Anti-Influenza Strategies Based on Nanoparticle Applications
Abstract
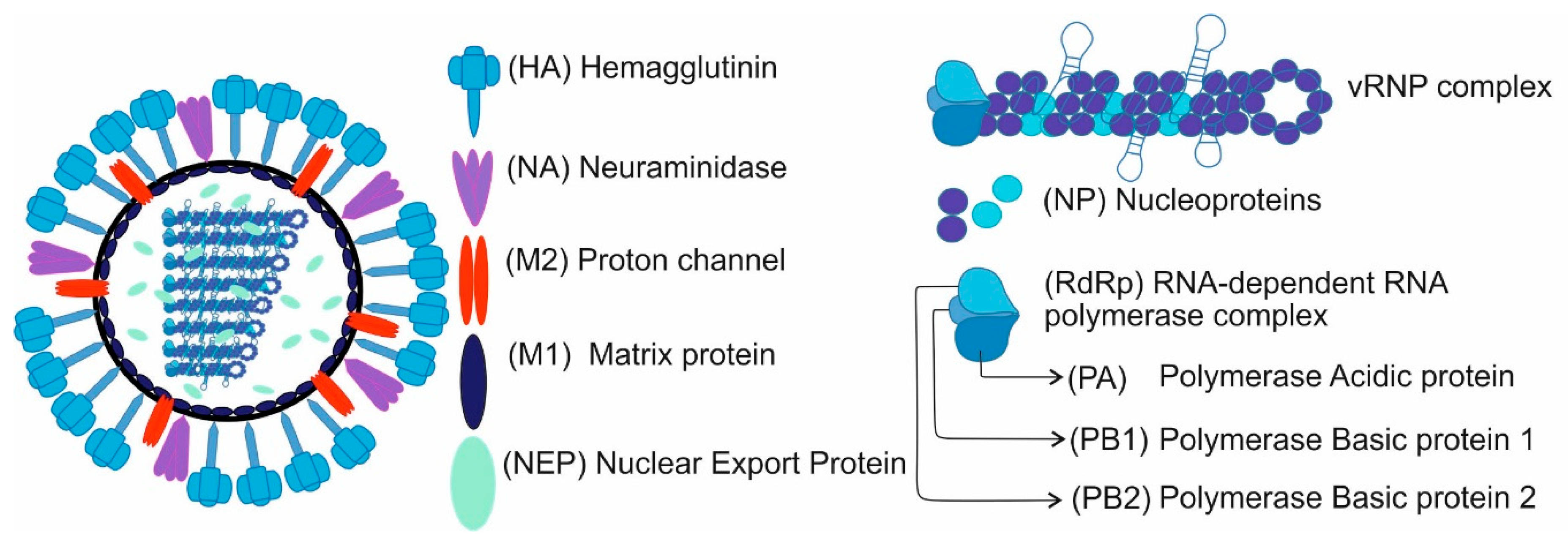
1. Introduction
2. Influenza Virus Inhibition Strategies Based on the Use of Nanoparticles
2.1. Nanotechnology in Vaccination Strategies
2.1.1. Viral Antigen Vaccination Strategy Based on NPs Delivery
Hemagglutinin Protein as a Viral Antigen in Vaccine Based on Nanoparticles
External Domain of the Matrix Protein 2 as a Viral Antigen in Vaccine Based on Nanoparticles
Mix of Influenza Membrane Proteins as Viral Antigens Vaccines Based on Nanoparticles
2.1.2. Inactivated Influenza Virus Strategy Based on NPs Delivery
2.1.3. mRNA Vaccines Strategy Based on NPs Delivery
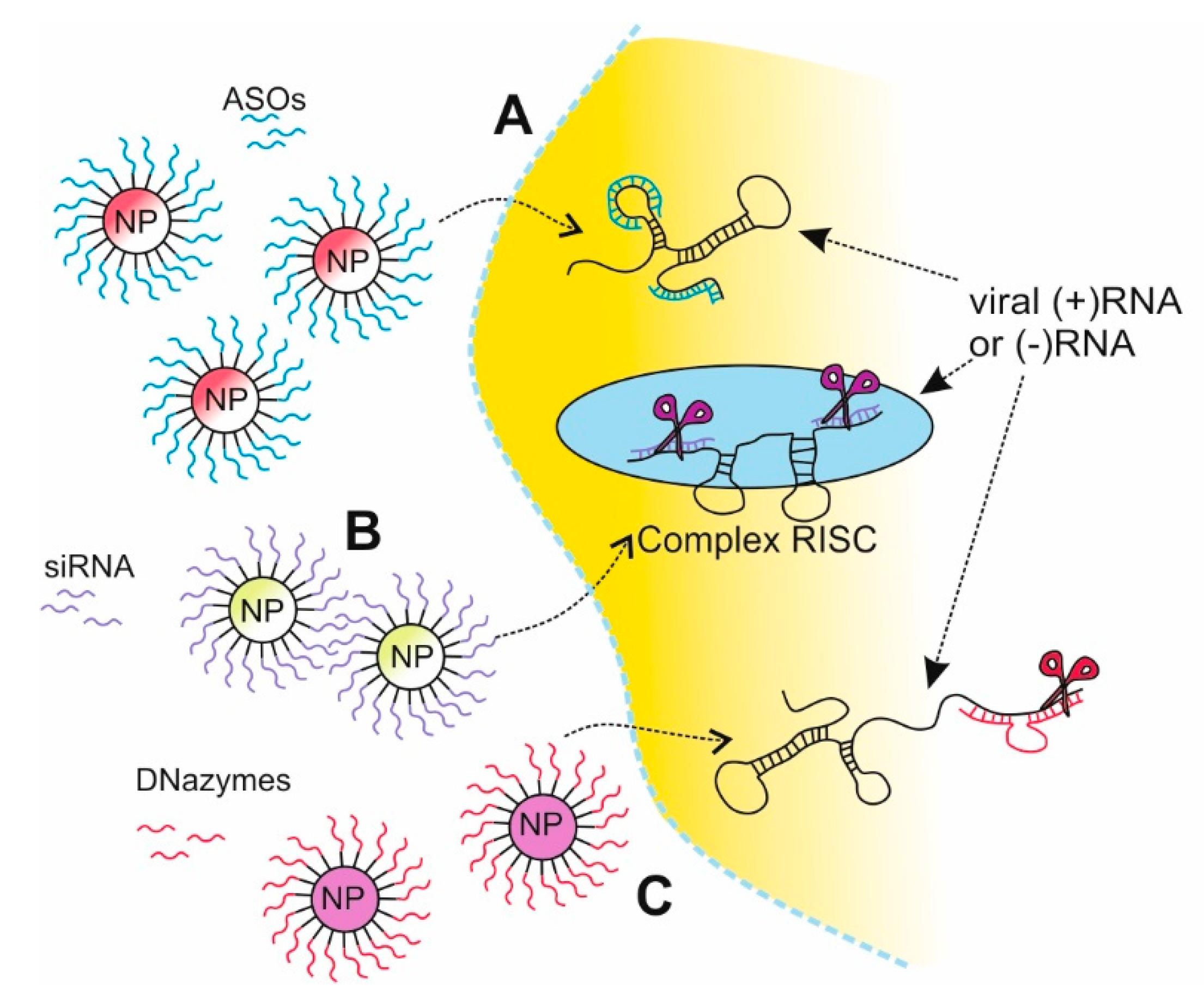
2.2. Nanotechnology in Gene Silencing Strategies
2.2.1. Antisense Oligonucleotides Strategy Based on NPs Delivery
2.2.2. siRNA Strategy Based on NPs Delivery
2.2.3. DNAzymes Strategy Based on NPs Delivery
2.3. Bare Nanoparticles Antiviral Strategy
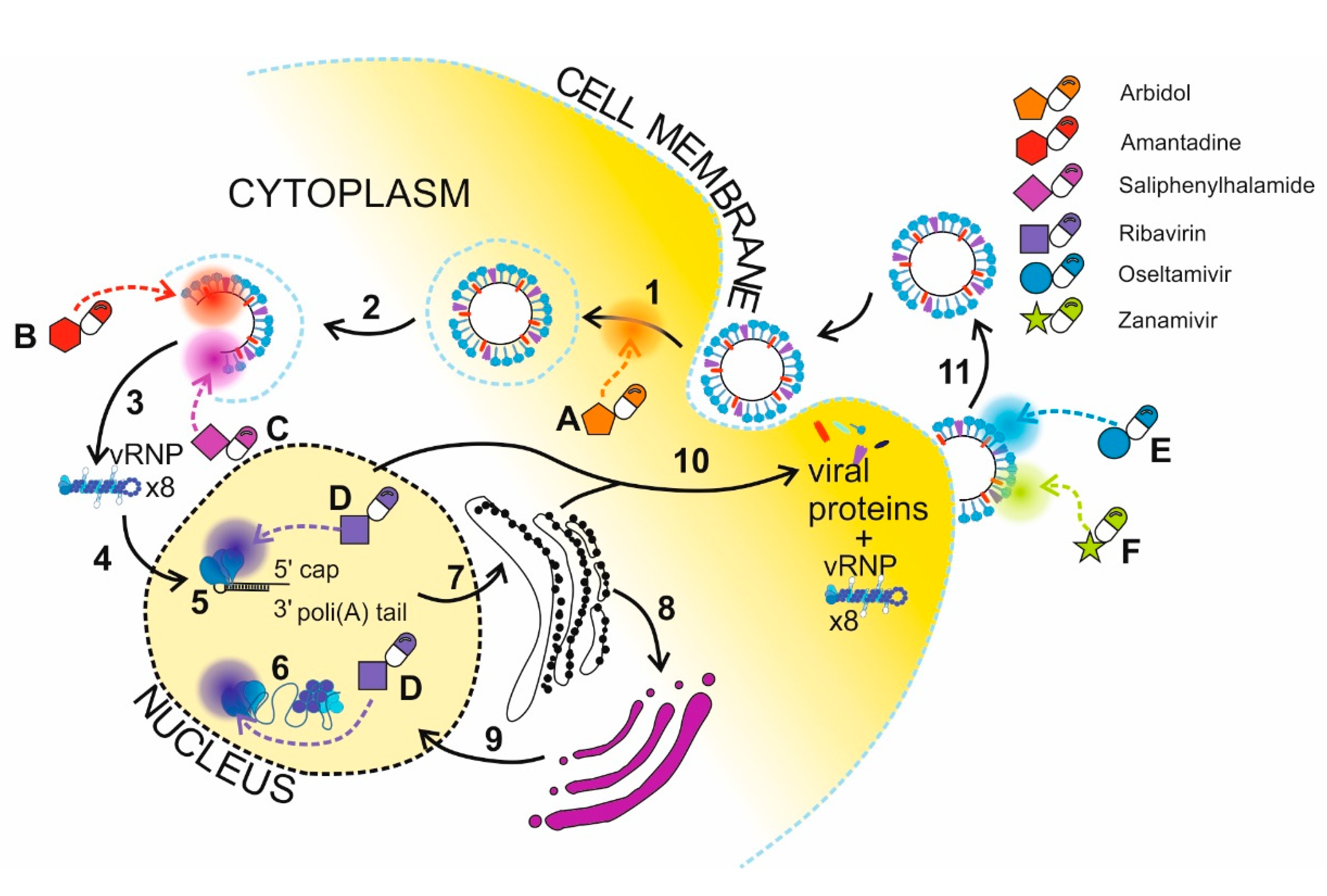
2.4. Nanotechnology in Delivery of Drugs Targeting Proteins
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Woolhouse, M.E.J.; Brierley, L. Epidemiological characteristics of human-infective RNA viruses. Sci. Data 2018, 5, 1–6. [Google Scholar] [CrossRef]
- Gounder, A.P.; Boon, A.C.M. Influenza Pathogenesis: The Effect of Host Factors on Severity of Disease. J. Immunol. 2019, 202, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, S.; Kawaoka, Y. The pathogenesis of influenza virus infections: The contributions of virus and host factors. Curr. Opin. Immunol. 2011, 23, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, N.M.; Palese, P. The biology of influenza viruses. Vaccine 2008, 26, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Webster, R.G.; Webby, R.J. Influenza Virus: Dealing with a Drifting and Shifting Pathogen. Viral Immunol. 2018, 31, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Rejmanek, D.; Hosseini, P.R.; Mazet, J.A.K.; Daszak, P.; Goldstein, T. Evolutionary Dynamics and Global Diversity of Influenza A Virus. J. Virol. 2015, 89, 10993–11001. [Google Scholar] [CrossRef]
- Shao, W.; Li, X.; Goraya, M.U.; Wang, S.; Chen, J.L. Evolution of influenza a virus by mutation and re-assortment. Int. J. Mol. Sci. 2017, 18, 1650. [Google Scholar] [CrossRef]
- Asadi, S.; Gaaloul, N.; Barre, R.S.; Ristenpart, W.D.; Bouvier, N.M.; Wexler, A.S. Influenza A virus is transmissible via aerosolized fomites. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Dunning, J.; Thwaites, R.S.; Openshaw, P.J.M. Seasonal and pandemic influenza: 100 years of progress, still much to learn. Mucosal Immunol. 2020, 13, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Saunders-Hastings, P.R.; Krewski, D. Reviewing the history of pandemic influenza: Understanding patterns of emergence and transmission. Pathogens 2016, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Martellucci, C.A.; Flacco, M.E.; Cappadona, R.; Bravi, F.; Mantovani, L.; Manzoli, L. SARS-CoV-2 pandemic: An overview. Adv. Biol. Regul. 2020, 77, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Triggle, C.R.; Bansal, D.; Farag, E.A.B.A.; Ding, H.; Sultan, A.A. COVID-19: Learning from Lessons To Guide Treatment and Prevention Interventions. Am. Soc. Microbiol. 2020, 5, e00317-20. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Wu, J.; Chang, L. Co-infection of influenza B virus and SARS-CoV-2: A case report from Taiwan. J. Formos. Med. Assoc. 2020, 19–20. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Safamanesh, S.; Ghasemzadeh-moghaddam, H.; Ghafouri, M.; Amir, A. High prevalence of SARS-CoV-2 and influenza A virus (H1N1) co-infection in dead patients in Northeastern Iran. J. Med. Virol. 2020, 1–5. [Google Scholar] [CrossRef]
- Kondo, Y.; Miyazaki, S.; Yamashita, R.; Ikeda, T. Coinfection with SARS-CoV-2 and influenza A virus. BMJ Case Rep. 2020, 13. [Google Scholar] [CrossRef]
- Singh, B.; Kaur, P.; Reid, R.-J.; Shamoon, F.; Bikkina, M. COVID-19 and Influenza Co-Infection: Report of Three Cases. Cureus 2020, 12, e9852. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases (NCIRD) How to Prevent Flu. Available online: https://www.cdc.gov/flu/prevent/prevention.htm (accessed on 28 September 2020).
- World Health Organization. WHO Rcommendations on the Composition of Influenza Virus Vaccines. Available online: https://www.who.int/influenza/vaccines/virus/recommendations/en/ (accessed on 28 September 2020).
- Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases (NCIRD) Past Seasons Vaccine Effectiveness Estimates. Available online: https://www.cdc.gov/flu/vaccines-work/past-seasons-estimates.html (accessed on 28 September 2020).
- Kissling, E.; Pozo, F.; Buda, S.; Gherasim, A.; Brytting, M.; Domegan, L.; Gómez, V.; Meijer, A.; Lazar, M.; Vučina, V.V.; et al. Low 2018/19 vaccine effectiveness against influenza A(H3N2) among 15–64-year-olds in Europe: Exploration by birth cohort. Eurosurveillance 2019, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kissling, E.; Rose, A.; Embor, H.A.; Gherasim, A.; Pebody, R.; Pozo, F.; Trebbien, R.; Mazagatos, C.; Whitaker, H.; Valenciano, M.; et al. Interim 2018/19 influenza vaccine effectiveness: Six European studies, October 2018 to January 2019. Eurosurveillance 2019, 24, 1900121. [Google Scholar] [CrossRef]
- Estrada Leonardo, D.; Shultz-Cherry, S. Development of a universal influenza vaccine. J. Immunol. 2019, 202, 392–398. [Google Scholar] [CrossRef]
- Eshaghi, A.; Shalhoub, S.; Rosenfeld, P.; Li, A.; Higgins, R.R.; Stogios, P.J.; Savchenko, A.; Bastien, N.; Li, Y.; Rotstein, C.; et al. Multiple influenza a (H3N2) mutations conferring resistance to neuraminidase inhibitors in a bone marrow transplant recipient. Antimicrob. Agents Chemother. 2014, 58, 7188–7197. [Google Scholar] [CrossRef]
- Dobrovolny, H.M.; Beauchemin, C.A.A. Modelling the emergence of influenza drug resistance: The roles of surface proteins, the immune response and antiviral mechanisms. PLoS ONE 2017, 12, e18058. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Castaldo, N.; Carnelutti, A. Neuraminidase inhibitors as a strategy for influenza treatment: Pros, cons and future perspectives. Expert Opin. Pharmacother. 2019, 20, 1711–1718. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, X.; Sun, Q.; Zhang, C.; Yang, S.Y.; Li, L.; Jia, Z. Progress of small molecular inhibitors in the development of anti-influenza virus agents. Theranostics 2017, 7, 826–845. [Google Scholar] [CrossRef]
- Davidson, S. Treating influenza infection, from now and into the future. Front. Immunol. 2018, 9, 1946. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, M.; Dianat-Moghadam, H.; Sofiani, V.H.; Karimzadeh, M.; Zargar, M.; Moghoofei, M.; Biglari, H.; Ghorbani, S.; Nahand, J.S.; Mirzaei, H. MiRNA-based strategy for modulation of influenza A virus infection. Epigenomics 2018, 10, 829–844. [Google Scholar] [CrossRef] [PubMed]
- Bhagyaraj, S.M.; Oluwafemi, O.S. Synthesis of Inorganic Nanomaterials, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; ISBN 978008019764. [Google Scholar]
- Lungu, M.; Neculae, A.; Bunoiu, M.; Biris, C. Nanoparticles’ Promises and Risks; Springer Nature: Basel, Switzerland, 2015; ISBN 9783319117287. [Google Scholar]
- Vert, M.; Doi, Y.; Hellwich, K.A.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for biorelated polymers applications (IUPACRecommendations, 2.0.1.2.). Pure Appl. Chem. 2012, 84, 377–410. [Google Scholar] [CrossRef]
- Kroll, A. Nanobiology-convergence of disciplines inspires great applications. Cell. Mol. Life Sci. 2012, 69, 335–336. [Google Scholar] [CrossRef]
- Schmidt, C.; Storsberg, J. Nanomaterials-tools, technology and methodology of nanotechnology based biomedical systems for diagnostics and therapy. Biomedicines 2015, 3, 203–223. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Sanna, V.; Sechi, M. Therapeutic Potential of Targeted Nanoparticles and Perspective on Nanotherapies. ACS Med. Chem. Lett. 2020, 11, 1069–1073. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, L.; Kong, X.; Sun, L. Application of nanodiagnostics in point-of-care tests for infectious diseases. Int. J. Nanomed. 2017, 12, 4789–4803. [Google Scholar] [CrossRef] [PubMed]
- Gamage, S.; Howard, M.; Makita, H.; Cross, B.; Hastings, G.; Luo, M.; Abate, Y. Probing structural changes in single enveloped virus particles using nano-infrared spectroscopic imaging. PLoS ONE 2018, 13, e0199112. [Google Scholar] [CrossRef] [PubMed]
- Toy, R.; Roy, K. Engineering nanoparticles to overcome barriers to immunotherapy. Bioeng. Transl. Med. 2016, 1, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Shukoor, M.I.; Chen, Y.; Yuan, Q.; Zhu, Z.; Zhao, Z.; Gulbakan, B.; Tan, W. Aptamer-conjugated nanomaterials for bioanalysis and biotechnology applications. Nanoscale 2011, 3, 546–556. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, W.; Wang, B.Z. Dual-linker gold nanoparticles as adjuvanting carriers for multivalent display of recombinant influenza hemagglutinin trimers and flagellin improve the immunological responses in vivo and in vitro. Int. J. Nanomed. 2017, 12, 4747–4762. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, W.; Luo, Y.; Wang, B.Z. Gold nanoparticles conjugating recombinant influenza hemagglutinin trimers and flagellin enhanced mucosal cellular immunity. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1349–1360. [Google Scholar] [CrossRef]
- Knuschke, T.; Sokolova, V.; Rotan, O.; Wadwa, M.; Tenbusch, M.; Hansen, W.; Staeheli, P.; Epple, M.; Buer, J.; Westendorf, A.M. Immunization with Biodegradable Nanoparticles Efficiently Induces Cellular Immunity and Protects against Influenza Virus Infection. J. Immunol. 2013, 190, 6221–6229. [Google Scholar] [CrossRef]
- Sokolova, V.; Knuschke, T.; Kovtun, A.; Buer, J.; Epple, M.; Westendorf, A.M. The use of calcium phosphate nanoparticles encapsulating Toll-like receptor ligands and the antigen hemagglutinin to induce dendritic cell maturation and T cell activation. Biomaterials 2010, 31, 5627–5633. [Google Scholar] [CrossRef]
- Sawaengsak, C.; Mori, Y.; Yamanishi, K.; Mitrevej, A.; Sinchaipanid, N. Chitosan nanoparticle encapsulated hemagglutinin-split influenza virus mucosal vaccine. AAPS PharmSciTech 2014, 15, 317–325. [Google Scholar] [CrossRef]
- Kanekiyo, M.; Wei, C.J.; Yassine, H.M.; McTamney, P.M.; Boyington, J.C.; Whittle, J.R.R.; Rao, S.S.; Kong, W.P.; Wang, L.; Nabel, G.J. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature 2013, 499, 102–106. [Google Scholar] [CrossRef]
- Yassine, H.M.; Boyington, J.C.; McTamney, P.M.; Wei, C.J.; Kanekiyo, M.; Kong, W.P.; Gallagher, J.R.; Wang, L.; Zhang, Y.; Joyce, M.G.; et al. Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat. Med. 2015, 21, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Ross, K.A.; Lyod, H.; Wu, W.; Huntimer, L.; Ahmed, S.; Sambol, A.; Broderick, S.; Flickinger, Z.; Rajan, K.; Bronich, T.; et al. Hemagglutinin-based polyanhydride nanovaccines against H5N1 infuenza elicit protective virus neutralizing titers and cell-mediated immunity. Int. J. Nanomed. 2015, 10, 229–243. [Google Scholar] [CrossRef][Green Version]
- Ross, K.; Adams, J.; Loyd, H.; Ahmed, S.; Sambol, A.; Broderick, S.; Rajan, K.; Kohut, M.; Bronich, T.; Wannemuehler, M.J.; et al. Combination Nanovaccine Demonstrates Synergistic Enhancement in Efficacy against Influenza. ACS Biomater. Sci. Eng. 2016, 2, 368–374. [Google Scholar] [CrossRef]
- Kingstad-Bakke, B.A.; Chandrasekar, S.S.; Phanse, Y.; Ross, K.A.; Hatta, M.; Suresh, M.; Kawaoka, Y.; Osorio, J.E.; Narasimhan, B.; Talaat, A.M. Effective mosaic-based nanovaccines against avian influenza in poultry. Vaccine 2019, 37, 5051–5058. [Google Scholar] [CrossRef]
- Tao, W.; Ziemer, K.S.; Gill, H.S. Gold nanoparticle–M2e conjugate coformulated with CpG induces protective immunity against influenza A virus. Nanomedicine (Lond.) 2014, 9, 237–251. [Google Scholar] [CrossRef]
- Tao, W.; Gill, H.S. M2e-immobilized gold nanoparticles as influenza A vaccine: Role of soluble M2e and longevity of protection. Vaccine 2015, 33, 2307–2315. [Google Scholar] [CrossRef]
- Tao, W.; Hurst, B.; Shakya, A.K.; Uddin, M.J.; Ingrole, R.S.; Hernandez-Sanabria, M.; Arya, R.; Bimler, L.; Paust, S.; Tarbet, E.B.; et al. Consensus M2e peptide conjugated to gold nanoparticles confers protection against H1N1, H3N2 and H5N1 influenza A viruses. Antivir. Res. 2017, 141, 62–72. [Google Scholar] [CrossRef]
- Yong, C.Y.; Yeap, S.K.; Ho, K.L.; Omar, A.R.; Tan, W.S. Potential recombinant vaccine against influenza A virus based on M2e displayed on nodaviral capsid nanoparticles. Int. J. Nanomed. 2015, 10, 2751–2763. [Google Scholar] [CrossRef]
- de Filette, M.; Jou, W.M.; Birkett, A.; Lyons, K.; Schultz, B.; Tonkyro, A.; Resch, S.; Fiers, W. Universal influenza A vaccine: Optimization of M2-based constructs. Virology 2005, 337, 149–161. [Google Scholar] [CrossRef]
- de Filette, M.; Martens, W.; Smet, A.; Schotsaert, M.; Birkett, A.; Londoño-Arcila, P.; Fiers, W.; Saelens, X. Universal influenza A M2e-HBc vaccine protects against disease even in the presence of pre-existing anti-HBc antibodies. Vaccine 2008, 26, 6503–6507. [Google Scholar] [CrossRef]
- Liu, Q.; Zheng, X.; Zhang, C.; Shao, X.; Zhang, X.; Zhang, Q.; Jiang, X. Conjugating Influenza A (H1N1) Antigen to N-Trimethylaminoethylmethacrylate Chitosan Nanoparticles Improves the Immunogenicity of the Antigen After Nasal Administration. J. Med. Virol. 2015, 87, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.M.J.; Chien, C.Y.; Liu, M.T.; Fang, Z.S.; Chang, S.Y.; Juang, R.H.; Chang, S.C.; Chen, H.W. Multi-antigen avian influenza a (H7N9) virus-like particles: Particulate characterizations and immunogenicity evaluation in murine and avian models. BMC Biotechnol. 2017, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Ross, K.; Senapati, S.; Alley, J.; Darling, R.; Goodman, J.; Jefferson, M.; Uz, M.; Guo, B.; Yoon, K.J.; Verhoeven, D.; et al. Single dose combination nanovaccine provides protection against influenza A virus in young and aged mice. Biomater. Sci. 2019, 7, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Hiremath, J.; Kang, K.I.; Xia, M.; Elaish, M.; Binjawadagi, B.; Ouyang, K.; Dhakal, S.; Arcos, J.; Torrelles, J.B.; Jiang, X.; et al. Entrapment of H1N1 influenza virus derived conserved peptides in PLGA nanoparticles enhances T cell response and vaccine efficacy in pigs. PLoS ONE 2016, 11, e0151922. [Google Scholar] [CrossRef]
- Dehghan, S.; Kheiri, M.T.; Tabatabaiean, M.; Darzi, S.; Tafaghodi, M. Dry-powder form of chitosan nanospheres containing influenza virus and adjuvants for nasal immunization. Arch. Pharm. Res. 2013, 36, 981–992. [Google Scholar] [CrossRef]
- Dehghan, S.; Tafaghodi, M.; Bolourieh, T.; Mazaheri, V.; Torabi, A.; Abnous, K.; Tavassoti Kheiri, M. Rabbit nasal immunization against influenza by dry-powder form of chitosan nanospheres encapsulated with influenza whole virus and adjuvants. Int. J. Pharm. 2014, 475, 1–8. [Google Scholar] [CrossRef]
- Dhakal, S.; Goodman, J.; Bondra, K.; Lakshmanappa, Y.S.; Hiremath, J.; Shyu, D.L.; Ouyang, K.; Kang, K.I.; Krakowka, S.; Wannemuehler, M.J.; et al. Polyanhydride nanovaccine against swine influenza virus in pigs. Vaccine 2017, 35, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Morçöl, T.; Hurst, B.L.; Tarbet, E.B. Calcium phosphate nanoparticle (CaPNP) for dose-sparing of inactivated whole virus pandemic influenza A (H1N1) 2009 vaccine in mice. Vaccine 2017, 35, 4569–4577. [Google Scholar] [CrossRef] [PubMed]
- Alkie, T.N.; Yitbarek, A.; Taha-Abdelaziz, K.; Astill, J.; Sharif, S. Characterization of immunogenicity of avian influenza antigens encapsulated in PLGA nanoparticles following mucosal and subcutaneous delivery in chickens. PLoS ONE 2018, 13, e0206324. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Krishnan, S.; Lenzen, G.; Magné, R.; Gomard, E.; Guillet, J.-G.; Lévy, J.-P.; Meulien, P. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur. J. Immunol. 1993, 23, 1719–1722. [Google Scholar] [CrossRef]
- Bahl, K.; Senn, J.J.; Yuzhakov, O.; Bulychev, A.; Brito, L.A.; Hassett, K.J.; Laska, M.E.; Smith, M.; Almarsson, Ö.; Thompson, J.; et al. Preclinical and Clinical Demonstration of Immunogenicity by mRNA Vaccines against H10N8 and H7N9 Influenza Viruses. Mol. Ther. 2017, 25, 1316–1327. [Google Scholar] [CrossRef] [PubMed]
- Magini, D.; Giovani, C.; Mangiavacchi, S.; MacCari, S.; Cecchi, R.; Ulmer, J.B.; De Gregorio, E.; Geall, A.J.; Brazzoli, M.; Bertholet, S. Self-amplifying mRNA vaccines expressing multiple conserved influenza antigens confer protection against homologous and heterosubtypic viral challenge. PLoS ONE 2016, 11, e0161193. [Google Scholar] [CrossRef] [PubMed]
- Freyn, A.W.; Ramos da Silva, J.; Rosado, V.C.; Bliss, C.M.; Pine, M.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; de Souza Ferreira, L.C.; Weissman, D.; et al. A Multi-Targeting, Nucleoside-Modified mRNA Influenza Virus Vaccine Provides Broad Protection in Mice. Mol. Ther. 2020, 28, 1569–1584. [Google Scholar] [CrossRef] [PubMed]
- Stöhr, K.; Bucher, D.; Colgate, T.; Wood, J. Influenza Virus Surveillance, Vaccine Strain Selection, and Manufacture. Methods Mol. Biol. 2012, 865, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.; Robinson, J.M.; Cunningham, G.; Iqbal, R.; Larsen, S. The complexity and cost of vaccine manufacturing—An overview. Vaccine 2017, 35, 4064–4071. [Google Scholar] [CrossRef] [PubMed]
- Global, W.H.O.; Programme, I.; September, B.; Europe, E. Recommended composition of influenza virus vaccines for use in the 2020 southern hemisphere influenza season. Wkly. Epidemiol. Rec. 2019, 94, 473–484. [Google Scholar]
- World Health Organization. Recommended Composition of Influenza Virus Vaccines for Use in the 2018–2019 Northern Hemisphere Influenza Season. Available online: https://www.who.int/influenza/vaccines/virus/recommendations/201802_recommendation.pdf (accessed on 28 September 2020).
- Hollingsworth, R.; El Guerche-Séblain, C.; Tsai, T.; Vasiliev, Y.; Lee, S.; Bright, H.; Barbosa, P. Assessment of the benefits of seasonal influenza vaccination: Elements of a framework to interpret estimates of vaccine effectiveness estimates and support robust decision-making and communication. Influenza Other Respir. Viruses 2020. [Google Scholar] [CrossRef]
- Padilla-Quirarte, H.O.; Lopez-Guerrero, D.V.; Gutierrez-Xicotencatl, L.; Esquivel-Guadarrama, F. Protective antibodies against influenza proteins. Front. Immunol. 2019, 10, 1677. [Google Scholar] [CrossRef]
- Kosik, I.; Yewdell, J.W. Influenza hemagglutinin and neuraminidase: Yin–yang proteins coevolving to thwart immunity. Viruses 2019, 11, 346. [Google Scholar] [CrossRef]
- Garten, W.; Klenk, H. Understanding influenza virus pathogenicity Septic shock caused by Gram-positive bacteria. Trends Microbiol. 1999, 7, 99–100. [Google Scholar] [CrossRef]
- Pinto, L.H.; Lamb, R.A. Influenza virus proton channels. Photochem. Photobiol. Sci. 2006, 5, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, R.; Igarashi, M.; Takada, A. Influenza A virus M2 protein: Roles from ingress to egress. Int. J. Mol. Sci. 2017, 18, 2649. [Google Scholar] [CrossRef]
- Zebedee, S.L.; Lamb, R.A. Influenza A virus M2 protein: Monoclonal antibody restriction of virus growth and detection of M2 in virions. J. Virol. 1988, 62, 2762–2772. [Google Scholar] [CrossRef] [PubMed]
- Drummond, J.E.; Shaw, E.E.; Antonello, J.M.; Green, T.; Page, G.J.; Motley, C.O.; Wilson, K.A.; Finnefrock, A.C.; Liang, X.; Casimiro, D.R. Design and optimization of a multiplex anti-influenza peptide immunoassay. J. Immunol. Methods 2008, 334, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Kitikoon, P.; Strait, E.L.; Thacker, E.L. The antibody responses to swine influenza virus (SIV) recombinant matrix 1 (rM1), matrix 2 (M2), and hemagglutinin (HA) proteins in pigs with different SIV exposure. Vet. Microbiol. 2008, 126, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Schepens, B.; De Vlieger, D.; Saelens, X. Vaccine options for influenza: Thinking small. Curr. Opin. Immunol. 2018, 53, 22–29. [Google Scholar] [CrossRef]
- Mezhenskaya, D.; Isakova-Sivak, I.; Rudenko, L. M2e-based universal influenza vaccines: A historical overview and new approaches to development. J. Biomed. Sci. 2019, 26, 1–15. [Google Scholar] [CrossRef]
- Vasou, A.; Sultanoglu, N.; Goodbourn, S.; Randall, R.E.; Kostrikis, L.G. Targeting pattern recognition receptors (PRR) for vaccine adjuvantation: From synthetic PRR agonists to the potential of defective interfering particles of viruses. Viruses 2017, 9, 186. [Google Scholar] [CrossRef]
- Demento, S.L.; Eisenbarth, S.C.; Foellmer, H.G.; Platt, C.; Caplan, J.; Saltzman, W.M.; Mellman, I.; Ledizet, M.; Fikrig, E.; Richard, A.; et al. Inflammasome-activating nanoparticles as modulac systems for optimizing vaccine efficacy. Vaccine 2010, 27, 3013–3021. [Google Scholar] [CrossRef]
- Zhang, C.; Maruggi, G.; Shan, H.; Li, J. Advances in mRNA vaccines for infectious diseases. Front. Immunol. 2019, 10, 564. [Google Scholar] [CrossRef]
- Piasecka, J.; Lenartowicz, E.; Soszynska-Jozwiak, M.; Szutkowska, B.; Kierzek, R.; Kierzek, E. RNA Secondary Structure Motifs of the Influenza A Virus as Targets for siRNA-Mediated RNA Interference. Mol. Ther. Nucleic Acids 2020, 19, 627–642. [Google Scholar] [CrossRef]
- Huang, D.T.-N.; Lu, C.-Y.; Shao, P.-L.; Chang, L.-Y.; Wang, J.-Y.; Chang, Y.-H.; Lai, M.-J.; Chi, Y.-H.; Huang, L.-M. In vivo inhibition of influenza A virus replication by RNA interference targeting the PB2 subunit via intratracheal delivery. PLoS ONE 2017, 12, e0174523. [Google Scholar] [CrossRef] [PubMed]
- Kesy, J.; Patil, K.M.; Kumar, S.R.; Shu, Z.; Yong, H.Y.; Zimmermann, L.; Ong, A.A.L.; Toh, D.F.K.; Krishna, M.S.; Yang, L.; et al. A Short Chemically Modified dsRNA-Binding PNA (dbPNA) Inhibits Influenza Viral Replication by Targeting Viral RNA Panhandle Structure. Bioconjug. Chem. 2019, 30, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Lenartowicz, E.; Nogales, A.; Kierzek, E.; Kierzek, R.; Martínez-Sobrido, L.; Turner, D.H. Antisense Oligonucleotides Targeting Influenza A Segment 8 Genomic RNA Inhibit Viral Replication. Nucleic Acid Ther. 2016, 26, 277–285. [Google Scholar] [CrossRef]
- Michalak, P.; Soszynska-Jozwiak, M.; Biala, E.; Moss, W.N.; Kesy, J.; Szutkowska, B.; Lenartowicz, E.; Kierzek, R.; Kierzek, E. Secondary structure of the segment 5 genomic RNA of influenza A virus and its application for designing antisense oligonucleotides. Sci. Rep. 2019, 9, 3801. [Google Scholar] [CrossRef] [PubMed]
- Reed, C.A.; Peralta, E.R.; Wenrich, L.M.; Wong, C.A.; Bennett, C.F.; Freier, S.; Lollo, B. Transfection protocol for antisense oligonucleotides affects uniformity of transfection in cell culture and efficiency of mRNA target reduction. Oligonucleotides 2005, 15, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Pavlin, M.; Kandušer, M.; Reberšek, M.; Pucihar, G.; Hart, F.X.; Magjarević, R.; Miklavčič, D. Effect of cell electroporation on the conductivity of a cell suspension. Biophys. J. 2005, 88, 4378–4390. [Google Scholar] [CrossRef]
- Kim, T.K.; Eberwine, J.H. Mammalian cell transfection: The present and the future. Anal. Bioanal. Chem. 2010, 397, 3173–3178. [Google Scholar] [CrossRef]
- Mescalchin, A.; Restle, T. Oligomeric nucleic acids as antivirals. Molecules 2011, 16, 1271–1296. [Google Scholar] [CrossRef]
- Frazier, K.S. Antisense Oligonucleotide Therapies:The Promise and the Challenges from a Toxicologic Pathologist’s Perspective. Toxicol. Pathol. 2015, 43, 78–89. [Google Scholar] [CrossRef]
- Levina, A.S.; Repkova, M.N.; Ismagilov, Z.R.; Shikina, N.V.; Malygin, E.G.; Mazurkova, N.A.; Zinov’ev, V.V.; Evdokimov, A.A.; Baiborodin, S.I.; Zarytova, V.F. High-performance method for specific effect on nucleic acids in cells using TiO2~DNA nanocomposites. Sci. Rep. 2012, 2, 756. [Google Scholar] [CrossRef] [PubMed]
- Levina, A.S.; Repkova, M.N.; Ismagilov, Z.R.; Shikina, N.V.; Mazurkova, N.A.; Zarytova, V.F. Efficient inhibition of human influenza a virus by oligonucleotides electrostatically fixed on polylysine-containing TiO2 nanoparticles. Russ. J. Bioorg. Chem. 2014, 40, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Levina, A.S.; Repkova, M.N.; Mazurkova, N.A.; Makarevich, E.V.; Ismagilov, Z.R.; Zarytova, V.F. Knockdown of different influenza A virus subtypes in cell culture by a single antisense oligodeoxyribonucleotide. Int. J. Antimicrob. Agents 2015, 46, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Levina, A.S.; Repkova, M.N.; Bessudnova, E.V.; Filippova, E.I.; Mazurkova, N.A.; Zarytova, V.F. High antiviral effect of TiO2·PL-DNA nanocomposites targeted to conservative regions of (-)RNA and (+)RNA of influenza A virus in cell culture. Beilstein J. Nanotechnol. 2016, 7, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Amirkhanov, R.N.; Mazurkova, N.A.; Amirkhanov, N.V.; Zarytova, V.F. Composites of peptide nucleic acids with titanium dioxide nanoparticles. IV. Antiviral activity of nanocomposites containing DNA/PNA duplexes. Russ. J. Bioorg. Chem. 2015, 41, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Levina, A.S.; Repkova, M.N.; Shikina, N.V.; Ismagilov, Z.R.; Yashnik, S.A.; Semenov, D.V.; Savinovskaya, Y.I.; Mazurkova, N.A.; Pyshnaya, I.A.; Zarytova, V.F. Non-agglomerated silicon-organic nanoparticles and their nanocomplexes with oligonucleotides: Synthesis and properties. Beilstein J. Nanotechnol. 2018, 9, 2516–2525. [Google Scholar] [CrossRef]
- Frede, A.; Neuhaus, B.; Knuschke, T.; Wadwa, M.; Kollenda, S.; Klopfleisch, R.; Hansen, W.; Buer, J.; Bruder, D.; Epple, M.; et al. Local delivery of siRNA-loaded calcium phosphate nanoparticles abates pulmonary inflammation. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2395–2403. [Google Scholar] [CrossRef]
- Jamali, A.; Mottaghitalab, F.; Abdoli, A.; Dinarvand, M.; Esmailie, A.; Kheiri, M.T.; Atyabi, F. Inhibiting influenza virus replication and inducing protection against lethal influenza virus challenge through chitosan nanoparticles loaded by siRNA. Drug Deliv. Transl. Res. 2018, 8, 12–20. [Google Scholar] [CrossRef]
- Repkova, M.; Levina, A.; Chelobanov, B.; Ismagilov, Z.; Shatskaya, N.; Baiborodin, S.; Filippova, E.; Mazurkova, N.; Zarytova, V. Efficient inhibition of influenza A viral replication in cells by deoxyribozymes delivered by nanocomposites. Int. J. Antimicrob. Agents 2017, 49, 703–708. [Google Scholar] [CrossRef]
- Ding, S.-W.; Han, Q.; Wang, J.; Li, W.-X. Antiviral RNA interference in mammals. Curr. Opin. Immunol. 2019, 54, 109–114. [Google Scholar] [CrossRef]
- Qureshi, A.; Tantray, V.G.; Kirmani, A.R.; Ahangar, A.G. A review on current status of antiviral siRNA. Rev. Med. Virol. 2018, 28, e1976. [Google Scholar] [CrossRef] [PubMed]
- Brandelli, A.; Ritter, A.C.; Veras, F.F. Antimicrobial Activities of Metal Nanoparticles. In Metal Nanoparticles in Pharma; Springer: Cham, Switzerland, 2017; pp. 337–363. [Google Scholar] [CrossRef]
- Mehrbod, P.; Motamed, N.; Tabatabaian, M.; Soleimani Estyar, R.; Amini, E.; Shahidi, M.; Kheiri, M.T. In vitro antiviral effect of “nanosilver” on influenza virus. Daru 2009, 17, 88–93. [Google Scholar]
- Xiang, D.X.; Chen, Q.; Pang, L.; Zheng, C.L. Inhibitory effects of silver nanoparticles on H1N1 influenza A virus in vitro. J. Virol. Methods 2011, 178, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Zheng, Y.; Duan, W.; Li, X.; Yin, J.; Shigdar, S.; O’Connor, M.L.; Marappan, M.; Zhao, X.; Miao, Y.; et al. Inhibition of A/Human/Hubei/3/2005 (H3N2) influenza virus infection by silver nanoparticles in vitro and in vivo. Int. J. Nanomed. 2013, 8, 4103–4114. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Ono, T.; Miyahira, Y.; Nguyen, V.Q.; Matsui, T.; Ishihara, M. Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Res. Lett. 2013, 8, 93. [Google Scholar] [CrossRef]
- Fatima, M.; Zaidi, N.u.S.S.; Amraiz, D.; Afzal, F. In vitro antiviral activity of Cinnamomum cassia and its nanoparticles against H7N3 influenza a virus. J. Microbiol. Biotechnol. 2015, 26, 151–159. [Google Scholar] [CrossRef]
- Sreekanth, T.V.M.; Nagajyothi, P.C.; Muthuraman, P.; Enkhtaivan, G.; Vattikuti, S.V.P.; Tettey, C.O.; Kim, D.H.; Shim, J.; Yoo, K. Ultra-sonication-assisted silver nanoparticles using Panax ginseng root extract and their anti-cancer and antiviral activities. J. Photochem. Photobiol. B Biol. 2018, 188, 6–11. [Google Scholar] [CrossRef]
- Liang, J.-J.; Wei, J.-C.; Lee, Y.-L.; Hsu, S.-H.; Lin, J.-J.; Lin, Y.-L. Surfactant-Modified Nanoclay Exhibits an Antiviral Activity with High Potency and Broad Spectrum. J. Virol. 2014, 88, 4218–4228. [Google Scholar] [CrossRef]
- Kumar, S.R.; Paulpandi, M.; Manivelraja, M.; Mangalaraj, D.; Viswanathan, C.; Kannan, S.; Ponpandian, N. An in vitro analysis of H1N1 viral inhibition using polymer coated superparamagnetic Fe3O4 nanoparticles. RSC Adv. 2014, 4, 13409–13418. [Google Scholar] [CrossRef]
- Kumar, R.; Nayak, M.; Sahoo, G.C.; Pandey, K.; Sarkar, M.C.; Ansari, Y.; Das, V.N.R.; Topno, R.K.; Bhawna; Madhukar, M.; et al. Iron oxide nanoparticles based antiviral activity of H1N1 influenza A virus. J. Infect. Chemother. 2019, 25, 325–329. [Google Scholar] [CrossRef]
- Ghaffari, H.; Tavakoli, A.; Moradi, A.; Tabarraei, A.; Bokharaei-Salim, F.; Zahmatkeshan, M.; Farahmand, M.; Javanmard, D.; Kiani, S.J.; Esghaei, M.; et al. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: Another emerging application of nanomedicine. J. Biomed. Sci. 2019, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sametband, M.; Shukla, S.; Meningher, T.; Hirsh, S.; Mendelson, E.; Sarid, R.; Gedanken, A.; Mandelboim, M. Effective multi-strain inhibition of influenza virus by anionic gold nanoparticles. Med. Chem. Commun. 2011, 2, 421–423. [Google Scholar] [CrossRef]
- Papp, I.; Sieben, C.; Ludwig, K.; Roskamp, M.; Böttcher, C.; Schlecht, S.; Herrmann, A.; Haag, R. Inhibition of influenza virus infection by multivalent sialic-acid-functionalized gold nanoparticles. Small 2010, 6, 2900–2906. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Jiang, J.; Gu, W.; Sun, C.; Wu, D.; Yang, T.; Yang, G. Photocatalytic inactivation efficiency of anatase nano-TiO2 sol on the H9N2 avian influenza virus. Photochem. Photobiol. 2010, 86, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Thammakarn, C.; Satoh, K.; Suguro, A.; Hakim, H.; Ruenphet, S.; Takehara, K. Inactivation of avian influenza virus, Newcastle disease virus and goose parvovirus using solution of nano-sized scallop shell powder. J. Vet. Med. Sci. 2014, 76, 1277–1280. [Google Scholar] [CrossRef]
- Huo, C.; Xiao, J.; Xiao, K.; Zou, S.; Wang, M.; Qi, P.; Liu, T.; Hu, Y. Pre-treatment with zirconia nanoparticles reduces inflammation induced by the pathogenic H5N1 influenza virus. Int. J. Nanomed. 2020, 15, 661–674. [Google Scholar] [CrossRef]
- Qin, T.; Ma, R.; Yin, Y.; Miao, X.; Chen, S.; Fan, K.; Xi, J.; Liu, Q.; Gu, Y.; Yin, Y.; et al. Catalytic inactivation of influenza virus by iron oxide nanozyme. Theranostics 2019, 9, 6920–6935. [Google Scholar] [CrossRef]
- Belshe, R.B.; Burk, B.; Newman, F.; Cerrutie, R.L.; Sim, I.S. Resistance of influenza A virus to amantadine and rimantadine: Results of the one decade of surveillance. J. Infect. Dis. 1989, 159, 430–435. [Google Scholar] [CrossRef]
- Influenza (Flu) Antiviral Drugs and Related Information—Get Information on Medicines for the Flu. Available online: https://www.fda.gov/drugs/information-drug-class/influenza-flu-antiviral-drugs-and-related-information (accessed on 28 September 2020).
- Cheng, P.K.C.; Leung, T.W.C.; Ho, E.C.M.; Leung, P.C.K.; Ng, A.Y.Y.; Lai, M.Y.Y.; Lim, W.W.L. Oseltamivir-and amantadine-resistant influenza viruses A (H1N1). Emerg. Infect. Dis. 2009, 15, 966–968. [Google Scholar] [CrossRef]
- Zaraket, H.; Saito, R.; Suzuki, Y.; Baranovich, T.; Dapat, C.; Caperig-Dapat, I.; Suzuki, H. Genetic makeup of amantadine-resistant and oseltamivir-resistant human influenza A/H1N1 viruses. J. Clin. Microbiol. 2010, 48, 1085–1092. [Google Scholar] [CrossRef]
- Mishin, V.P.; Hayden, F.G.; Gubareva, L.V. Susceptibilities of antiviral-resistant influenza viruses to novel neuraminidase inhibitors. Antimicrob. Agents Chemother. 2005, 49, 4515–4520. [Google Scholar] [CrossRef] [PubMed]
- Sagandira, C.R.; Mathe, F.M.; Guyo, U.; Watts, P. The evolution of Tamiflu synthesis, 20 years on: Advent of enabling technologies the last piece of the puzzle? Tetrahedron 2020, 76, 131440. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.P.; Wang, T.C.; Yu, R.; Li, M.; Huang, J.W. Design, synthesis and biological evaluation of novel zanamivir derivatives as potent neuraminidase inhibitors. Bioorg. Med. Chem. Lett. 2018, 28, 3622–3629. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Alpivab (Peramivir). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/alpivab (accessed on 28 September 2020).
- Ison, M.G.; Hayden, F.G. Antiviral Agents Against Respiratory Viruses, 4th ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Heo, Y.A. Baloxavir: First Global Approval. Drugs 2018, 78, 693–697. [Google Scholar] [CrossRef] [PubMed]
- O’Hanlon, R.; Shaw, M.L. Baloxavir marboxil: The new influenza drug on the market. Curr. Opin. Virol. 2019, 35, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, Z.; Guo, M.; Xia, Y.; Zhao, M.; Wang, C.; Xu, T.; Chen, T.; Zhu, B. Inhibitory activity of selenium nanoparticles functionalized with oseltamivir on H1N1 influenza virus. Int. J. Nanomed. 2017, 12, 5733–5743. [Google Scholar] [CrossRef]
- Lin, Z.; Li, Y.; Guo, M.; Xiao, M.; Wang, C.; Zhao, M.; Xu, T.; Xia, Y.; Zhu, B. Inhibition of H1N1 influenza virus by selenium nanoparticles loaded with zanamivir through p38 and JNK signaling pathways. RSC Adv. 2017, 7, 35290–35296. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Z.; Guo, M.; Zhao, M.; Xia, Y.; Wang, C.; Xu, T.; Zhu, B. Inhibition of H1N1 influenza virus-induced apoptosis by functionalized selenium nanoparticles with amantadine through ROS-mediated AKT signaling pathways. Int. J. Nanomed. 2018, 13, 2005–2016. [Google Scholar] [CrossRef]
- Lin, Z.; Li, Y.; Gong, G.; Xia, Y.; Wang, C.; Chen, Y.; Hua, L.; Zhong, J.; Tang, Y.; Liu, X.; et al. Restriction of H1N1 influenza virus infection by selenium nanoparticles loaded with ribavirin via resisting caspase-3 apoptotic pathway. Int. J. Nanomed. 2018, 13, 5787–5797. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Z.; Gong, G.; Guo, M.; Xu, T.; Wang, C.; Zhao, M.; Xia, Y.; Tang, Y.; Zhong, J.; et al. Inhibition of H1N1 influenza virus-induced apoptosis by selenium nanoparticles functionalized with arbidol through ROS-mediated signaling pathways. J. Mater. Chem. B 2019, 7, 4252–4262. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Z.; Zhao, M.; Guo, M.; Xu, T.; Wang, C.; Xia, H.; Zhu, B. The reversal of H1N1 influenza virus-induced apoptosis by silver nanoparticles functionalized with Amantadine. RSC Adv. 2016, 6, 89679–89686. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Z.; Zhao, M.; Xu, T.; Wang, C.; Hua, L.; Wang, H.; Xia, H.; Zhu, B. Silver Nanoparticle Based Codelivery of Oseltamivir to Inhibit the Activity of the H1N1 Influenza Virus through ROS-Mediated Signaling Pathways. ACS Appl. Mater. Interfaces 2016, 8, 24385–24393. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Li, Y.; Guo, M.; Xu, T.; Wang, C.; Zhao, M.; Wang, H.; Chen, T.; Zhu, B. The inhibition of H1N1 influenza virus-induced apoptosis by silver nanoparticles functionalized with zanamivir. RSC Adv. 2017, 7, 742–750. [Google Scholar] [CrossRef]
- Bimbo, L.M.; Denisova, O.V.; Makila, E.; Kaasalainen, M.; De Brabander, J.K.; Hirvonen, J.; Salonen, J.; Kakkola, L.; Kainov, D.; Santos, A.H. Inhibition of Influenza A Virus Infection in Vitro by Saliphenylhalamide-Loaded Porous Silicon Nanoparticles. ACS Nano 2013, 7, 6884–6893. [Google Scholar] [CrossRef]
- Chakravarty, M.; Vora, A. Nanotechnology-based antiviral therapeutics. Drug Deliv. Transl. Res. 2020. [Google Scholar] [CrossRef]
- Rai, M.; Deshmukh, S.D.; Ingle, A.P.; Gupta, I.R.; Galdiero, M.; Galdiero, S. Metal nanoparticles: The protective nanoshield against virus infection. Crit. Rev. Microbiol. 2016, 42, 46–56. [Google Scholar] [CrossRef]
- Cagno, V.; Andreozzi, P.; D’Alicarnasso, M.; Silva, P.J.; Mueller, M.; Galloux, M.; Goffic, R.L.; Jones, S.T.; Vallino, M.; Hodek, J.; et al. Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nat. Mater. 2018, 17, 195–203. [Google Scholar] [CrossRef]
- Jones, A.A.D.; Mi, G.; Webster, T.J. A Status Report on FDA Approval of Medical Devices Containing Nanostructured Materials. Trends Biotechnol. 2019, 37, 117–120. [Google Scholar] [CrossRef]
- Cojocaru, F.D.; Botezat, D.; Gardikiotis, I.; Uritu, C.M.; Dodi, G.; Trandafir, L.; Rezus, C.; Rezus, E.; Tamba, B.I.; Mihai, C.T. Nanomaterials designed for antiviral drug delivery transport across biological barriers. Pharmaceutics 2020, 12, 171. [Google Scholar] [CrossRef]
- Abo-Zeid, Y.; Ismail, N.S.M.; McLean, G.R.; Hamdy, N.M. A molecular docking study repurposes FDA approved iron oxide nanoparticles to treat and control COVID-19 infection. Eur. J. Pharm. Sci. 2020, 153, 105465. [Google Scholar] [CrossRef]

| Strategy | NPs Material/Size | Influenza Virus Strain (Subtype) | In Vitro/in Vivo Studies | Vaccine | Ref. |
|---|---|---|---|---|---|
| HA as an viral antigen | Au NPs/18 nm | A/Aichi/2/1968 (H3N2) | JAWS II, BMDC/mice | IN | [40] |
| A/Aichi/2/1968 (H3N2) | HEK 293/mice | IN | [41] | ||
| CaP NPs/250 nm | A/Puerto Rico/8/1934 (H1N1) | Splenocytes, dendritic cells, T cells/mice | SC, IN | [42] | |
| - | Splenocytes, dendritic cells, T cells/- | - | [43] | ||
| chitosan NPs/300 nm | A/Brisbane/59/2007(H1N1) | -/mice | IN | [44] | |
| ferritin NPs/37 nm | A/New Caledonia/20/1999 (H1N1), A/Puerto Rico/8/1934 (H1N1), A/Singapore/6/1986 (H1N1), A/Brisbane/59/2007 (H1N1), A/California/04/2009 (H1N1), A/Beijing/262/1995 (H1N1), A/Solomon Islands/3/2006 (H1N1), A/Perth/16/2009 (H3N2), B/Florida/04/2006 | -/mice, ferrets | SC | [45] | |
| ferritin NPs/no data | A/New Caledonia/20/1999 (H1N1), A/South Carolina/1/1918 (H1N1), A/California/04/2009 (H1N1), A/Singapore/6/1986 (H1N1), A/Canada/720/2005 (H2N2), A/Vietnam/1203/2004 (H5N1), A/Indonesia/5/2005 (H5N1), A/Anhui/1/2013 (H7N9), A/Hong Kong/1074/1999 (H9N2) | -/mice, ferrets | SC | [46] | |
| polyanhydride NPs/200 nm | A/Whooper Swan/Mongolia/244/2005 (H5N1), A/Vietnam/1203/2004 (H5N1) | -/mice | SC, IN | [47] | |
| polyanhydride NPs/200 nm | VNH5N1-PR8/CDC-RG (a reassortant virus strain containing the HA gene with a modified basic amino acid cleavage site) segment, the neuraminidase gene segment from the A/Vietnam/1203/2004 (H5N1) virus and the six internal gene segments of A/Puerto Rico/8/1934 (H1N1) virus generated by reverse genetics technology) | -/mice | SC | [48] | |
| polyanhydride NPs/200 nm | A/Vietnam/1203/2004 (H5N1), A/Indonesia/5/2005 (H5N1), A/Hong Kong/482/1997 (H5N1), A/Chicken/Qalubia-Egypt/1/2008 (H5N1), A/northern pintail/Alaska/622/2012 (H5N2), A/northern pintail/Alaska/622/2012 (H5N2), A/American green-winged teal/Alaska/472/2014 (H5N2), recombinant A/Puerto Rico/8/1934 (H1N1), recombinant A/chicken/Vietnam/NCVD5/2003 (H5N1) | -/chickens | SC | [49] | |
| Au NPs/12 nm | A/Puerto Rico/8/1934 (H1N1) | -/mice | IN | [50] | |
| IN | [51] | ||||
| A/California/04/2009 (H1N1), A/Victoria/3/1975 (H3N2), A/Vietnam/1203/2004 (H5N1) | MDCK/mice | IN | [52] | ||
| VLPs/30 nm | no data | -/mice | SC | [53] | |
| VLPs/no data | mouse adapted recombinant X47virus (H3N2) (A/Victoria/3/1975 (H3N2) x A/PuertoRico/8/1934 (H1N1)) | -/mice | IN | [54] | |
| mouse adapted recombinant X47virus(H3N2) (A/Victoria/3/1975 (H3N2) x A/PuertoRico/8/1934 (H1N1)) | -/mice | SC | [55] | ||
| mix of virus membrane proteins as a viral antigen | chitosan NPs/140 nm | A/California/7/2009 (H1N1) | RAW 264.7/ mice | IN | [56] |
| VLPs/114 nm | A/Taiwan/S02076/2013 (H7N9) | -/mice, chickens | SC | [57] | |
| polyanhydride NPs/200 nm | A/Puerto Rico/8/1934 (H1N1) | -/mice | SC | [58] | |
| PLGA NPs/260 nm | A/South Carolina/1/1918 (H1N1), A/New Caledonia/20/1999 (H1N1) | -/pigs | IN, IT | [59] | |
| inactivated virus strategy | chitosan NPs/581 nm | A/New Caledonia/20/1999 (H1N1) | - | - | [60] |
| A/New Caledonia/20/1999 (H1N1) | -/rabbits | SC, IN | [61] | ||
| polyanhydride NPs/200 nm | A/Swine/Ohio/24366/2007 (H1N1), A/Swine/Ohio/FAH10-1/2010 (H1N2) | -/pigs | IN | [62] | |
| CaP NPs/450–500 nm | A/California/7/2009 (H1N1) | MDCK/mice | IM | [63] | |
| PLGA NPs/624 nm chitosan@PLGA NPs/819 nm mannan@PLGA NPs/719 nm | A/Duck/Czech/1956 (H4N6) | chicken | MC, SC | [64] | |
| mRNA strategy | lipid NPs/ no data | A/Northern Territory/60/1968 (H3N2) | -/mice | IJ | [65] |
| lipid NPs/80–100 nm | A/Anhui/1/2013 (H7N9), A/Jiangxi-Donghu/346/2013 (H10N8) | -/mice, ferrets, cynomolgus monkeys (cynos) | IJ | [66] | |
| lipid NPs/130–142 nm | A/Puerto Rico/8/1934 (H1N1), mouse-adapted A/HongKong/1/1968 (H3N2), A/California/7/2009 (H1N1) | BHK/mice | IM | [67] | |
| lipid NPs/~80 nm | Recombinant IAV with: the HA and NA from A/Singapore/GP1908/2015 (H1N1) and remaining proteins from A/Texas/1/1977 (H3N2), A/Michigan/45/2015 (H1N1), A/New Caledonia/20/1999 (H1N1) and A/Puerto Rico/8/1934 (H1N1). Recombinant chimeric IAV with: HA head domain from A/mallard/Sweden/81/2002 (H6N1), HA stalk domain from A/California/04/2009 (H1N1), NA from A/mallard/Sweden/86/2003 (H12N5) and remaining proteins from A/Puerto Rico/8/1934 (H1N1). Recombinant IAV with the polybasic cleavage site removed from the A/Vietnam/1203/2004 (H5N1), the N8 from A/mallard/Sweden/50/2002 (H3N8) and remaining proteins from A/Puerto Rico/8/1934 (H1N1). Recombinant IAV with: the HA and NA from A/chicken/Hong Kong/G9/1997 (H9N2) and remaining proteins from A/Puerto Rico/8/1934 (H1N1) and A/Hong Kong/4801/2014 (H3N2). | NIH-3T3, MDCK, HEK293T/ mice | IJ | [68] |
| Strategy | NPs Material/Size | Influenza Virus Strain (Subtype) | In Vitro/In Vivo Studies | Vaccine | Ref. |
|---|---|---|---|---|---|
| antisense oligonucleotides | TiO2 NPs/ 5 nm | A/Aichi/2/1968 (H3N2) | MDCK/- | - | [97] |
| A/Aichi/2/1968 (H3N2) | MDCK/- | - | [98] | ||
| A/Salekhard/01/2009 (H1N1), Aichi/2/68 (H3N2), A/chicken/Kurgan/05/2005(H5N1) | MDCK/- | - | [99] | ||
| A/Salekhard/01/2009 (H1N1), Aichi/2/68 (H3N2), A/chicken/Kurgan/05/2005(H5N1) | MDCK/- | - | [100] | ||
| TiO2 NPs/ 4–6 nm | Aichi/2/1968 (H3N2) | MDCK/- | - | [101] | |
| Si-NH2 NPs/ 1.2–1.5 nm | A/chicken/Kurgan/05/2005 (H5N1) | MDCK/- | - | [102] | |
| siRNA | CaP NPs/ 145 nm | A/Puerto Rico/8/1934 (H1N1) | -/mice | IN | [103] |
| chitosan NPs/278 nm | A/Puerto Rico/8/1934 (H1N1) | Vero/mice | IN | [104] | |
| DNAzymes | TiO2 NPs/5 nm | A/chicken/Kurgan/05/2005 (H5N1) | MDCK, HeLa/- | - | [105] |
| Strategy | NPs Material/Size | Influenza Virus Strain (Subtype) | In Vitro/In Vivo Studies | Vaccine | Ref. |
|---|---|---|---|---|---|
| silver NPs | Ag NPs/no data | A/New Caledonia/20/1999 (H1N1) | MDCK/- | - | [109] |
| Ag NPs/10 nm | A/Puerto Rico/8/1934 (H1N1) | MDCK/- | - | [110] | |
| Ag NPs/10 nm | A/Human/Hubei/3/2005 (H3N2) | MDCK/mice | IN | [111] | |
| Ag NPs/4–13 nm | A/Puerto Rico/8/1934 (H1N1) | MDCK/- | - | [112] | |
| Ag NPs/42 nm | no data (H7N3) | Vero/- | - | [113] | |
| Ag NPs/ 5–15 nm | A/Puerto Rico/8/1934 (H1N1) | MDCK/- | - | [114] | |
| silicate platelet@Ag NPs/ 80 × 80 × 1 nm | A/WSN/1933 (H1N1) | MDCK, A549/- | - | [115] | |
| iron oxide NPs | Fe3O4 NPs/8–10 nm | A/2009 (H1N1) | MDCK/- | - | [116] |
| Fe3O4 NPs/8–10 nm | A/Puerto Rico/8/1934 (H1N1) | MA104/- | - | [117] | |
| zinc oxide NPs | ZnO NPs/16–50 nm | A/Puerto Rico/8/1934 (H1N1) | MDCK/- | - | [118] |
| gold NPs | Au NPs/5 nm | A/Israel/119/2009 (H1N1), A/Brisbane/10/2007 (H3N2), A/Puerto Rico/8/1934 (H1N1), B/Brisbane/60/2008, B/Shandong/7/1997 | MDCK/- | - | [119] |
| sialic-acid@Au NPs/2 and 14 nm | A/X-31 (H3N2) | MDCK, CHO/- | - | [120] | |
| titanium dioxide NPs | TiO2 NPs/50 nm | no data (H9N2) | MDCK/- | - | [121] |
| calcium oxide NPs | CaO NPs/500 nm | A/duck/Aomori/395/2004 (H7N1) | MDCK/- | - | [122] |
| zirconium dioxide NPs | ZrO2 NPs/200 nm | A/chicken/Henan/1/2004 (H5N1) | -/mice | IN | [123] |
| Drug | NPs Material/Size | Influenza Virus Strain (Subtype) | In Vitro/In Vivo Studies | Vaccine | Ref. |
|---|---|---|---|---|---|
| oseltamivir | Se NPs/ 70–200 nm | no data (H1N1) | MDCK/- | - | [136] |
| zanamivir | A/Hubei/74/2009 (H1N1) | MDCK/- | - | [137] | |
| ribavirin | no data (H1N1) | MDCK/- | - | [138] | |
| amantadine | A/Guangdong/1/2009 (H1N1) | MDCK/mice | IN | [139] | |
| arbidol | no data (H1N1) | MDCK/mice | IN | [140] | |
| amantadine | Ag NPs/ 2–3 nm | no data (H1N1) | MDCK/- | - | [141] |
| oseltamivir | no data (H1N1) | MDCK/- | - | [142] | |
| zanamivir | A/Hubei/74/2009 (H1N1) | MDCK/- | - | [143] | |
| saliphenylhalamide | THCPSi NPs/129 nm | A/WSN/1933 (H1N1), A/Puerto Rico/8/1934-NS116-GFP (H1N1) | MDCK, monkey Vero-E6/- | - | [144] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wieczorek, K.; Szutkowska, B.; Kierzek, E. Anti-Influenza Strategies Based on Nanoparticle Applications. Pathogens 2020, 9, 1020. https://doi.org/10.3390/pathogens9121020
Wieczorek K, Szutkowska B, Kierzek E. Anti-Influenza Strategies Based on Nanoparticle Applications. Pathogens. 2020; 9(12):1020. https://doi.org/10.3390/pathogens9121020
Chicago/Turabian StyleWieczorek, Klaudia, Barbara Szutkowska, and Elzbieta Kierzek. 2020. "Anti-Influenza Strategies Based on Nanoparticle Applications" Pathogens 9, no. 12: 1020. https://doi.org/10.3390/pathogens9121020
APA StyleWieczorek, K., Szutkowska, B., & Kierzek, E. (2020). Anti-Influenza Strategies Based on Nanoparticle Applications. Pathogens, 9(12), 1020. https://doi.org/10.3390/pathogens9121020






