenia * | 2 | Vomiting/Nausea ^ | 4 |
| Suicidal * | 2 | IBS * | 1 |
| Tourettes * | 2 | Bloating * | 1 |
| Paralysis ^ | 2 | Muscle Pain ^ | 15 |
| Mast Cell Activation Syndrome * | 2 | Respiratory ^ | 15 |
| MS-Like diagnosis * | 1 | Muscle Weakness ^ | 14 |
| Sudden Onset OCD * | 1 | Numbness ^ | 13 |
| PANS * | 1 | Tachycardia (Not POTS) ^ | 10 |
| Psychosis * | 1 | Tendonitis ^ | 8 |
| Stuttering * | 1 | Syncope ^ | 6 |
| Dental Issues ^ | 6 | ||
| Jaw Pain * | 5 | ||
| Gingival Recession * | 2 | ||
| Mouth Ulcers * | 1 | ||
| Fever ^ of Unknown Origin * | 5 | ||
| Ehrlos Danlos Syndrome * | 3 | ||
| Cardiac (not reported elsewhere) * | 2 |
| Reciprocal IFA Antibody Titers to: | Direct Blood PCR | BAPGM Enrichment Blood Culture/PCR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study No: | Bartonella vinsonii berkhoffii Type I | Bartonella vinsonii berkhoffii Type II | Bartonella vinsonii berkhoffii Type III | Bartonella henselae San Antonio 2 | Bartonella koehlerae | Bartonella quintana | qPCR * | ddPCR | qPCR * | ddPCR |
| 1 | 32 | 64 | 32 | 32 | 32 | 32 | Neg | Neg | Neg | Neg |
| 2 | 32 | 32 | 64 | 32 | 64 | 128 | Neg | Pos | Neg | Pos |
| 3 | 32 | 32 | 32 | 64 | 32 | 16 | Neg | Neg | Neg | Pos |
| 4 | <16 | 32 | 32 | 64 | 16 | <16 | Neg | Neg | Neg | Neg |
| 5 | 256 | 64 | 32 | 128 | 32 | 16 | Neg | Neg | Neg | Pos |
| 6 | 128 | 128 | 64 | 64 | 128 | 128 | Neg | Neg | Neg | Pos |
| 7 | <16 | 64 | 64 | 64 | <16 | 64 | Neg | Neg | Neg | Neg |
| 8 | 16 | <16 | <16 | <16 | <16 | <16 | Neg | Neg | Neg | Pos |
| 9 | <16 | <16 | 16 | <16 | <16 | 16 | Neg | Pos | Neg | Pos |
| 10 | <16 | <16 | <16 | 16 | <16 | <16 | Neg | Neg | Neg | Pos |
| 11 | <16 | 64 | 64 | 256 | 64 | 32 | Neg | Pos | Neg | Pos |
| 12 | <16 | 16 | 32 | 64 | <16 | 32 | Neg | Neg | Neg | Pos |
| 13 | <16 | <16 | <16 | 32 | <16 | <16 | Neg | Neg | Neg | Pos |
| 14 | <16 | <16 | <16 | <16 | <16 | <16 | Neg | Neg | Neg | Pos |
| 15 | 64 | 64 | 64 | 128 | 64 | 64 | Neg | Neg | Bh | Neg |
| 16 | 256 | 128 | 256 | 128 | 64 | 128 | Neg | Neg | Neg | Pos |
| 17 | <16 | 32 | 32 | 64 | <16 | 32 | Bh | Pos | Neg | Pos |
| 18 | 16 | 32 | 32 | 64 | 64 | 64 | Neg | Neg | Neg | Pos |
| 19 | 32 | 32 | 64 | 32 | 64 | 32 | Bk | Pos | Neg | Neg |
| 20 | 16 | 16 | 32 | 32 | <16 | 64 | Neg | Neg | Neg | Neg |
| 21 | 32 | 16 | 32 | 64 | 32 | 32 | Neg | Neg | Neg | Pos |
| 22 | <16 | 16 | <16 | 16 | <16 | 16 | Neg | Pos | Neg | Pos |
| 23 | 16 | 32 | 16 | 64 | 16 | 16 | Neg | Pos | Bh | Pos |
| 24 | 256 | 64 | 64 | 64 | 64 | 64 | Neg | Pos | Neg | Pos |
| 25 | <16 | <16 | 32 | 64 | 16 | <16 | Neg | Neg | Neg | Neg |
| 26 | <16 | <16 | 32 | 64 | 32 | 16 | Neg | Neg | Neg | Neg |
| 27 | <16 | 32 | 16 | 128 | 32 | <16 | Neg | Neg | Neg | Neg |
| 28 | 128 | 128 | 64 | 128 | 64 | 64 | Neg | Neg | Neg | Pos |
| 29 | <16 | 16 | 16 | 16 | <16 | 16 | Neg | Neg | Neg | Pos |
| Participant | Source of DNA Tested by ddPCR | |||
|---|---|---|---|---|
| Blood | BAPGM Incubation Day 7 | BAPGM Incubation Day 14 | BAPGM Incubation Day 21 | |
| 1 | Neg | Neg | Neg | Neg |
| 2 | Pos | Neg | Neg | Pos |
| 3 | Neg | Neg | Pos | Neg |
| 4 | Neg | Neg | Neg | Neg |
| 5 | Neg | Neg | Neg | Pos |
| 6 | Neg | Neg | Neg | Pos |
| 7 | Neg | Neg | Neg | Neg |
| 8 | Neg | Pos | Neg | Neg |
| 9 | Pos | Pos | Pos | Pos |
| 10 | Neg | Neg | Neg | Pos |
| 11 | Pos | Neg | Pos | Neg |
| 12 | Neg | Neg | Neg | Pos |
| 13 | Neg | Neg | Neg | Pos |
| 14 | Neg | Neg | Neg | Pos |
| 15 | Neg | Neg | Neg | Neg |
| 16 | Neg | Pos | Neg | Neg |
| 17 | Pos | Pos | Pos | Neg |
| 18 | Neg | Neg | Neg | Pos |
| 19 | Pos | Neg | Neg | Neg |
| 20 | Neg | Neg | Neg | Neg |
| 21 | Neg | Pos | Neg | Neg |
| 22 | Pos | Neg | Neg | Pos |
| 23 | Pos | Pos | Pos | Pos |
| 24 | Pos | Pos | Neg | Neg |
| 25 | Neg | Neg | Neg | Neg |
| 26 | Neg | Neg | Neg | Neg |
| 27 | Neg | Neg | Neg | Neg |
| 28 | Neg | Neg | Pos | Pos |
| 29 | Neg | Neg | Neg | Pos |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breitschwerdt, E.B.; Bradley, J.M.; Maggi, R.G.; Lashnits, E.; Reicherter, P. Bartonella Associated Cutaneous Lesions (BACL) in People with Neuropsychiatric Symptoms. Pathogens 2020, 9, 1023. https://doi.org/10.3390/pathogens9121023
Breitschwerdt EB, Bradley JM, Maggi RG, Lashnits E, Reicherter P. Bartonella Associated Cutaneous Lesions (BACL) in People with Neuropsychiatric Symptoms. Pathogens. 2020; 9(12):1023. https://doi.org/10.3390/pathogens9121023
Chicago/Turabian StyleBreitschwerdt, Edward B., Julie M. Bradley, Ricardo G. Maggi, Erin Lashnits, and Paul Reicherter. 2020. "Bartonella Associated Cutaneous Lesions (BACL) in People with Neuropsychiatric Symptoms" Pathogens 9, no. 12: 1023. https://doi.org/10.3390/pathogens9121023
APA StyleBreitschwerdt, E. B., Bradley, J. M., Maggi, R. G., Lashnits, E., & Reicherter, P. (2020). Bartonella Associated Cutaneous Lesions (BACL) in People with Neuropsychiatric Symptoms. Pathogens, 9(12), 1023. https://doi.org/10.3390/pathogens9121023






