Prevalence and Distribution of Avian Influenza Viruses in Domestic Ducks at the Waterfowl-Chicken Interface in Wetlands
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
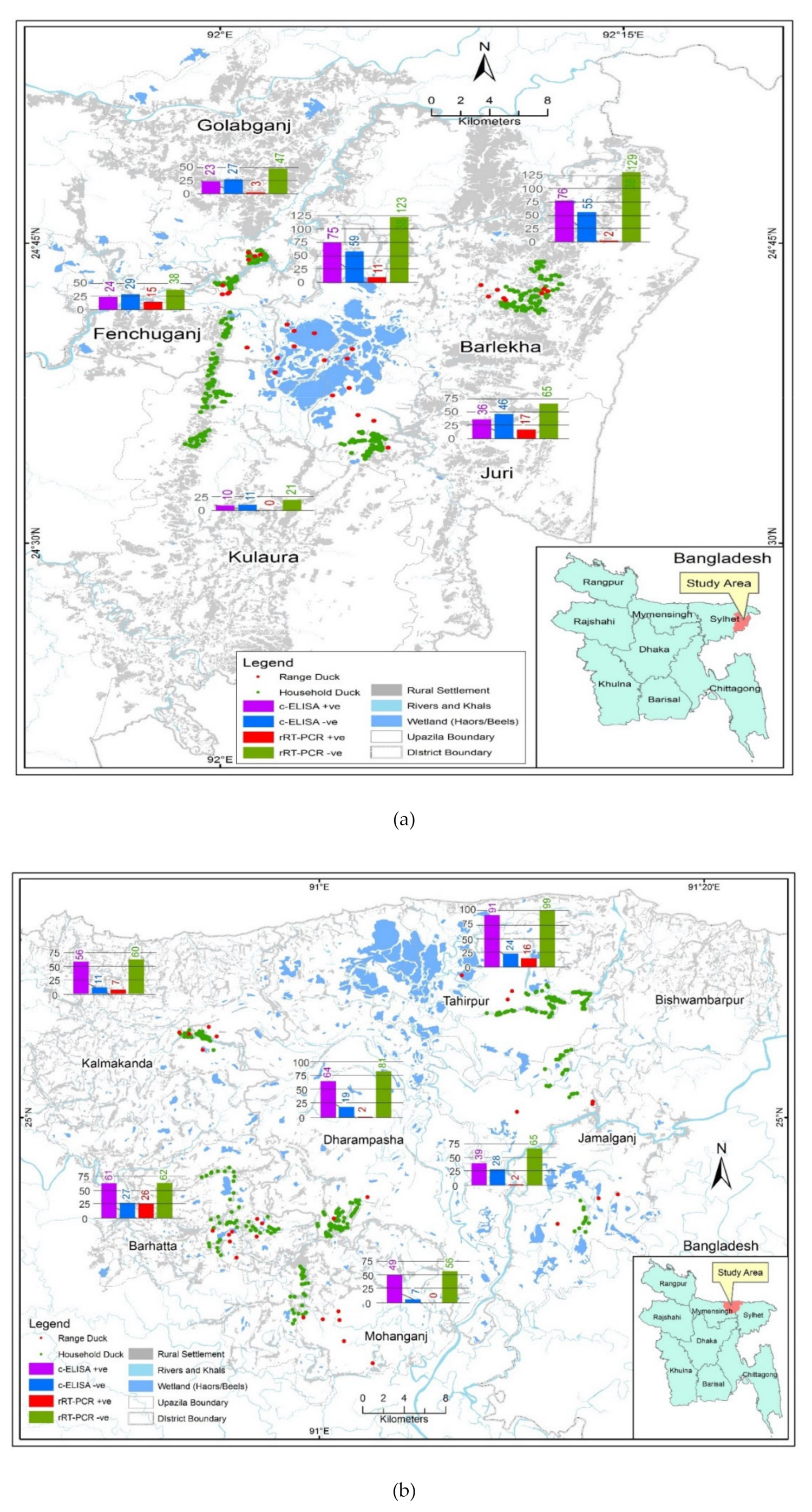
2.2. Study Locations
2.3. Study Design
2.4. Source Population and Sampling
2.5. Sample Collection
2.6. Data Collection
2.7. Competitive Enzyme-Linked Immunosorbent Assay (c-ELISA)
2.8. RNA Extraction and rRT-PCR
2.9. Sequencing and Phylogenetic Analysis
2.10. Statistical Analysis
3. Results
3.1. Free-Range and Household Ducks Samples
3.2. Avian Influenza Serological and Viral RNA Prevalence in Free-Range and Household Ducks
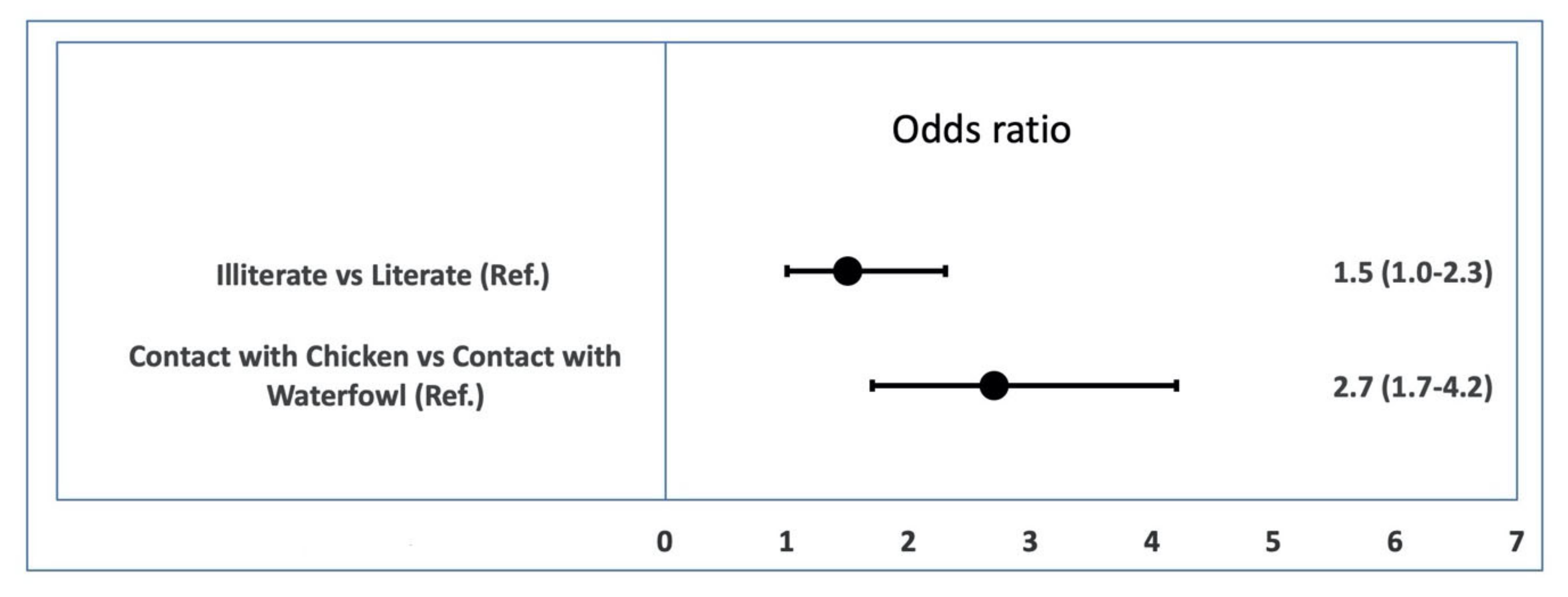
3.3. Risk Factor Analysis of Individual-Level AI Serological and Viral RNA Prevalence
3.4. Risk Factor Analysis of AI Serological and Viral RNA Prevalence in Free-Range Duck Flocks and Household Duck Flocks
3.5. Univariable Logistic Regression of the Effect of Risk Factors on AI Seroprevalence in Ducks
3.6. Univariable Logistic Regression of the Effect of Risk Factors on AI Viral RNA Prevalence in Ducks
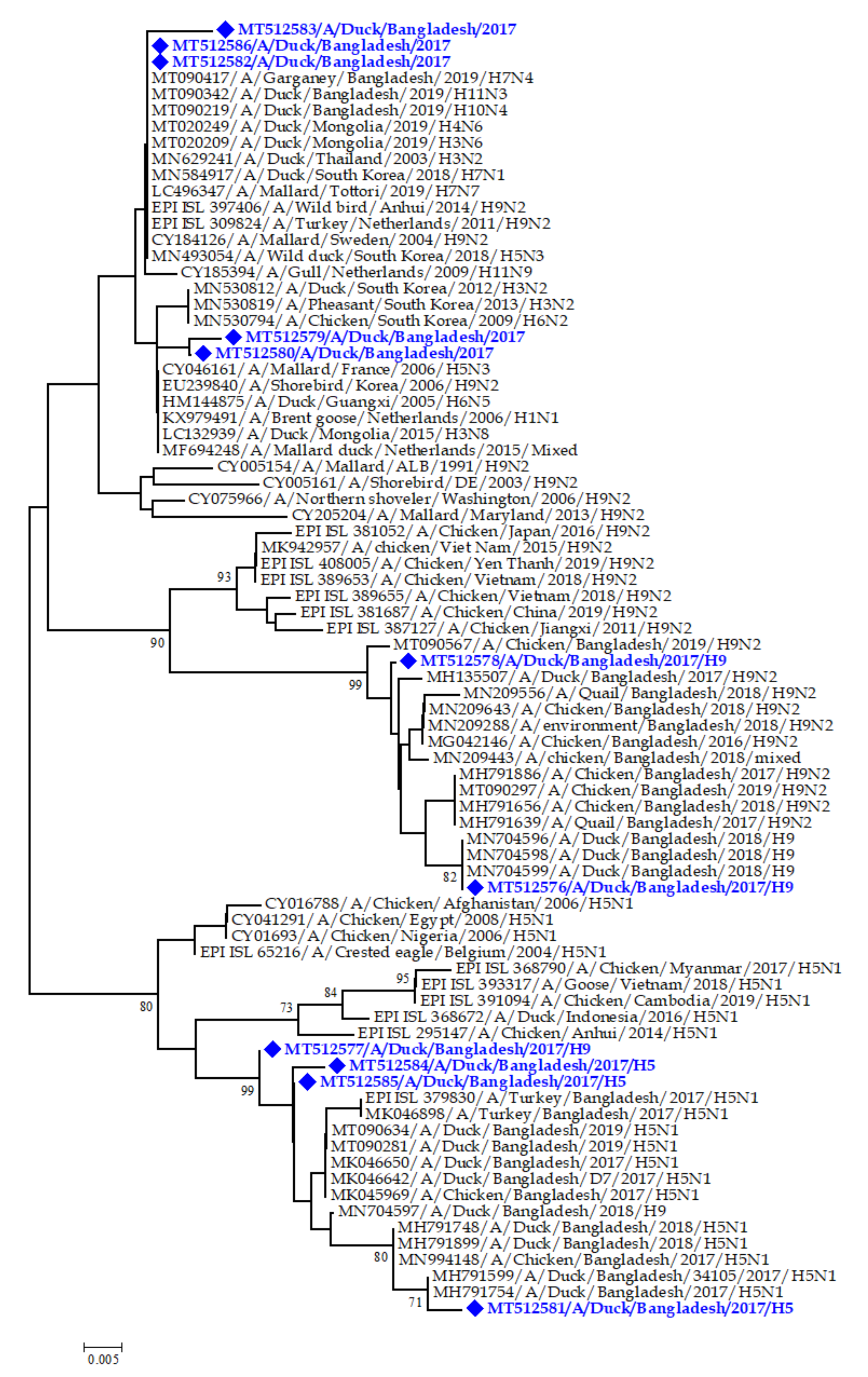
3.7. Phylogenetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Capua, I.; Alexander, D.J. Avian influenza infections in birds—A moving target. Influenza Other Respir. Viruses 2007, 1, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Riedel, S. Crossing the species barrier: The threat of an avian influenza pandemic. In Baylor University Medical Center Proceedings; Taylor & Francis: Abingdon, UK, 2006; Volume 19, pp. 16–20. [Google Scholar]
- Marchenko, V.Y.; Alekseev, A.; Sharshov, K.; Petrov, V.; Silko, N.; Susloparov, I.; Tserennorov, D.; Otgonbaatar, D.; Savchenko, I.; Shestopalov, A. Ecology of influenza virus in wild bird populations in Central Asia. Avian Dis. 2012, 56, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Liu, W.; Cao, Y.; Peng, D.; Wang, X.; Wan, H.; Zhao, G.; Xu, Q.; Zhang, W.; Li, Y.; et al. Novel reassortant highly pathogenic avian influenza (H5N5) viruses in domestic ducks, China. Emerg. Infect. Dis. 2011, 17, 1060–1063. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-R.; Park, C.-K.; Oem, J.-K.; Bae, Y.-C.; Choi, J.-G.; Lee, O.-S.; Lee, Y.-J. Characterization of H5N2 influenza viruses isolated in South Korea and their influence on the emergence of a novel H9N2 influenza virus. J. Gen. Virol. 2010, 91, 1978–1983. [Google Scholar] [CrossRef]
- Cappelle, J.; Zhao, D.; Gilbert, M.; Nelson, M.I.; Newman, S.H.; Takekawa, J.Y.; Gaidet, N.; Prosser, D.J.; Liu, Y.; Li, P. Risks of avian influenza transmission in areas of intensive free-ranging duck production with wild waterfowl. EcoHealth 2014, 11, 109–119. [Google Scholar] [CrossRef]
- Hassan, M.M.; Hoque, M.A.; Ujvari, B.; Klaassen, M. Live bird markets in Bangladesh as a potentially important source for Avian Influenza Virus transmission. Prev. Vet. Med. 2018, 156, 22–27. [Google Scholar] [CrossRef]
- Khatun, A.; Giasuddin, M.; Islam, K.M.; Khanom, S.; Samad, M.A.; Islam, M.R.; Noor, M.; Bhuiyan, J.U.; Kim, W.-I.; Eo, S.K. Surveillance of avian influenza virus type A in semi-scavenging ducks in Bangladesh. BMC Vet. Res. 2013, 9, 196. [Google Scholar] [CrossRef]
- Sarker, R.D.; Giasuddin, M.; Chowdhury, E.H.; Islam, M.R. Serological and virological surveillance of avian influenza virus in domestic ducks of the north-east region of Bangladesh. BMC Vet. Res. 2017, 13, 180. [Google Scholar] [CrossRef]
- Parvin, R.; Kamal, A.H.; Haque, M.E.; Chowdhury, E.H.; Giasuddin, M.; Islam, M.R.; Vahlenkamp, T.W. Genetic characterization of highly pathogenic H5N1 avian influenza virus from live migratory birds in Bangladesh. Virus Genes 2014, 49, 438–448. [Google Scholar] [CrossRef]
- Biswas, P.K.; Christensen, J.P.; Ahmed, S.S.; Barua, H.; Das, A.; Rahman, M.H.; Giasuddin, M.; Hannan, A.S.; Habib, M.A.; Ahad, A. Avian influenza outbreaks in chickens, Bangladesh. Emerg. Infect. Dis. 2008, 14, 1909. [Google Scholar] [CrossRef]
- Gerloff, N.A.; Khan, S.U.; Balish, A.; Shanta, I.S.; Simpson, N.; Berman, L.; Haider, N.; Poh, M.K.; Islam, A.; Gurley, E. Multiple reassortment events among highly pathogenic avian influenza A (H5N1) viruses detected in Bangladesh. Virology 2014, 450, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Das, B.; Mahmud, M.; Amin, M.; Yousuf, M.; Jaber, M.; Belal, S.; Hasan, M.; Hossen, A.; Karim, M. Seroprevalence and detection of avian influenza type A in ducks at Nikli and Bajitpur upazila of Bangladesh. Bangladesh J. Vet. Med. 2015, 13, 11–17. [Google Scholar] [CrossRef]
- Hassan, M.M.; El Zowalaty, M.E.; Islam, A.; Khan, S.A.; Rahman, M.K.; Järhult, J.D.; Hoque, M.A. Prevalence and Diversity of Avian Influenza Virus Hemagglutinin Sero-Subtypes in Poultry and Wild Birds in Bangladesh. Vet. Sci. 2020, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Haider, N.; Sturm-Ramirez, K.; Khan, S.; Rahman, M.; Sarkar, S.; Poh, M.; Shivaprasad, H.; Kalam, M.; Paul, S.; Karmakar, P. Unusually high mortality in waterfowl caused by highly pathogenic avian influenza A (H5N1) in Bangladesh. Transbound. Emerg. Dis. 2017, 64, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.C.; Feeroz, M.M.; Hasan, M.K.; Akhtar, S.; Walker, D.; Seiler, P.; Barman, S.; Franks, J.; Jones-Engel, L.; McKenzie, P. Insight into live bird markets of Bangladesh: An overview of the dynamics of transmission of H5N1 and H9N2 avian influenza viruses. Emerg. Microbes Infect. 2017, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Biswas, P.K.; Christensen, J.P.; Ahmed, S.S.; Das, A.; Rahman, M.H.; Barua, H.; Giasuddin, M.; Hannan, A.S.; Habib, M.A.; Debnath, N.C. Risk for infection with highly pathogenic avian influenza virus (H5N1) in backyard chickens, Bangladesh. Emerg. Infect. Dis. 2009, 15, 1931. [Google Scholar] [CrossRef]
- Loth, L.; Gilbert, M.; Osmani, M.G.; Kalam, A.M.; Xiao, X. Risk factors and clusters of highly pathogenic avian influenza H5N1 outbreaks in Bangladesh. Prev. Vet. Med. 2010, 96, 104–113. [Google Scholar] [CrossRef]
- Ahmed, S.S.; Ersbøll, A.K.; Biswas, P.K.; Christensen, J.P.; Hannan, A.S.; Toft, N. Ecological determinants of highly pathogenic avian influenza (H5N1) outbreaks in Bangladesh. PLoS ONE 2012, 7, e33938. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Shaw, R. Flood and sustainable agriculture in the Haor basin of Bangladesh: A review paper. Univers. J. Agric. Res. 2018, 6, 40–49. [Google Scholar] [CrossRef]
- Jha, B.; Hossain, M.; Baishnab, P.; Mandal, P.; Islam, M. Socio-economic status of duck farmers and duck farming in haor areas of Sylhet district in Bangladesh. Int. J. Nat. Sci. 2015, 5, 73–79. [Google Scholar] [CrossRef]
- Khanum, R.; Al Mahadi, M.S. Economic empowerment of haor women through duck farming in Bangladesh. Agriculturists 2015, 13, 18–25. [Google Scholar] [CrossRef][Green Version]
- Chakraborty, T.R. Management of haors, baors and beels in Bangladesh. Lessons Lake Basin Manag. 2009, 1, 15. [Google Scholar]
- Ghosh, S.; Haider, N.; Khan, M. Status of Household Ducks and their Associated Factors under Scavenging System in a Southern Area of Bangladesh. Int. J. Nat. Sci. 2012, 2, 108–111. [Google Scholar] [CrossRef]
- Hoque, M.; Skerratt, L.; Cook, A.; Khan, S.; Grace, D.; Alam, M.; Vidal-Diez, A.; Debnath, N. Factors limiting the health of semi-scavenging ducks in Bangladesh. Trop. Anim. Health Prod. 2011, 43, 441–450. [Google Scholar] [CrossRef]
- Hoque, M.A.; Hassan, M.M.; Haque, E.; Shaikat, A.H.; Khan, S.A.; Alim, A.; Skerratt, L.F.; Islam, A.; Tun, H.M.; Dissanayake, R. A survey of gastro-intestinal parasitic infection in domestic and wild birds in Chittagong and Greater Sylhet, Bangladesh. Prev. Vet. Med. 2014, 117, 305–312. [Google Scholar] [CrossRef]
- Sarkar, S.; Khan, S.U.; Mikolon, A.; Rahman, M.Z.; Abedin, J.; Zeidner, N.; Sturm-Ramirez, K.; Luby, S.P. An epidemiological study of avian influenza A (H5) virus in nomadic ducks and their raising practices in northeastern Bangladesh, 2011–2012. Influenza Other Respir. Viruses 2017, 11, 275–282. [Google Scholar] [CrossRef]
- Wan, X. Lessons from emergence of a/goose/guangdong/1996-like h5n1 highly pathogenic avian influenza viruses and recent influenza surveillance efforts in southern china. Zoonoses Public Health 2012, 59, 32–42. [Google Scholar] [CrossRef]
- World Health Organization. Cumulative Number of Confirmed Human Cases for Avian Influenza A (H5N1) Reported to WHO, 2003–2020; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/influenza/human_animal_interface/2020_OCT_tableH5N1.pdf (accessed on 11 November 2020).
- Chakma, D.; Rushton, J. Rapid Assessment on Socio-Economic Impact due to Highly Pathogenic Avian Influenza in Bangladesh; Report Submitted to FAO; FAO: Dhaka, Bangladesh, 2008; Volume 35. [Google Scholar]
- Hassan, M.M.; Hoque, M.A.; Debnath, N.C.; Yamage, M.; Klaassen, M. Are poultry or wild birds the main reservoirs for avian influenza in Bangladesh? Ecohealth 2017, 14, 490–500. [Google Scholar] [CrossRef]
- Choudhury, G.A.; Nishat, A. Hydro-Meteorological Characteristics of Hakaluki Haor; International Union for Conservation of Nature (IUCN)-The World Conservation Union, Bangladesh Country Office: Dhaka, Bangladesh, 2005. [Google Scholar]
- Nowreen, S.; Murshed, S.B.; Islam, A.S.; Bhaskaran, B.; Hasan, M.A. Changes of rainfall extremes around the haor basin areas of Bangladesh using multi-member ensemble RCM. Theor. Appl. Climatol. 2015, 119, 363–377. [Google Scholar] [CrossRef]
- Druce, J.; Garcia, K.; Tran, T.; Papadakis, G.; Birch, C. Evaluation of swabs, transport media and specimen transport conditions for optimal detection of viruses by PCR. J. Clin. Microbiol. 2012, 50, 1064–1065. [Google Scholar] [CrossRef]
- Selleck, P. Influenza A Virus: A Competitive ELISA for the Detection of Antibodies to Influenza a Viruses in Equine Sera, Equine Influenza c-ELISA Protocol; Australian Animal Health Laboratory, Disease Diagnostic Project CSIRO Animal Health Laboratory: Geelong, Australia, 2007. [Google Scholar]
- Spackman, E.; Senne, D.A.; Myers, T.; Bulaga, L.L.; Garber, L.P.; Perdue, M.L.; Lohman, K.; Daum, L.T.; Suarez, D.L. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J. Clin. Microbiol. 2002, 40, 3256–3260. [Google Scholar] [CrossRef] [PubMed]
- Monne, I.; Ormelli, S.; Salviato, A.; De Battisti, C.; Bettini, F.; Salomoni, A.; Drago, A.; Zecchin, B.; Capua, I.; Cattoli, G. Development and validation of a one-step real-time PCR assay for simultaneous detection of subtype H5, H7 and H9 avian influenza viruses. J. Clin. Microbiol. 2008, 46, 1769–1773. [Google Scholar] [CrossRef]
- Anthony, S.; Leger, J.S.; Pugliares, K.; Ip, H.S.; Chan, J.; Carpenter, Z.; Navarrete-Macias, I.; Sanchez-Leon, M.; Saliki, J.; Pedersen, J. Emergence of fatal avian influenza in New England harbor seals. MBio 2012, 3, e00166-12. [Google Scholar] [CrossRef] [PubMed]
- Puthavathana, P.; Auewarakul, P.; Charoenying, P.C.; Sangsiriwut, K.; Pooruk, P.; Boonnak, K.; Khanyok, R.; Thawachsupa, P.; Kijphati, R.; Sawanpanyalert, P. Molecular characterization of the complete genome of human influenza H5N1 virus isolates from Thailand. J. Gen. Virol. 2005, 86, 423–433. [Google Scholar] [CrossRef]
- Kimura, D.K. Likelihood methods for the von Bertalanffy growth curve. Fish. Bull. 1980, 77, 765–776. [Google Scholar]
- Ansari, W.K.; Parvej, M.S.; El Zowalaty, M.E.; Jackson, S.; Bustin, S.A.; Ibrahim, A.K.; El Zowalaty, A.E.; Rahman, M.T.; Zhang, H.; Khan, M.F.R. Surveillance, epidemiological and virological detection of highly pathogenic H5N1 avian influenza viruses in duck and poultry from Bangladesh. Vet. Microbiol. 2016, 193, 49–59. [Google Scholar] [CrossRef][Green Version]
- Hassan, M.M.; El Zowalaty, M.E.; Islam, A.; Rahman, M.M.; Chowdhury, M.N.; Nine, H.S.; Rahman, M.K.; Järhult, J.D.; Hoque, M.A. Serological Evidence of Avian Influenza in Captive Wild Birds in a Zoo and Two Safari Parks in Bangladesh. Vet. Sci. 2020, 7, 122. [Google Scholar] [CrossRef]
- Pfeiffer, D.U.; Minh, P.Q.; Martin, V.; Epprecht, M.; Otte, M.J. An analysis of the spatial and temporal patterns of highly pathogenic avian influenza occurrence in Vietnam using national surveillance data. Vet. J. 2007, 174, 302–309. [Google Scholar] [CrossRef]
- Khan, S.U.; Gurley, E.S.; Gerloff, N.; Rahman, M.Z.; Simpson, N.; Rahman, M.; Haider, N.; Chowdhury, S.; Balish, A.; Zaman, R.U. Avian influenza surveillance in domestic waterfowl and environment of live bird markets in Bangladesh, 2007–2012. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- El-Shesheny, R.; Feeroz, M.M.; Krauss, S.; Vogel, P.; McKenzie, P.; Webby, R.J.; Webster, R.G. Replication and pathogenic potential of influenza A virus subtypes H3, H7 and H15 from free-range ducks in Bangladesh in mammals. Emerg. Microbes Infect. 2018, 7, 1–13. [Google Scholar] [CrossRef]
- Latorre-Margalef, N.; Gunnarsson, G.; Munster, V.J.; Fouchier, R.A.; Osterhaus, A.D.; Elmberg, J.; Olsen, B.; Wallensten, A.; Haemig, P.D.; Fransson, T. Effects of influenza A virus infection on migrating mallard ducks. Proc. R. Soc. B Biol. Sci. 2009, 276, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.; Newman, S.H.; Takekawa, J.Y.; Loth, L.; Biradar, C.; Prosser, D.J.; Balachandran, S.; Rao, M.V.S.; Mundkur, T.; Yan, B. Flying over an infected landscape: Distribution of highly pathogenic avian influenza H5N1 risk in South Asia and satellite tracking of wild waterfowl. Ecohealth 2010, 7, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.S.; Ersbøll, A.K.; Biswas, P.K.; Christensen, J.P.; Toft, N. Spatio-temporal magnitude and direction of highly pathogenic avian influenza (H5N1) outbreaks in Bangladesh. PLoS ONE 2011, 6, e24324. [Google Scholar] [CrossRef]
- Giasuddin, M.; Haque, M.; Kamal, A.; Islam, M.; Jahangir, A.; Chowdhury, E.; Taimur, M.; Rahman, M.H. Outbreak evaluation of highly pathogenic avian influenza in Bangladesh. Bangladesh J. Livest. Res. 2012, 19, 44–49. [Google Scholar] [CrossRef][Green Version]
- Lowen, A.C.; Mubareka, S.; Steel, J.; Palese, P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 2007, 3, e151. [Google Scholar] [CrossRef]
- Barman, S.; Marinova-Petkova, A.; Hasan, M.K.; Akhtar, S.; El-Shesheny, R.; Turner, J.C.; Franks, J.; Walker, D.; Seiler, J.; Friedman, K. Role of domestic ducks in the emergence of a new genotype of highly pathogenic H5N1 avian influenza A viruses in Bangladesh. Emerg. Microbes Infect. 2017, 6, 1–13. [Google Scholar] [CrossRef]
- Lickfett, T.M.; Clark, E.; Gehring, T.M.; Alm, E.W. Detection of Influenza A viruses at migratory bird stopover sites in Michigan, USA. Infect. Ecol. Epidemiol. 2018, 8, 1474709. [Google Scholar] [CrossRef]
- Alam, A.; Chowdhury, M.; Sobhan, I. Biodiversity of Tanguar Haor: A Ramsar Site of Bangladesh. Wildl. IUCN Bangladesh Dhaka. 2012, 1, 234. [Google Scholar]
- Hassan, M.M. Who Is the Culprit: Ecology and Epidemiology of Avian Influenza at the Wildlife-Poultry Interface in Bangladesh. Ph.D. Thesis, Deakin Univeristy, Geelong, VIC, Australia, 2017. [Google Scholar]
- Gilbert, M.; Chaitaweesub, P.; Parakamawongsa, T.; Premashthira, S.; Tiensin, T.; Kalpravidh, W.; Wagner, H.; Slingenbergh, J. Free-grazing ducks and highly pathogenic avian influenza, Thailand. Emerg. Infect. Dis. 2006, 12, 227. [Google Scholar] [CrossRef]
- Conan, A.; Goutard, F.L.; Sorn, S.; Vong, S. Biosecurity measures for backyard poultry in developing countries: A systematic review. BMC Vet. Res. 2012, 8, 240. [Google Scholar] [CrossRef]
- Sultana, R.; Rimi, N.A.; Azad, S.; Islam, M.S.; Khan, M.S.U.; Gurley, E.S.; Nahar, N.; Luby, S.P. Bangladeshi backyard poultry raisers’ perceptions and practices related to zoonotic transmission of avian influenza. J. Infect. Dev. Ctries. 2012, 6, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-W.; Suarez, D.L.; Tumpey, T.M.; Sung, H.-W.; Kwon, Y.-K.; Lee, Y.-J.; Choi, J.-G.; Joh, S.-J.; Kim, M.-C.; Lee, E.-K. Characterization of highly pathogenic H5N1 avian influenza A viruses isolated from South Korea. J. Virol. 2005, 79, 3692–3702. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganatham, K.; Feeroz, M.M.; Jones-Engel, L.; Walker, D.; Alam, S.; Hasan, M.; McKenzie, P.; Krauss, S.; Webby, R.J.; Webster, R.G. Genesis of avian influenza H9N2 in Bangladesh. Emerg. Microbes Infect. 2014, 3, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.A.; Tun, H.M.; Hassan, M.M.; Khan, S.A.; Islam, S.A.; Islam, M.N.; Giasuddin, M.; Osmani, T.M.G.; Islam, A.; Thornton, R.N. Molecular epidemiology of influenza A (H5N1) viruses, Bangladesh, 2007–2011. Prev. Vet. Med. 2013, 111, 314–318. [Google Scholar] [CrossRef] [PubMed]


| Type of Sample | Test (n) | Positive Number (n) | Percentage (%) | 95% CI |
|---|---|---|---|---|
| Total ducks | c-ELISA (947) | 604 | 63.8 | 60.6–66.8 |
| rRT-PCR (947) | 101 | 10.7 | 8.8–12.8 | |
| Free-range duck flocks | c-ELISA (62) * | 56 | 90.3 | 80.1–96.4 |
| rRT-PCR (62) * | 8 | 12.9 | 5.7–23.9 | |
| Household duck flocks | c-ELISA (635) | 421 | 66.3 | 62.5–69.9 |
| rRT-PCR (635) | 71 | 11.2 | 8.8–13.9 |
| Factor | Category | c-ELISA | rRT-PCR | ||||
|---|---|---|---|---|---|---|---|
| Number of Positive Samples (%) | 95% CI | p | Number of Positive Samples (%) | 95% CI | p | ||
| Wetlands | Hakaluki haor (471) | 244 (51.8) | 47.2–56.4 | <0.001 | 48 (10.2) | 7.6–13.3 | 0.638 |
| Tanguar haor (476) | 360 (75.6) | 71.5–79.4 | 53 (11.1) | 8.5–14.3 | |||
| Year | 2015 (316) | 201 (63.6) | 58.0–68.9 | 0.912 | 35 (11.1) | 7.8–15.1 | 0.936 |
| 2016 (314) | 198 (63.1) | 57.5–68.4 | 32 (10.2) | 7.1–14.1 | |||
| 2017 (317) | 205 (65.7) | 59.1–69.9 | 34 (10.7) | 7.5–14.6 | |||
| Education (Farmer) | Illiterate (382) | 266 (69.6) | 64.8–74.2 | 0.002 | 50 (13.1) | 9.8–16.9 | 0.047 |
| Literate (565) | 338 (59.8) | 55.6–63.9 | 51 (9.0) | 6.8–11.7 | |||
| Duck type | Household (635) | 421 (66.3) | 62.5–69.9 | 0.021 | 71 (11.2) | 8.8–13.9 | 0.463 |
| Free-range (312) | 183 (58.7) | 52.9–64.2 | 30 (9.6) | 6.5–13.4 | |||
| Contact type | Contact with Chicken (321) | 251 (78.2) | 73.3–82.6 | <0.001 | 71 (22.1) | 17.7–27.1 | <0.001 |
| No contact with Chicken (314) | 170 (54.1) | 48.5–59.7 | 0(0) | 0–1.16 | |||
| Contact with Waterfowl (312) | 183 (58.7) | 52.9–64.2 | 30 (9.6) | 6.6–13.4 | |||
| Factor | Category | c-ELISA | rRT-PCR | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Free-Range Duck Flocks | Household Duck Flocks | Free-Range Duck Flocks | Household Duck Flocks | ||||||
| Number of Positives (%) | p | Number of Positives (%) | p | Number of Positives (%) | p | Number of Positives (%) | p | ||
| Wetlands | Hakaluki haor | 28 (90.3) | 1.000 | 153 (48.4) | <0.001 | 3 (9.7) | 0.449 | 39 (12.3) | 0.356 |
| Tanguar haor | 28 (90.3) | 268 (84.0) | 5 (16.1) | 32 (10.0) | |||||
| Year | 2015 | 19 (90.5) | 0.998 | 140 (66.4) | 0.971 | 3 (14.3) | 0.849 | 25 (11.9) | 0.922 |
| 2016 | 19 (90.5) | 136 (65.7) | 2 (9.5) | 22 (10.6) | |||||
| 2017 | 18 (90) | 145 (66.8) | 3 (15) | 24 (11.1) | |||||
| Education (Farmer) | Illiterate | 18 (90) | 0.953 | 201 (73.6) | 0.001 | 6 (30) | 0.006 | 23 (8.4) | 0.056 |
| Literate | 38 (90.5) | 220 (60.8) | 2 (4.8) | 48 (13.3) | |||||
| Contact type | Contact with Chicken | - | - | 251 (78.2) | <0.001 | - | - | 71 (22.1) | <0.001 |
| No contact with Chicken | - | - | 170 (54.1) | - | - | 0 | |||
| <10 | - | - | 392 (66.2) | 0.870 | - | - | 7 (16.3) | 0.272 | |
| Farm/Flock size | ≥10 | - | - | 29 (67.4) | - | - | 64 (10.8) | ||
| <500 | 38 (92.7) | 0.380 | - | - | 5 (12.2) | 0.816 | - | - | |
| ≥500 | 18 (85.7) | - | - | 3 (14.3) | - | - | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, M.M.; Islam, A.; Hasan, R.B.; Rahman, M.K.; Webby, R.J.; Hoque, M.A.; El Zowalaty, M.E. Prevalence and Distribution of Avian Influenza Viruses in Domestic Ducks at the Waterfowl-Chicken Interface in Wetlands. Pathogens 2020, 9, 953. https://doi.org/10.3390/pathogens9110953
Hassan MM, Islam A, Hasan RB, Rahman MK, Webby RJ, Hoque MA, El Zowalaty ME. Prevalence and Distribution of Avian Influenza Viruses in Domestic Ducks at the Waterfowl-Chicken Interface in Wetlands. Pathogens. 2020; 9(11):953. https://doi.org/10.3390/pathogens9110953
Chicago/Turabian StyleHassan, Mohammad M., Ariful Islam, Rubyath B. Hasan, Md. K. Rahman, Richard J. Webby, Md. A. Hoque, and Mohamed E. El Zowalaty. 2020. "Prevalence and Distribution of Avian Influenza Viruses in Domestic Ducks at the Waterfowl-Chicken Interface in Wetlands" Pathogens 9, no. 11: 953. https://doi.org/10.3390/pathogens9110953
APA StyleHassan, M. M., Islam, A., Hasan, R. B., Rahman, M. K., Webby, R. J., Hoque, M. A., & El Zowalaty, M. E. (2020). Prevalence and Distribution of Avian Influenza Viruses in Domestic Ducks at the Waterfowl-Chicken Interface in Wetlands. Pathogens, 9(11), 953. https://doi.org/10.3390/pathogens9110953









