Disappearance of TBEV Circulation among Rodents in a Natural Focus in Alsace, Eastern France
Abstract
1. Introduction
2. Results
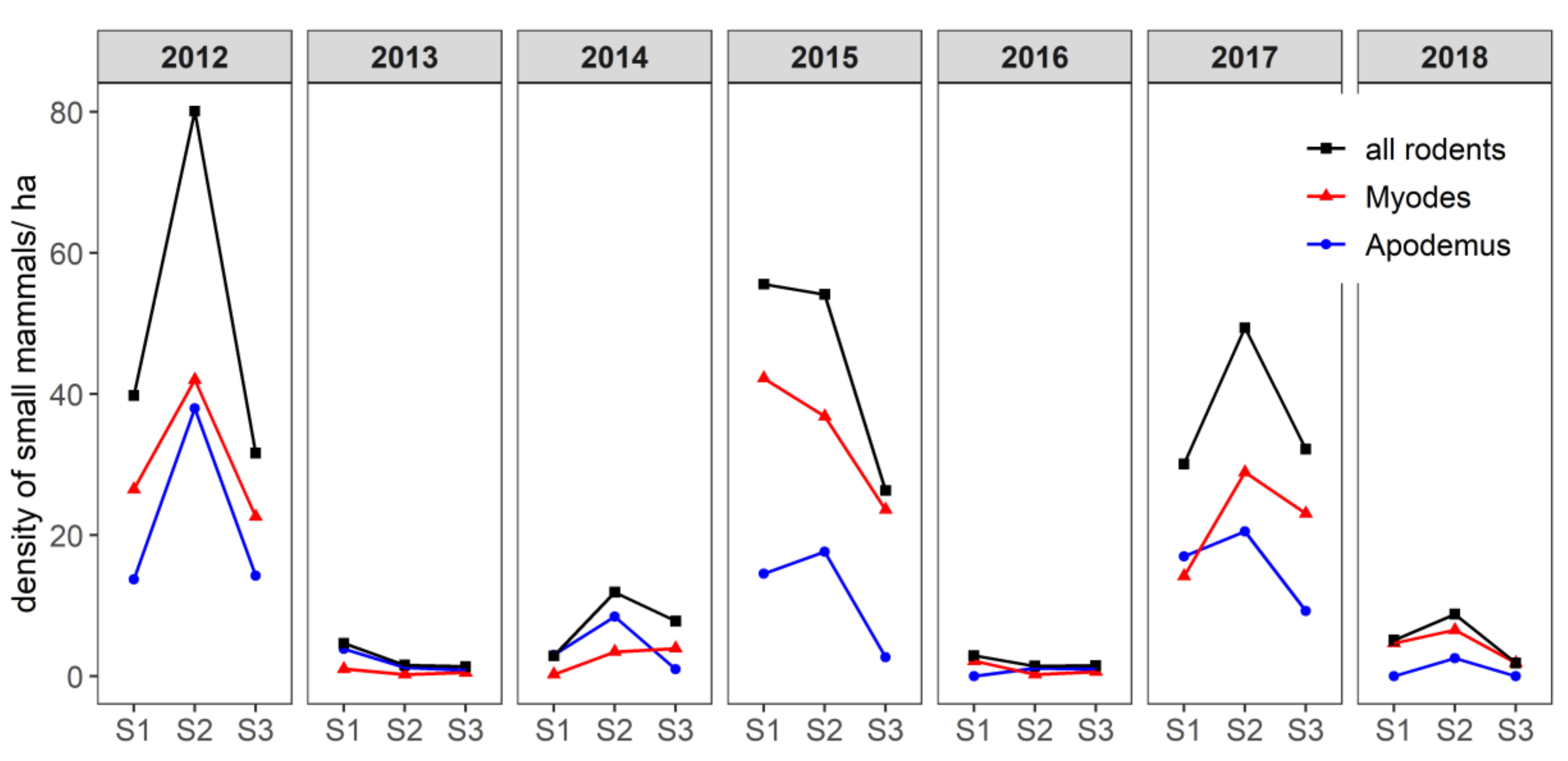
2.1. Small Mammal Abundance
2.2. Prevalence of Tick Infestation in Small Mammals
2.3. Detection of TBEV Antibodies in Small Mammals
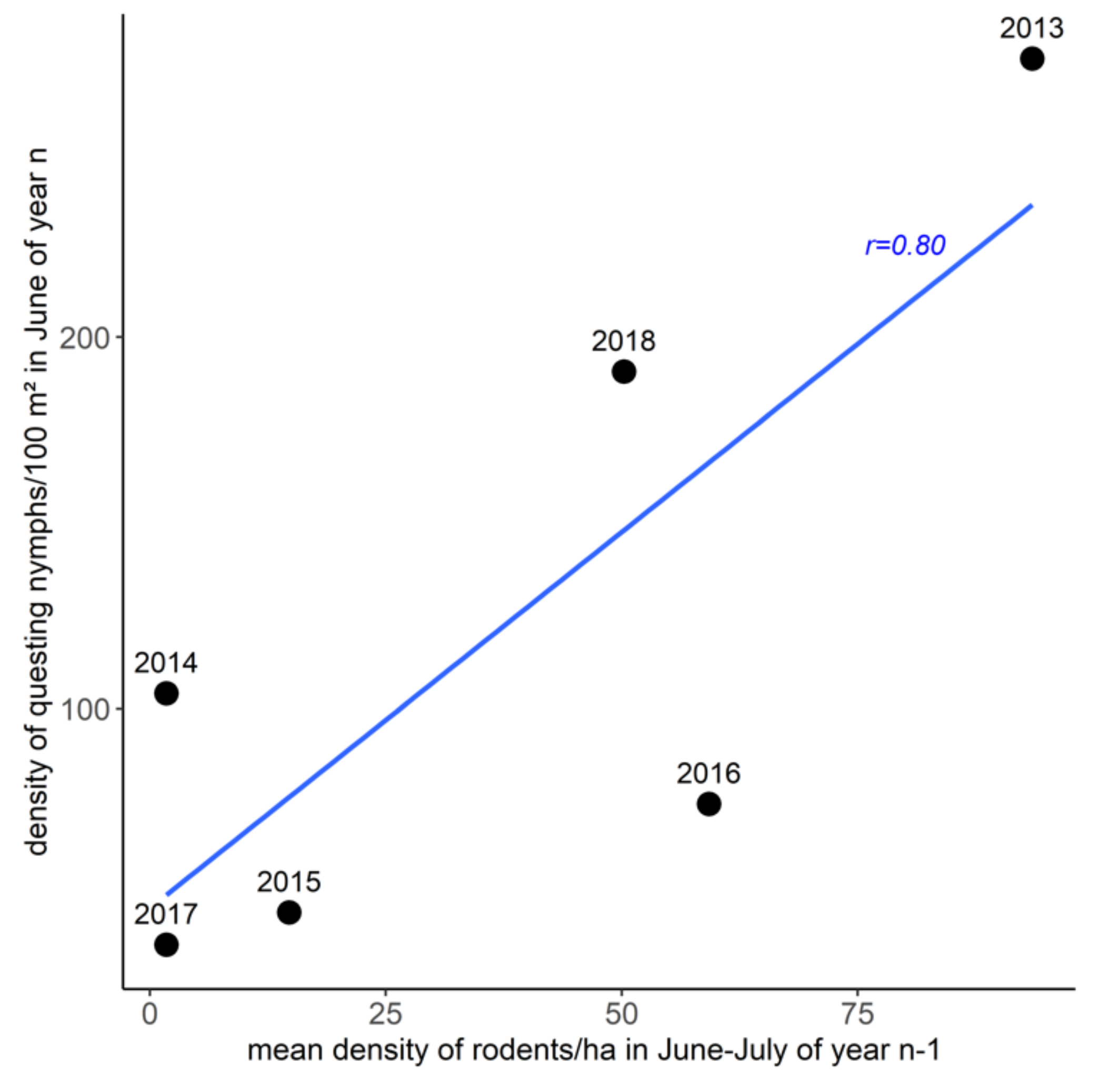
2.4. Questing Tick Densities
3. Discussion
3.1. Synchronous Activity of Larvae and Nymphs in 2012–2018
3.2. Disappearance of TBEV on the Studied Site
4. Conclusions
5. Materials and Methods
5.1. Study Area
5.2. Small Mammals: TBEV-Seroprevalence and Prevalence of Tick Infestation
5.3. Questing Tick Sampling
5.4. Ethical Statement
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beauté, J.; Spiteri, G.; Warns-Petit, E.; Zeller, H. Tick-borne encephalitis in Europe, 2012 to 2016. Eurosurveillance 2018, 23, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Süss, J. Tick-borne encephalitis 2010: Epidemiology, risk areas, and virus strains in Europe and Asia—An overview. Ticks Tick-Borne Dis. 2011, 2, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Hartemink, N.A.; Randolph, S.E.; Davis, S.A.; Heesterbeek, J.A.P. The basic reproduction number for complex disease systems: Defining R0 for tick-borne infections. Am. Nat. 2008, 171, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Randolph, S.E. Transmission of tick-borne pathogens between co-feeding ticks: Milan Labuda’s enduring paradigm. Ticks Tick-Borne Dis. 2011, 2, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, L.; Vapalahti, O. Tick-borne encephalitis. Lancet 2008, 371, 1861–1871. [Google Scholar] [CrossRef]
- Zeman, P. Objective assessment of risk maps of tick-borne encephalitis and Lyme borreliosis based on spatial patterns of located cases. Int. J. Epidemiol. 1997, 26, 1121–1129. [Google Scholar] [CrossRef]
- Randolph, S.E.; Green, R.M.; Peacey, M.F.; Rogers, D.J. Seasonal synchrony: The key to tick-borne encephalitis foci identified by satellite data. Parasitology 2000, 121, 15–23. [Google Scholar] [CrossRef]
- Randolph, S.E. The shifting landscape of tick-borne zoonoses: Tick-borne encephalitis and Lyme borreliosis in Europe. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2001, 356, 1045–1056. [Google Scholar] [CrossRef]
- Rosà, R.; Tagliapietra, V.; Manica, M.; Arnoldi, D.; Hauffe, H.C.; Rossi, C.; Rosso, F.; Henttonen, H.; Rizzoli, A. Changes in host densities and co-feeding pattern efficiently predict tick-borne encephalitis hazard in an endemic focus in northern Italy. Int. J. Parasitol. 2019, 49, 779–787. [Google Scholar] [CrossRef]
- Randolph, S.E.; Storey, K. Impact of microclimate on immature tick-rodent host interactions (Acari: Ixodidae): Implications for parasite transmission. J. Med. Entomol. 1999, 36, 741–748. [Google Scholar] [CrossRef]
- Burri, C.; Bastic, V.; Maeder, G.; Patalas, E.; Gern, L. Microclimate and the zoonotic cycle of tick-borne encephalitis virus in Switzerland. J. Med. Entomol. 2011, 48, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Cagnacci, F.; Bolzoni, L.; Rosà, R.; Carpi, G.; Hauffe, H.C.; Valent, M.; Tagliapietra, V.; Kazimirova, M.; Koci, J.; Stanko, M.; et al. Effects of deer density on tick infestation of rodents and the hazard of tick-borne encephalitis. I: Empirical assessment. Int. J. Parasitol. 2012, 42, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Bolzoni, L.; Rosà, R.; Cagnacci, F.; Rizzoli, A. Effect of deer density on tick infestation of rodents and the hazard of tick-borne encephalitis. II: Population and infection models. Int. J. Parasitol. 2012, 42, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Frimmel, S.; Krienke, A.; Riebold, D.; Loebermann, M.; Littmann, M.; Fiedler, K.; Klaus, C.; Süss, J.; Reisinger, E.C. Tick-borne encephalitis virus habitats in north East Germany: Reemergence of TBEV in ticks after 15 years of inactivity. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Velay, A.; Solis, M.; Kack-Kack, W.; Gantner, P.; Maquart, M.; Martinot, M.; Augereau, O.; De Briel, D.; Kieffer, P.; Lohmann, C.; et al. A new hot spot for tick-borne encephalitis (TBE): A marked increase of TBE cases in France in 2016. Ticks Tick-Borne Dis. 2018, 9, 120–125. [Google Scholar] [CrossRef]
- Bournez, L.; Umhang, G.; Moinet, M.; Richomme, C.; Demerson, J.-M.; Caillot, C.; Devillers, E.; Boucher, J.-M.; Hansmann, Y.; Boué, F.; et al. Tick-borne rncephalitis virus: Seasonal and annual variation of epidemiological parameters related to nymph-to-larva transmission and exposure of small mammals. Pathogens 2020, 9, 518. [Google Scholar] [CrossRef]
- Cannon, R.M. Sense and sensitivity—Designing surveys based on an imperfect test. Prev. Vet. Med. 2001, 49, 141–163. [Google Scholar] [CrossRef]
- Achazi, K.; Růžek, D.; Donoso-Mantke, O.; Schlegel, M.; Ali, H.S.; Wenk, M.; Schmidt-Chanasit, J.; Ohlmeyer, L.; Rühe, F.; Vor, T.; et al. Rodents as sentinels for the prevalence of tick-borne encephalitis virus. Vector-Borne Zoonotic Dis. 2011, 11, 641–647. [Google Scholar] [CrossRef]
- Burri, C.; Korva, M.; Bastic, V.; Knap, N.; Avšič-Županc, T.; Gern, L. Serological evidence of tick-borne encephalitis virus infection in rodents captured at four sites in Switzerland. J. Med. Entomol. 2012, 49, 436–439. [Google Scholar] [CrossRef]
- Perez-Eid, C.; Hannoun, C.; Rodhain, F. The Alsatian tick-borne encephalitis focus: Presence of the virus among ticks and small mammals. Eur. J. Epidemiol. 1992, 8. [Google Scholar] [CrossRef] [PubMed]
- Rosà, R.; Pugliese, A.; Ghosh, M.; Perkins, S.E.; Rizzoli, A. Temporal variation of Ixodes ricinus intensity on the rodent host Apodemus flavicollis in relation to local climate and host dynamics. Vector-Borne Zoonotic Dis. 2007, 7, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Harrison, A.; Bennett, N.C. The importance of the aggregation of ticks on small mammal hosts for the establishment and persistence of tick-borne pathogens: An investigation using the R0 model. Parasitology 2012, 139, 1605–1613. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, J. Ixodes ricinus in relation to its physical environment: II. The factors governing survival and activity. Parasitology 1935, 27, 123–144. [Google Scholar] [CrossRef]
- Herrmann, C.; Gern, L. Survival of Ixodes ricinus (Acari: Ixodidae) nymphs under cold conditions is negatively influenced by frequent temperature variations. Ticks Tick-Borne Dis. 2013, 4, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Hofmeester, T.R.; Coipan, E.C.; van Wieren, S.E.; Prins, H.H.T.; Takken, W.; Sprong, H. Few vertebrate species dominate the Borrelia burgdorferi s.l. life cycle. Environ. Res. Lett. 2016, 11, 043001. [Google Scholar] [CrossRef]
- Gern, L.; Morán Cadenas, F.; Burri, C. Influence of some climatic factors on Ixodes ricinus ticks studied along altitudinal gradients in two geographic regions in Switzerland. Int. J. Med. Microbiol. 2008, 298, 55–59. [Google Scholar] [CrossRef]
- Tagliapietra, V.; Rosà, R.; Arnoldi, D.; Cagnacci, F.; Capelli, G.; Montarsi, F.; Hauffe, H.C.; Rizzoli, A. Saturation deficit and deer density affect questing activity and local abundance of Ixodes ricinus (Acari, Ixodidae) in Italy. Vet. Parasitol. 2011, 183, 114–124. [Google Scholar] [CrossRef]
- Mishaeva, N.P. Effect of abiotic factors on tick-borne encephalitis virus in ixodid ticks. Zdravookhr. Belorus. 1975, 6, 16–17. [Google Scholar]
- Mishaeva, N.P.; Erofeeva, N.I. The effect of diapause of the tick Ixodes ricinus (Ixodidae) upon the multiplication of the tick encephalitis virus in them. Parazitologiya 1979, 13, 218–222. [Google Scholar]
- Korenberg, E.I. Chapter 4 Recent Epidemiology of Tick-Borne Encephalitis: An Effect of Climate Change? In Advances in Virus Research; Elsevier: Burlington, VT, USA, 2009; Volume 74, pp. 123–144. ISBN 978-0-12-378587-9. [Google Scholar]
- Lloyd-Smith, J.O.; Cross, P.C.; Briggs, C.J.; Daugherty, M.; Getz, W.M.; Latto, J.; Sanchez, M.S.; Smith, A.B.; Swei, A. Should we expect population thresholds for wildlife disease? Trends Ecol. Evol. 2005, 20, 511–519. [Google Scholar] [CrossRef]
- Randolph, S.E.; Miklisová, D.; Lysy, J.; Rogers, D.J.; Labuda, M. Incidence from coincidence: Patterns of tick infestations on rodents facilitate transmission of tick-borne encephalitis virus. Parasitology 1999, 118, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Labuda, M.; Randolph, S.E. Survival strategy of tick-borne encephalitis virus: Cellular basis and environmental determinants. Zentralblatt für Bakteriologie 1999, 289, 513–524. [Google Scholar] [CrossRef]
- Dizij, A.; Kurtenbach, K. Clethrionomys glareolus, but not Apodemus flavicollis, acquires resistance to lxodes ricinus L, the main European vector of Borrelia burgdorferi. Parasite Immunol. 1995, 17, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Kurtenbach, K.; Kampen, H.; Dizij, A.; Arndt, S.; Seitz, H.M.; Schaible, U.E.; Simon, M.M. Infestation of rodents with larval Ixodes ricinus (Acari; Ixodidae) is an important factor in the transmission cycle of Borrelia burgdorferi s.l. in German woodlands. J. Med. Entomol. 1995, 32, 807–817. [Google Scholar] [CrossRef]
- Pérez, D.; Kneubühler, Y.; Rais, O.; Gern, L. Seasonality of Ixodes ricinus ticks on vegetation and on rodents and Borrelia burgdorferi sensu lato genospecies diversity in two Lyme borreliosis-endemic areas in Switzerland. Vector-Borne Zoonotic Dis. 2012, 12, 633–644. [Google Scholar] [CrossRef]
- Perez, G.; Bastian, S.; Chastagner, A.; Agoulon, A.; Plantard, O.; Vourc’h, G.; Butet, A. Ecological factors influencing small mammal infection by Anaplasma phagocytophilum and Borrelia burgdorferi s.l. in agricultural and forest landscapes: Tick-borne infection in small mammals. Environ. Microbiol. 2017, 19, 4205–4219. [Google Scholar] [CrossRef]
- Vincent, J.; Gaillard, J.; Bideau, E. Kilometric index as biological indicator for monitoring forest roe deer populations. Acta Theriol. 1991, 36, 315–328. [Google Scholar] [CrossRef]
- Perret, J.-L.; Guigoz, E.; Rais, O.; Gern, L. Influence of saturation deficit and temperature on Ixodes ricinus tick questing activity in a Lyme borreliosis-endemic area (Switzerland). Parasitol. Res. 2000, 86, 554–557. [Google Scholar] [CrossRef]
- Schnabel, Z.E. The estimation of total fish populations of a lake. Am. Math. Mon. 1938, 45, 348–352. [Google Scholar]
- Ostfeld, R.S.; Levi, T.; Keesing, F.; Oggenfuss, K.; Canham, C.D. Tick-borne disease risk in a forest food web. Ecology 2018, 99, 1562–1573. [Google Scholar] [CrossRef]
- Brugger, K.; Walter, M.; Chitimia-Dobler, L.; Dobler, G.; Rubel, F. Forecasting next season’s Ixodes ricinus nymphal density: The example of southern Germany 2018. Exp. Appl. Acarol. 2018, 75, 281–288. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. The R Project for Statistical Computing. Available online: http://www.R-project.org/ (accessed on 20 October 2019).
| Year | Season | No. Infested by Ticks/No. Inspected (%) | No. TBEV-Positive/No. Tested (%) | ||||
|---|---|---|---|---|---|---|---|
| Tot | Myodes | Apodemus | Tot | Myodes | Apodemus | ||
| 2012 | Season 1 | 37/104 (35.6) | 24/72 (33.3) | 13/32 (40.6) | 4/95 (4.2) | 2/65 (3.1) | 2/30 (6.7) |
| Season 2 | 158/359 (44.0) | 54/182 (29.7) | 104/177 (58.8) | 11/349 (3.2) | 5/178 (2.8) | 6/171 (3.5) | |
| Season 3 | 28/121 (23.1) | 22/93 (23.7) | 6/28 (21.4) | 5/97 (5.2) | 4/75 (5.3) | 1/22 (4.5) | |
| 2013 | Season 1 | 2/16 (12.5) | 0/4 (0) | 2/12 (16.7) | 0/16 (0) | 0/4 (0) | 0/12 (0) |
| Season 2 | 11/11 (100) | 3/3 (100) | 8/8 (100) | 1/10 (1.0) | 0/3 (0) | 1/7 (14.3) | |
| Season 3 | 8/10 (80.0) | 2/3 (66.7) | 6/7 (85.7) | 0/10 (0) | 0/3 (0) | 0/7 (0) | |
| 2014 | Season 1 | 4/7 (57.1) | 1/1 (100) | 3/6 (50) | 0/7 (0) | 0/1 (0) | 0/6 (0) |
| Season 2 | 65/69 (94.2) | 17/20 (85.0) | 48/49 (98.0) | 0/60 (0) | 0/16 (0) | 0/44 (0) | |
| Season 3 | 11/20 (55.0) | 8/12 (66.7) | 3/8 (37.5) | 1/20 (5.0) | 1/12 (8.3) | 0/8 (0) | |
| 2015 | Season 1 | 5/129 (3.9) | 4/89 (4.5) | 1/40 (2.5) | 1/79 (1.3) | 1/51 (2.0) | 0/28 (0) |
| Season 2 | 87/246 (35.4) | 42/163 (25.8) | 45/83 (54.2) | 3/220 (1.4) | 2/143 (1.3) | 1/77 (1.3) | |
| Season 3 | 25/138 (18.1) | 19/123 (15.5) | 6/15 (33.3) | 2/122 (1.6) | 2/109 (1.8) | 0/13 (0) | |
| 2016 | Season 1 | 8/8 (100) | 6/6 (100) | 2/2 (100) | 0/10 (0) | 0/8 (0) | 0/2 (0) |
| Season 2 | 9/9 (100) | 2/2 (100) | 7/7 (100) | 0/9 (0) | 0/2 (0) | 0/7 (0) | |
| Season 3 | 2/7 (28.6) | 2/4 (50.0) | 0/3 (0) | 0/5 (0) | 0/2 (0) | 0/3 (0) | |
| 2017 | Season 1 | 77 * | 41 * | 36 * | 0/75 (0) | 0/40 (0) | 0/35 (0) |
| Season 2 | 12/253 (4.7) | 7/142 (5.0) | 5/111 (4.5) | 0/241 (0) | 0/136 (0) | 0/105 (0) | |
| Season 3 | 53/166 (31.9) | 33/106 (31.3) | 20/60 (33.3) | 0/162 (0) | 0/106 (0) | 0/56 (0) | |
| 2018 | Season 1 | 10/20 (50.0) | 9/18 (50.0) | 1/2 (50.0) | 0/20 (0) | 0/18 (0) | 0/2 (0) |
| Season 2 | 39/45 (86.7) | 28/32 (87.5) | 11/13 (84.6) | 0/42 (0) | 0/31 (0) | 0/11 (0) | |
| Season 3 | 6/6 (100.0) | 6/6 (100.0) | 0 | 0/6 (0) | 0/6 (0) | 0/6 (0) | |
| Year | Density of Questing Ticks (/100 m2) | |
|---|---|---|
| Early June | Early September | |
| 2012 | 86.5 | 6.3 |
| 2013 | 275.0 | 39.0 |
| 2014 | 104.2 | 6.0 |
| 2015 | 45.2 | 27.7 |
| 2016 | 74.4 | 5.6 |
| 2017 | 36.5 | 19.6 |
| 2018 | 190.8 | 2.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bournez, L.; Umhang, G.; Moinet, M.; Boucher, J.-M.; Demerson, J.-M.; Caillot, C.; Legras, L.; Devillers, E.; Hansmann, Y.; Velay, A.; et al. Disappearance of TBEV Circulation among Rodents in a Natural Focus in Alsace, Eastern France. Pathogens 2020, 9, 930. https://doi.org/10.3390/pathogens9110930
Bournez L, Umhang G, Moinet M, Boucher J-M, Demerson J-M, Caillot C, Legras L, Devillers E, Hansmann Y, Velay A, et al. Disappearance of TBEV Circulation among Rodents in a Natural Focus in Alsace, Eastern France. Pathogens. 2020; 9(11):930. https://doi.org/10.3390/pathogens9110930
Chicago/Turabian StyleBournez, Laure, Gerald Umhang, Marie Moinet, Jean-Marc Boucher, Jean-Michel Demerson, Christophe Caillot, Léo Legras, Elodie Devillers, Yves Hansmann, Aurélie Velay, and et al. 2020. "Disappearance of TBEV Circulation among Rodents in a Natural Focus in Alsace, Eastern France" Pathogens 9, no. 11: 930. https://doi.org/10.3390/pathogens9110930
APA StyleBournez, L., Umhang, G., Moinet, M., Boucher, J.-M., Demerson, J.-M., Caillot, C., Legras, L., Devillers, E., Hansmann, Y., Velay, A., Richomme, C., Moutailler, S., & Boué, F. (2020). Disappearance of TBEV Circulation among Rodents in a Natural Focus in Alsace, Eastern France. Pathogens, 9(11), 930. https://doi.org/10.3390/pathogens9110930






