Antibody-Based Immunotherapeutic Strategies for COVID-19
Abstract
1. Introduction
2. Some Recent Findings on the Immunobiology of SARS-CoV-2 Infection
3. Some Putative Mechanisms for the Immunopathology of SARS-CoV2 Infection
4. The Antibody-Based Therapies for SARS-CoV-2 Infections
4.1. Antiviral Neutralizing Antibody-Based Therapeutics
4.2. SARS-CoV-2 Immunomodulatory Antibody-Based Therapeutics
5. Overview and Analysis of the Current Clinical Trials
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Munster, V.J.; Koopmans, M.; van Doremalen, N.; van Riel, D.; de Wit, E. A Novel Coronavirus Emerging in China—Key Questions for Impact Assessment. N. Engl. J. Med. 2020, 382, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Iacobazzi, D.; Baquedano, M.; Madeddu, P.; Caputo, M. COVID-19, State of the Adult and Pediatric Heart: From Myocardial Injury to Cardiac Effect of Potential Therapeutic Intervention. Front. Cardiovasc Med. 2020, 7, 140. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020, 130, 2620–2629. [Google Scholar] [CrossRef] [PubMed]
- Shereen, M.A.; Khan, S.; Kazmi, A.; Bashir, N.; Siddique, R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020, 24, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, S. COVID-19: Face masks and human-to-human transmission. Influenza Other Respir. Viruses 2020, 14, 472–473. [Google Scholar] [CrossRef] [PubMed]
- Awadasseid, A.; Wu, Y.; Tanaka, Y.; Zhang, W. Initial success in the identification and management of the coronavirus disease 2019 (COVID-19) indicates human-to-human transmission in Wuhan, China. Int. J. Biol. Sci. 2020, 16, 1846–1860. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Bouadma, L.; Wiedemann, A.; Patrier, J.; Surenaud, M.; Wicky, P.H.; Foucat, E.; Diehl, J.L.; Hejblum, B.P.; Sinnah, F.; de Montmollin, E.; et al. Immune Alterations in a Patient with SARS-CoV-2-Related Acute Respiratory Distress Syndrome. J. Clin. Immunol. 2020. [Google Scholar] [CrossRef]
- Badraoui, R.; Alrashedi, M.M.; El-May, M.V.; Bardakci, F. Acute respiratory distress syndrome: A life threatening associated complication of SARS-CoV-2 infection inducing COVID-19. J. Biomol. Struct. Dyn. 2020, 1–10. [Google Scholar] [CrossRef]
- Barbeta, E.; Motos, A.; Torres, A.; Ceccato, A.; Ferrer, M.; Cilloniz, C.; Bueno, L.; Badia, J.R.; Castro, P.; Ferrando, C.; et al. SARS-CoV-2-induced Acute Respiratory Distress Syndrome: Pulmonary Mechanics and Gas-Exchange Abnormalities. Ann. Am. Thorac. Soc. 2020, 17, 1164–1168. [Google Scholar] [CrossRef]
- D’Abramo, A.; Lepore, L.; Palazzolo, C.; Barreca, F.; Liuzzi, G.; Lalle, E.; Nicastri, E. Acute respiratory distress syndrome due to SARS-CoV-2 and Influenza A co-infection in an Italian patient: Mini-review of the literature. Int. J. Infect. Dis. 2020, 97, 236–239. [Google Scholar] [CrossRef]
- Peiris, J.S. Severe Acute Respiratory Syndrome (SARS). J. Clin. Virol. 2003, 28, 245–247. [Google Scholar] [CrossRef]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620. [Google Scholar] [CrossRef]
- Bristow, M.R.; Zisman, L.S.; Altman, N.L.; Gilbert, E.M.; Lowes, B.D.; Minobe, W.A.; Slavov, D.; Schwisow, J.A.; Rodriguez, E.M.; Carroll, I.A.; et al. Dynamic Regulation of SARS-Cov-2 Binding and Cell Entry Mechanisms in Remodeled Human Ventricular Myocardium. JACC Basic Transl. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Darvish-Damavandi, M.; Laycock, J.; Ward, C.; van Driel, M.S.; Goldgraben, M.A.; Buczacki, S.J. An analysis of SARS-CoV-2 cell entry genes identifies the intestine and colorectal cancer as susceptible tissues. Br. J. Surg. 2020. [Google Scholar] [CrossRef]
- Bilinska, K.; Jakubowska, P.; Von Bartheld, C.S.; Butowt, R. Expression of the SARS-CoV-2 Entry Proteins, ACE2 and TMPRSS2, in Cells of the Olfactory Epithelium: Identification of Cell Types and Trends with Age. ACS Chem. Neurosci. 2020, 11, 1555–1562. [Google Scholar] [CrossRef]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef]
- Keam, S.; Megawati, D.; Patel, S.K.; Tiwari, R.; Dhama, K.; Harapan, H. Immunopathology and immunotherapeutic strategies in severe acute respiratory syndrome coronavirus 2 infection. Rev. Med. Virol. 2020. [Google Scholar] [CrossRef]
- Florindo, H.F.; Kleiner, R.; Vaskovich-Koubi, D.; Acurcio, R.C.; Carreira, B.; Yeini, E.; Tiram, G.; Liubomirski, Y.; Satchi-Fainaro, R. Immune-mediated approaches against COVID-19. Nat. Nanotechnol. 2020, 15, 630–645. [Google Scholar] [CrossRef]
- Kawamura, Y.; Higashimoto, Y.; Miura, H.; Ihira, M.; Inaba, M.; Ito, R.; Kozawa, K.; Yoshikawa, T. Immune response against SARS-CoV-2 in pediatric patients including young infants. J. Med. Virol. 2020. [Google Scholar] [CrossRef]
- Portela Sousa, C.; Brites, C. Immune response in SARS-CoV-2 infection: The role of interferons type I and type III. Braz. J. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Herroelen, P.H.; Martens, G.A.; De Smet, D.; Swaerts, K.; Decavele, A.S. Humoral Immune Response to SARS-CoV-2. Am. J. Clin. Pathol. 2020. [Google Scholar] [CrossRef]
- Shurin, M.R.; Morris, A.; Wells, A.; Wheeler, S.E. Assessing Immune Response to SARS-CoV-2 Infection. ImmunoTargets Ther. 2020, 9, 111–114. [Google Scholar] [CrossRef]
- Garcia-Salido, A. Narrative review of the immune response against coronavirus: An overview, applicability for SARS-COV-2, and therapeutic implications. An. Pediatr. (Barc.) 2020, 93, 60.e1–60.e7. [Google Scholar] [CrossRef]
- Azkur, A.K.; Akdis, M.; Azkur, D.; Sokolowska, M.; van de Veen, W.; Bruggen, M.C.; O’Mahony, L.; Gao, Y.; Nadeau, K.; Akdis, C.A. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy 2020, 75, 1564–1581. [Google Scholar] [CrossRef] [PubMed]
- di Mauro, G.; Cristina, S.; Concetta, R.; Francesco, R.; Annalisa, C. SARS-Cov-2 infection: Response of human immune system and possible implications for the rapid test and treatment. Int. Immunopharmacol. 2020, 84, 106519. [Google Scholar] [CrossRef]
- Kruse, R.L. Therapeutic strategies in an outbreak scenario to treat the novel coronavirus originating in Wuhan, China. F1000Research 2020, 9, 72. [Google Scholar] [CrossRef]
- Abdullahi, I.N.; Emeribe, A.U.; Adekola, H.A.; Abubakar, S.D.; Dangana, A.; Shuwa, H.A.; Nwoba, S.T.; Mustapha, J.O.; Haruna, M.T.; Olowookere, K.A.; et al. Leveraging on the genomics and immunopathology of SARS-CoV-2 for vaccines development: Prospects and challenges. Hum. Vaccin. Immunother. 2020, 1–18. [Google Scholar] [CrossRef]
- Beacon, T.H.; Su, R.C.; Lakowski, T.M.; Delcuve, G.P.; Davie, J.R. SARS-CoV-2 multifaceted interaction with the human host. Part II: Innate immunity response, immunopathology, and epigenetics. IUBMB Life 2020. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Di Gioacchino, M.; Tonacci, A.; Musolino, C.; Gangemi, S. Immunopathology of SARS-CoV-2 Infection: Immune Cells and Mediators, Prognostic Factors, and Immune-Therapeutic Implications. Int. J. Mol. Sci. 2020, 21, 4782. [Google Scholar] [CrossRef]
- Dhama, K.; Patel, S.K.; Pathak, M.; Yatoo, M.I.; Tiwari, R.; Malik, Y.S.; Singh, R.; Sah, R.; Rabaan, A.A.; Bonilla-Aldana, D.K.; et al. An update on SARS-CoV-2/COVID-19 with particular reference to its clinical pathology, pathogenesis, immunopathology and mitigation strategies. Travel Med. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Li, G.; Fan, Y.; Lai, Y.; Han, T.; Li, Z.; Zhou, P.; Pan, P.; Wang, W.; Hu, D.; Liu, X.; et al. Coronavirus infections and immune responses. J. Med. Virol. 2020, 92, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Prompetchara, E.; Ketloy, C.; Palaga, T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020, 38, 1–9. [Google Scholar] [CrossRef]
- Garcia, L.F. Immune Response, Inflammation, and the Clinical Spectrum of COVID-19. Front. Immunol. 2020, 11, 1441. [Google Scholar] [CrossRef]
- Thevarajan, I.; Nguyen, T.H.O.; Koutsakos, M.; Druce, J.; Caly, L.; van de Sandt, C.E.; Jia, X.; Nicholson, S.; Catton, M.; Cowie, B.; et al. Breadth of concomitant immune responses prior to patient recovery: A case report of non-severe COVID-19. Nat. Med. 2020, 26, 453–455. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.; Tsang, O.T.; Leung, W.S.; Tam, A.R.; Wu, T.C.; Lung, D.C.; Yip, C.C.; Cai, J.P.; Chan, J.M.; Chik, T.S.; et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 2020, 20, 565–574. [Google Scholar] [CrossRef]
- D’Elia, R.V.; Harrison, K.; Oyston, P.C.; Lukaszewski, R.A.; Clark, G.C. Targeting the “cytokine storm” for therapeutic benefit. Clin. Vaccine Immunol. 2013, 20, 319–327. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J.; Hlh Across Speciality Collaboration, U.K. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Li, J.; Gong, X.; Wang, Z.; Chen, R.; Li, T.; Zeng, D.; Li, M. Clinical features of familial clustering in patients infected with 2019 novel coronavirus in Wuhan, China. Virus Res. 2020, 286, 198043. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Correction to: Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 1294–1297. [Google Scholar] [CrossRef]
- Cao, X. COVID-19: Immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020, 20, 269–270. [Google Scholar] [CrossRef]
- Zheng, M.; Gao, Y.; Wang, G.; Song, G.; Liu, S.; Sun, D.; Xu, Y.; Tian, Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol. Immunol. 2020, 17, 533–535. [Google Scholar] [CrossRef]
- Epidemiology Working Group for Ncip Epidemic Response; Chinese Center for Disease Control and Prevention. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi 2020, 41, 145–151. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, X.; Zhu, C.; Song, Y.; Feng, F.; Qiu, Y.; Feng, J.; Jia, Q.; Song, Q.; Zhu, B.; et al. Immune Phenotyping Based on the Neutrophil-to-Lymphocyte Ratio and IgG Level Predicts Disease Severity and Outcome for Patients With COVID-19. Front. Mol. Biosci. 2020, 7, 157. [Google Scholar] [CrossRef]
- Zhao, J.; Yuan, Q.; Wang, H.; Liu, W.; Liao, X.; Su, Y.; Wang, X.; Yuan, J.; Li, T.; Li, J.; et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Zhao, Q. Perspectives on therapeutic neutralizing antibodies against the Novel Coronavirus SARS-CoV-2. Int. J. Biol. Sci. 2020, 16, 1718–1723. [Google Scholar] [CrossRef]
- AminJafari, A.; Ghasemi, S. The possible of immunotherapy for COVID-19: A systematic review. Int. Immunopharmacol. 2020, 83, 106455. [Google Scholar] [CrossRef]
- Abraham, J. Passive antibody therapy in COVID-19. Nat. Rev. Immunol. 2020, 20, 401–403. [Google Scholar] [CrossRef]
- Xi, Y. Convalescent plasma therapy for COVID-19: A tried-and-true old strategy? Signal Transduct. Target. Ther. 2020, 5, 203. [Google Scholar] [CrossRef]
- Casadevall, A.; Pirofski, L.A. The convalescent sera option for containing COVID-19. J. Clin. Invest. 2020, 130, 1545–1548. [Google Scholar] [CrossRef]
- Duan, K.; Liu, B.; Li, C.; Zhang, H.; Yu, T.; Qu, J.; Zhou, M.; Chen, L.; Meng, S.; Hu, Y.; et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. USA 2020, 117, 9490–9496. [Google Scholar] [CrossRef]
- Patel, A.; Bah, M.A.; Weiner, D.B. In Vivo Delivery of Nucleic Acid-Encoded Monoclonal Antibodies. BioDrugs 2020, 34, 273–293. [Google Scholar] [CrossRef]
- Baum, A.; Ajithdoss, D.; Copin, R.; Zhou, A.; Lanza, K.; Negron, N.; Ni, M.; Wei, Y.; Mohammadi, K.; Musser, B.; et al. REGN-COV2 antibodies prevent and treat SARS-CoV-2 infection in rhesus macaques and hamsters. Science 2020. [Google Scholar] [CrossRef]
- Tortorici, M.A.; Beltramello, M.; Lempp, F.A.; Pinto, D.; Dang, H.V.; Rosen, L.E.; McCallum, M.; Bowen, J.; Minola, A.; Jaconi, S.; et al. Ultrapotent human antibodies protect against SARS-CoV-2 challenge via multiple mechanisms. Science 2020. [Google Scholar] [CrossRef]
- Gustine, J.N.; Jones, D. Immunopathology of Hyperinflammation in COVID-19. Am. J. Pathol. 2020. [Google Scholar] [CrossRef]
- Boziki, M.K.; Mentis, A.A.; Shumilina, M.; Makshakov, G.; Evdoshenko, E.; Grigoriadis, N. COVID-19 Immunopathology and the Central Nervous System: Implication for Multiple Sclerosis and Other Autoimmune Diseases with Associated Demyelination. Brain Sci. 2020, 10, 345. [Google Scholar] [CrossRef]
- Lega, S.; Naviglio, S.; Volpi, S.; Tommasini, A. Recent Insight into SARS-CoV2 Immunopathology and Rationale for Potential Treatment and Preventive Strategies in COVID-19. Vaccines (Basel) 2020, 8, 224. [Google Scholar] [CrossRef]
- Chiappelli, F.; Khakshooy, A.; Greenberg, G. CoViD-19 Immunopathology and Immunotherapy. Bioinformation 2020, 16, 219–222. [Google Scholar] [CrossRef]
- Henderson, L.A.; Canna, S.W.; Schulert, G.S.; Volpi, S.; Lee, P.Y.; Kernan, K.F.; Caricchio, R.; Mahmud, S.; Hazen, M.M.; Halyabar, O.; et al. On the Alert for Cytokine Storm: Immunopathology in COVID-19. Arthritis Rheumatol. 2020, 72, 1059–1063. [Google Scholar] [CrossRef]
- Alzghari, S.K.; Acuna, V.S. Supportive Treatment with Tocilizumab for COVID-19: A Systematic Review. J. Clin. Virol. 2020, 127, 104380. [Google Scholar] [CrossRef]
- Marovich, M.; Mascola, J.R.; Cohen, M.S. Monoclonal Antibodies for Prevention and Treatment of COVID-19. JAMA 2020, 324, 131–132. [Google Scholar] [CrossRef]
- Zhao, M. Cytokine storm and immunomodulatory therapy in COVID-19: Role of chloroquine and anti-IL-6 monoclonal antibodies. Int. J. Antimicrob. Agents 2020, 55, 105982. [Google Scholar] [CrossRef]
- Sheng, C.C.; Sahoo, D.; Dugar, S.; Prada, R.A.; Wang, T.K.M.; Abou Hassan, O.K.; Brennan, D.; Culver, D.A.; Rajendram, P.; Duggal, A.; et al. Canakinumab to reduce deterioration of cardiac and respiratory function in SARS-CoV-2 associated myocardial injury with heightened inflammation (canakinumab in Covid-19 cardiac injury: The three C study). Clin. Cardiol. 2020. [Google Scholar] [CrossRef]
- Ucciferri, C.; Auricchio, A.; Di Nicola, M.; Potere, N.; Abbate, A.; Cipollone, F.; Vecchiet, J.; Falasca, K. Canakinumab in a subgroup of patients with COVID-19. Lancet Rheumatol. 2020, 2, e457–e458. [Google Scholar] [CrossRef]
- Akinosoglou, K.; Gogos, C. Severe COVID-19 and interleukin-6 receptor antagonist tocilizumab: Some notes of concern. Respirology 2020. [Google Scholar] [CrossRef]
- Zeng, J.; Xie, M.H.; Yang, J.; Chao, S.W.; Xu, E.L. Clinical efficacy of tocilizumab treatment in severe and critical COVID-19 patients. World J. Clin. Cases 2020, 8, 3763–3773. [Google Scholar] [CrossRef] [PubMed]
- Masia, M.; Fernandez-Gonzalez, M.; Padilla, S.; Ortega, P.; Garcia, J.A.; Agullo, V.; Garcia-Abellan, J.; Telenti, G.; Guillen, L.; Gutierrez, F. Impact of interleukin-6 blockade with tocilizumab on SARS-CoV-2 viral kinetics and antibody responses in patients with COVID-19: A prospective cohort study. EBioMedicine 2020, 60, 102999. [Google Scholar] [CrossRef]
- Aziz, M.; Haghbin, H.; Sitta, E.A.; Nawras, Y.; Fatima, R.; Sharma, S.; Lee-Smith, W.; Duggan, J.; Kammeyer, J.A.; Hanarahan, J.; et al. Efficacy of Tocilizumab in COVID-19: A Systematic review and Meta-Analysis. J. Med. Virol. 2020. [Google Scholar] [CrossRef]
- Piano, S.; Vettor, R.; Angeli, P. COVID-LIVER study group. Tocilizumab for severe COVID-19 pneumonia. Lancet Rheumatol. 2020. [Google Scholar] [CrossRef]
- Soy, M.; Keser, G.; Atagunduz, P.; Tabak, F.; Atagunduz, I.; Kayhan, S. Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020, 39, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- Pacha, O.; Sallman, M.A.; Evans, S.E. COVID-19: A case for inhibiting IL-17? Nat. Rev. Immunol. 2020, 20, 345–346. [Google Scholar] [CrossRef]
- Megna, M.; Napolitano, M.; Fabbrocini, G. May IL-17 have a role in COVID-19 infection? Med. Hypotheses 2020, 140, 109749. [Google Scholar] [CrossRef]
- Mendoza, V.M.M. Interleukin-17: A potential therapeutic target in COVID-19. J. Infect. 2020, 81, e136–e138. [Google Scholar] [CrossRef] [PubMed]
- Quartuccio, L.; Semerano, L.; Benucci, M.; Boissier, M.C.; De Vita, S. Urgent avenues in the treatment of COVID-19: Targeting downstream inflammation to prevent catastrophic syndrome. Joint Bone Spine 2020, 87, 191–193. [Google Scholar] [CrossRef]
- Risitano, A.M.; Mastellos, D.C.; Huber-Lang, M.; Yancopoulou, D.; Garlanda, C.; Ciceri, F.; Lambris, J.D. Complement as a target in COVID-19? Nat. Rev. Immunol. 2020, 20, 343–344. [Google Scholar] [CrossRef]
- Carvelli, J.; Demaria, O.; Vely, F.; Batista, L.; Benmansour, N.C.; Fares, J.; Carpentier, S.; Thibult, M.L.; Morel, A.; Remark, R.; et al. Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis. Nature 2020. [Google Scholar] [CrossRef]
- Hamilton, J.A. GM-CSF-Dependent Inflammatory Pathways. Front. Immunol. 2019, 10, 2055. [Google Scholar] [CrossRef]
- De Luca, G.; Cavalli, G.; Campochiaro, C.; Della-Torre, E.; Angelillo, P.; Tomelleri, A.; Boffini, N.; Tentori, S.; Mette, F.; Farina, N.; et al. GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: A single-centre, prospective cohort study. Lancet Rheumatol. 2020, 2, e465–e473. [Google Scholar] [CrossRef]
- Bonaventura, A.; Vecchie, A.; Wang, T.S.; Lee, E.; Cremer, P.C.; Carey, B.; Rajendram, P.; Hudock, K.M.; Korbee, L.; Van Tassell, B.W.; et al. Targeting GM-CSF in COVID-19 Pneumonia: Rationale and Strategies. Front. Immunol. 2020, 11, 1625. [Google Scholar] [CrossRef]
- Lang, F.M.; Lee, K.M.; Teijaro, J.R.; Becher, B.; Hamilton, J.A. GM-CSF-based treatments in COVID-19: Reconciling opposing therapeutic approaches. Nat. Rev. Immunol. 2020, 20, 507–514. [Google Scholar] [CrossRef]
- Neri, T.; Nieri, D.; Celi, A. P-selectin blockade in COVID-19-related ARDS. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L1237–L1238. [Google Scholar] [CrossRef]
- Man, Y.; Goreke, U.; Kucukal, E.; Hill, A.; An, R.; Liu, S.; Bode, A.; Solis-Fuentes, A.; Nayak, L.V.; Little, J.A.; et al. Leukocyte adhesion to P-selectin and the inhibitory role of Crizanlizumab in sickle cell disease: A standardized microfluidic assessment. Blood Cells Mol. Dis. 2020, 83, 102424. [Google Scholar] [CrossRef]
- Grobler, C.; Maphumulo, S.C.; Grobbelaar, L.M.; Bredenkamp, J.C.; Laubscher, G.J.; Lourens, P.J.; Steenkamp, J.; Kell, D.B.; Pretorius, E. Covid-19: The Rollercoaster of Fibrin(Ogen), D-Dimer, Von Willebrand Factor, P-Selectin and Their Interactions with Endothelial Cells, Platelets and Erythrocytes. Int. J. Mol. Sci. 2020, 21, 5168. [Google Scholar] [CrossRef]
- Patterson, B.K.; Seethamraju, H.; Dhody, K.; Corley, M.J.; Kazempour, K.; Lalezari, J.P.; Pang, A.P.; Sugai, C.; Francisco, E.B.; Pise, A.; et al. Disruption of the CCL5/RANTES-CCR5 Pathway Restores Immune Homeostasis and Reduces Plasma Viral Load in Critical COVID-19. medRxiv 2020. [Google Scholar] [CrossRef]
- Gamrekelashvili, J.; Kapanadze, T.; Sablotny, S.; Ratiu, C.; Dastagir, K.; Lochner, M.; Karbach, S.; Wenzel, P.; Sitnow, A.; Fleig, S.; et al. Notch and TLR signaling coordinate monocyte cell fate and inflammation. Elife 2020, 9. [Google Scholar] [CrossRef]
- Scheenstra, M.R.; van Harten, R.M.; Veldhuizen, E.J.A.; Haagsman, H.P.; Coorens, M. Cathelicidins Modulate TLR-Activation and Inflammation. Front. Immunol. 2020, 11, 1137. [Google Scholar] [CrossRef]
- Martin, T.R.; Wurfel, M.M.; Zanoni, I.; Ulevitch, R. Targeting innate immunity by blocking CD14: Novel approach to control inflammation and organ dysfunction in COVID-19 illness. EBioMedicine 2020, 57, 102836. [Google Scholar] [CrossRef]
- Tian, R.R.; Zhang, M.X.; Liu, M.; Fang, X.; Li, D.; Zhang, L.; Zheng, P.; Zheng, Y.T.; Liu, Y. CD24Fc protects against viral pneumonia in simian immunodeficiency virus-infected Chinese rhesus monkeys. Cell. Mol. Immunol. 2020, 17, 887–888. [Google Scholar] [CrossRef]
- Helal, M.A.; Shouman, S.; Abdelwaly, A.; Elmehrath, A.O.; Essawy, M.; Sayed, S.M.; Saleh, A.H.; El-Badri, N. Molecular basis of the potential interaction of SARS-CoV-2 spike protein to CD147 in COVID-19 associated-lymphopenia. J. Biomol. Struct. Dyn. 2020, 1–11. [Google Scholar] [CrossRef]
- Sehirli, A.O.; Sayiner, S.; Serakinci, N. Role of melatonin in the treatment of COVID-19; as an adjuvant through cluster differentiation 147 (CD147). Mol. Biol. Rep. 2020. [Google Scholar] [CrossRef]
- Liu, C.; von Brunn, A.; Zhu, D. Cyclophilin A and CD147: Novel therapeutic targets for the treatment of COVID-19. Med. Drug Discov. 2020, 7, 100056. [Google Scholar] [CrossRef] [PubMed]
- Raony, I.; de Figueiredo, C.S. Retinal outcomes of COVID-19: Possible role of CD147 and cytokine storm in infected patients with diabetes mellitus. Diabetes Res. Clin. Pract. 2020, 165, 108280. [Google Scholar] [CrossRef]
- Ulrich, H.; Pillat, M.M. CD147 as a Target for COVID-19 Treatment: Suggested Effects of Azithromycin and Stem Cell Engagement. Stem Cell Rev. Rep. 2020, 16, 434–440. [Google Scholar] [CrossRef]
- Lu, R.M.; Hwang, Y.C.; Liu, I.J.; Lee, C.C.; Tsai, H.Z.; Li, H.J.; Wu, H.C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 1–30. [Google Scholar] [CrossRef]
- Fu, Y.; Cheng, Y.; Wu, Y. Understanding SARS-CoV-2-Mediated Inflammatory Responses: From Mechanisms to Potential Therapeutic Tools. Virol. Sin. 2020, 35, 266–271. [Google Scholar] [CrossRef]
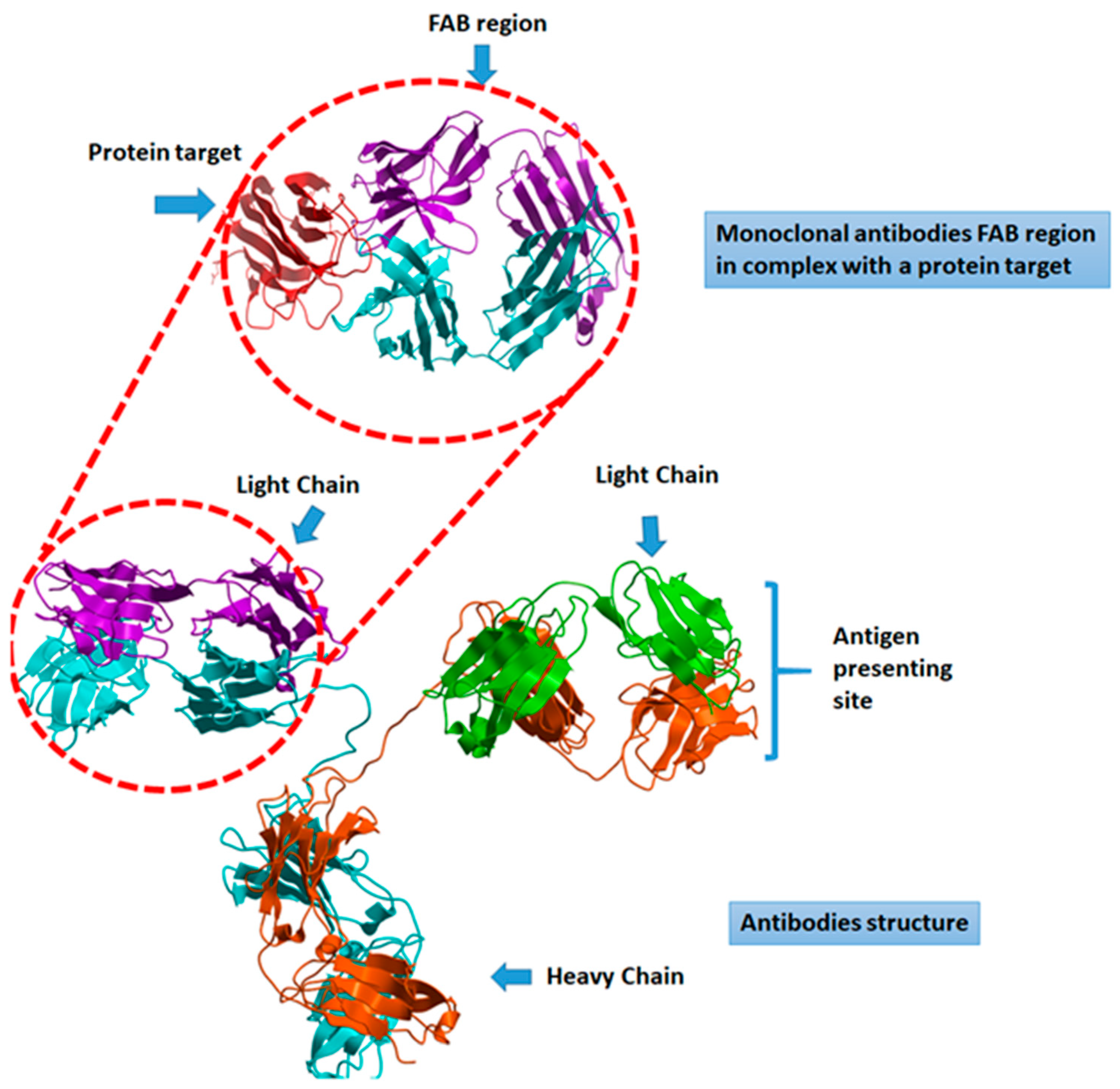
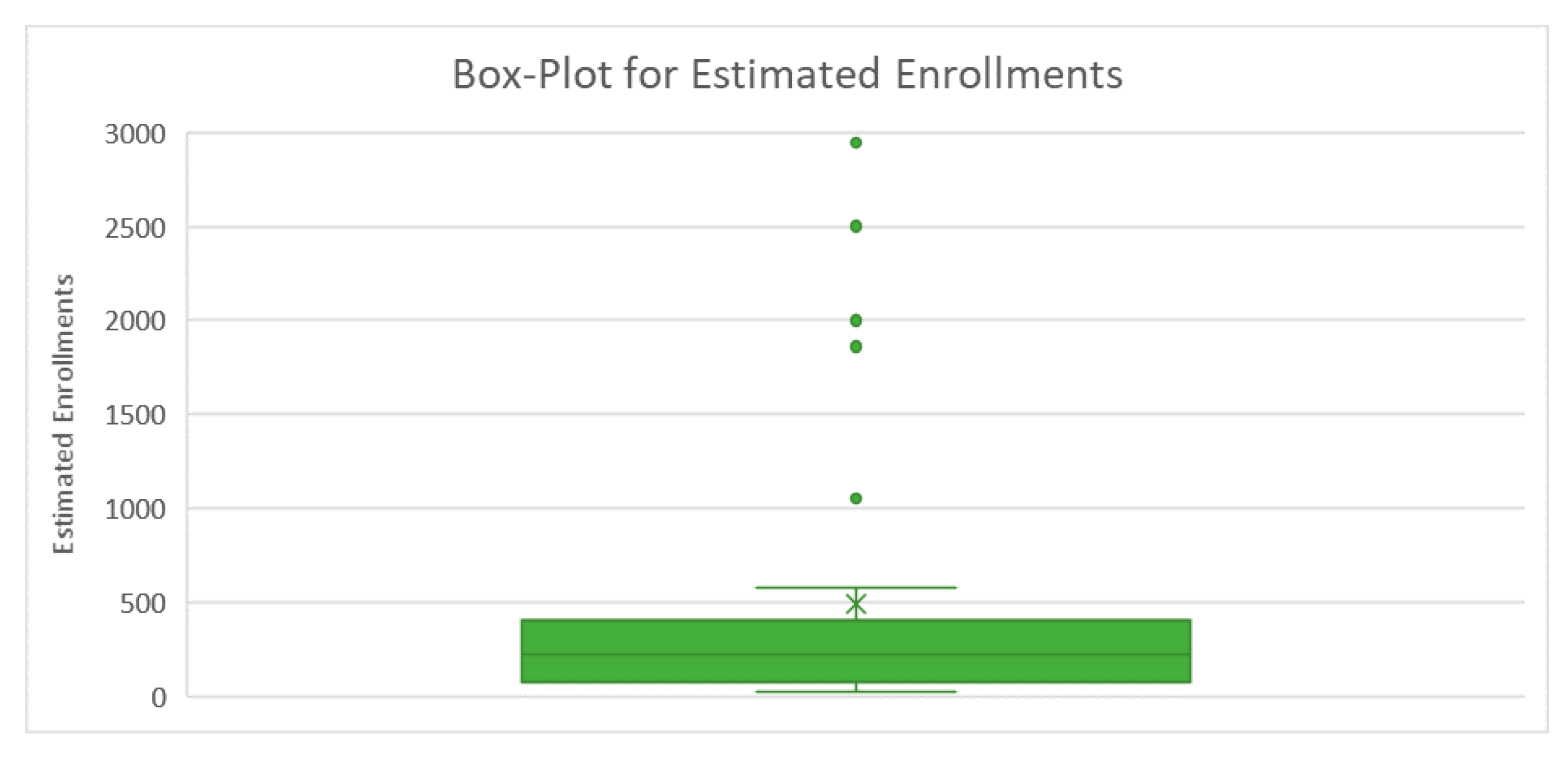
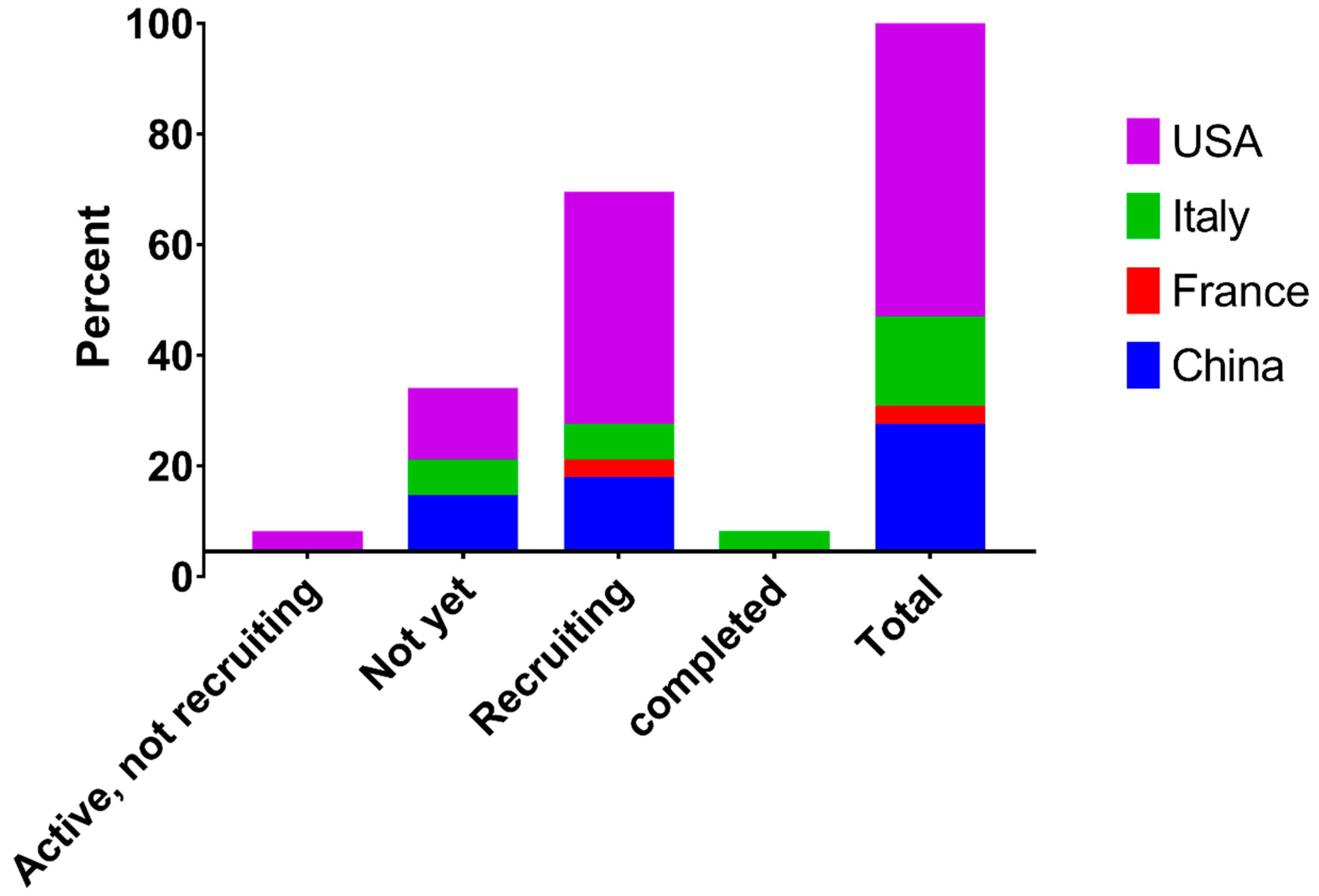
| ID | Recruiting | Country | Sponsor | Study Design | Estimated Enrollment | Intervention | Concept | Phase |
|---|---|---|---|---|---|---|---|---|
| NCT04261426 | Not yet | China | Medical college hospital | Single-center, randomized, open-label, controlled trial | 80 | Intravenous immunoglobulin therapy | Providing passive immunity and anti-inflammatory, immunomodulatory effect. | Phase 2/Phase 3 |
| NCT04268537 | Not yet | China | University | Randomized, parallel assessment | 120 | PD-1 blocking antibody | Evaluating the efficacy of the Programmed cell death (PD)-1 and thymosin in COVID-19 patients with severe pneumonia associated with lymphocytopenia | Phase 2 |
| NCT04275245 | Recruiting | China | Hospital | Single-group, randomized, open-label, trial | 20 | Meplazumab | Humanized anti-CD147 antibody | Phase 1/Phase 2 |
| NCT04293887 | Not yet | China | Medical college hospital | Randomized, Open label, parallel assessment | 328 | Recombinant human IFN-α2β | Efficacy and safety of IFN-α2β | Early Phase 1 |
| NCT04305106 | Recruiting | China | University hospital | Multicenter Randomized Controlled Clinical Trial | 140 | Bevacizumab | Antibody against vascular endothelial growth factor (VEGF), which is known as the most potent inducing factors to increase vascular permeability | |
| NCT04315298 | Recruiting | USA | Multicenter sponsored by pharmaceutical companies | Multicenter Randomized parallel assessment Clinical Trial | 2500 | Sarilumab | mAb targeting IL-6R | Phase 2/Phase 3 |
| NCT04317040 | Recruiting | USA | Multicenter sponsored by pharmaceutical company | Multicenter Randomized parallel assessment Clinical Trial | 230 | CD24Fc | Investigating the immunomodulatory effect of CD24Fc in COVID-19 treatment | Phase 3 |
| NCT04317092 | Recruiting | Italy | National institute | Open label single group assessment | 400 | Tocilizumab | IL-6 inhibitor | Phase 2 |
| NCT04320238 | Recruiting | China | University hospital | Nonrandomized open-label, parallel assessment Clinical Trial | 2944 | rhIFNα | Nasal Drops of recombinant hIFNα to prevent COVID-19 in medical staff | Phase 3 |
| NCT04320615 | Active, not recruiting | USA | Multicenter sponsored by pharmaceutical companies | Multicenter Randomized parallel assessment Clinical Trial | 450 | Tocilizumab | evaluate the efficacy, safety, pharmacodynamics, and pharmacokinetics of tocilizumab, IL-6 inhibitor | Phase 3 |
| NCT04322188 | completed | Italy | Hospital | Observational, retrospective study | 220 | Siltuximab | IL-6 inhibitor used for cancer therapy | |
| NCT04324021 | Recruiting | Italy | Biopharmaceutical company | Multicenter Randomized parallel assessment Clinical Trial | 54 | Emapalumab, Anakinra | A combination of an anti-IFNγ mAb (Emapalumab) and an IL-1 receptor antagonist (Anakinra) | Phase 2/Phase 3 |
| NCT04441918 | Recruiting | China | Biopharmaceutical company | Randomized open label, Clinical Trial | 40 | JS016 | Investigating the Safety, Tolerability, Pharmacokinetics, and immunogenicity of a recombinant humanized Anti-SARS-CoV-2 mAb (JS016) | Phase 1 |
| NCT04426695 | Recruiting | USA | Multicenter sponsored by pharmaceutical companies | Multicenter Randomized parallel assessment Clinical Trial | 1860 | Anti-Spike antibody | mAb against S Protein of SARS-CoV-2 | Phase 1/Phase 2/Phase 3 |
| NCT04425629 | Recruiting | USA | Multicenter sponsored by pharmaceutical companies | Multicenter Randomized parallel assessment Clinical Trial | 1054 | REGN10933 + REGN10987 antibody cocktail | Evaluating the Safety, Tolerability, and efficacy of mAb to SARS-CoV-2 S Protein for the treatment of ambulatory patients with COVID-19 | Phase 1/Phase 2/Phase 3 |
| NCT04351152 | Recruiting | USA | Multicenter sponsored by pharmaceutical companies | Multicenter Randomized parallel assessment Clinical Trial | 238 | Lenzilumab | For cytokine release syndrome mediated hyper-immune reaction (“cytokine storm”) | Phase 3 |
| NCT04371367 | Recruiting | France | Hospital, sponsored by pharmaceutical companies | Randomized parallel assessment Clinical Trial | 108 | Anti-C5aR | Complement component 5a receptor 1 or CD88 is a G protein-coupled receptor for C5a that regulates inflammation | Phase 2 |
| NCT04391309 | Not yet | USA | Hospital, sponsored by pharmaceutical companies | Randomized parallel assessment Clinical Trial | 300 | IC14 | IC14, monoclonal antibody to CD14 | Phase 2 |
| NCT04429529 | Recruiting | USA | sponsored by pharmaceutical companies | Randomized parallel assessment Clinical Trial | 25 | TY027 | Anti-SARS CoV2 antibody | Phase 1 |
| NCT04447469 | Recruiting | USA | sponsored by pharmaceutical companies | Randomized parallel assessment Clinical Trial | 573 | Mavrilimumab (KPL-301) | Mavrilimumab is a human mAb that inhibits human GM-CSF-R () | Phase 2/Phase 3 |
| NCT04370834 | Recruiting | USA | sponsored by pharmaceutical companies | Single group assignment | 219 | Tocilizumab | Using IL-6 mAb for treatment of COVID-19 patients with Cancer | Phase 2 |
| NCT04351243 | Recruiting | USA | Hospital, sponsored by pharmaceutical companies | Multicenter Randomized parallel assessment Clinical Trial | 270 | Gimsilumab | Gimsilumab acts on GM-CSF | Phase 2 |
| NCT04365153 | Recruiting | USA | Hospital, sponsored by pharmaceutical companies | Randomized, factorial assessment | 45 | Canakinumab | Canakinumab is antibody targeting interleukin-1 beta | Phase 2 |
| NCT04348448 | Not yet | Italy | sponsored by pharmaceutical companies | Retrospective and prospective observational study | 100 | Canakinumab | Canakinumab is antibody targeting interleukin-1 beta | |
| NCT04343651 | Not yet | USA | sponsored by pharmaceutical companies | Randomized parallel assessment Clinical Trial | 75 | Leronlimab | Anti CC chemokine receptor 5 (CCR5; CD195) antibody | Phase 2 |
| NCT04452318 | Not yet | USA | sponsored by pharmaceutical companies | Randomized parallel assessment Clinical Trial | 2000 | REGN10933 + REGN10987 | mAb against the S Protein of SARS CoV-2 | Phase 3 |
| NCT04432298 | Recruiting | USA | Hospital, sponsored by pharmaceutical companies | Randomized parallel assessment Clinical Trial | 130 | Pamrevlumab | Pamrevlumab against connective tissue growth factor (CTGF) | Phase 3 |
| NCT04341116 | Recruiting | USA | sponsored by pharmaceutical companies | Randomized parallel assessment Clinical Trial | 144 | TJ003234 | TJ003234 is an antibody against human GM-CSF | Phase 1/Phase 2 |
| NCT04347239 | Recruiting | USA | sponsored by pharmaceutical companies | Randomized parallel assessment Clinical Trial | 390 | Leronlimab | antibody against CC chemokine receptor 5 (CCR5; CD195) | Phase 2b/Phase 3 |
| NCT04397497 | Not yet | Italy | hospital | Randomized parallel assessment Clinical Trial | 50 | Mavrilimumab | Inhibits human GM-CSF-R | Phase 2 |
| NCT04435184 | Not yet | USA | Hospital, sponsored by pharmaceutical companies | Randomized parallel assessment Clinical Trial | 40 | Crizanlizumab | Active against P-selctin, a cell adhesion molecule on the surfaces of activated endothelial cells, which line the inner surface of blood vessels, and activated platelets. Prevent vaso-occulsive crises. | Phase 2 |
| Variables | Description |
|---|---|
| ID | Qualitative |
| Recruiting | Qualitative |
| Country | Qualitative |
| Sponsor | Qualitative |
| Study design | Qualitative |
| Estimated enrollment | Quantitative |
| Intervention | Qualitative |
| Concept | Qualitative |
| Phase | Qualitative |
| Parameter | Value |
|---|---|
| Mean | 489 |
| Standard Error | 137 |
| Median | 219 |
| Mode | 40 |
| Standard Deviation | 764 |
| Sample Variance | 584,098 |
| Kurtosis | 4 |
| Skewness | 2 |
| Range | 2924 |
| Minimum | 20 |
| Maximum | 2944 |
| Sum | 15,147 |
| Count | 31 |
| Sponsor | Country | ||||
|---|---|---|---|---|---|
| China | France | Italy | USA | Total | |
| Biopharmaceutical companies | 3.23 | 0 | 6.46 | 22.58 | 32.26 |
| Hospital, medical college, or university hospital | 19.36 | 3.23 | 6.46 | 16.13 | 45.16 |
| Multicenter sponsored | 0.00 | 0.00 | 0.00 | 19.36 | 19.36 |
| National institute | 0.00 | 0.00 | 3.23 | 0.00 | 3.23 |
| Total | 22.59 | 3.23 | 16.15 | 58.07 | 100.00 |
| Recruiting Status | Country | ||||
|---|---|---|---|---|---|
| China | France | Italy | USA | Total | |
| Active, not recruiting | 0.00 | 0.00 | 0.00 | 3.23 | 3.23 |
| Not yet | 9.68 | 0.00 | 6.45 | 12.90 | 29.03 |
| Recruiting | 12.90 | 3.23 | 6.45 | 41.94 | 64.52 |
| completed | 0.00 | 0.00 | 3.23 | 0.00 | 3.23 |
| Total | 22.58 | 3.23 | 16.13 | 58.06 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussen, J.; Kandeel, M.; Hemida, M.G.; Al-Mubarak, A.I.A. Antibody-Based Immunotherapeutic Strategies for COVID-19. Pathogens 2020, 9, 917. https://doi.org/10.3390/pathogens9110917
Hussen J, Kandeel M, Hemida MG, Al-Mubarak AIA. Antibody-Based Immunotherapeutic Strategies for COVID-19. Pathogens. 2020; 9(11):917. https://doi.org/10.3390/pathogens9110917
Chicago/Turabian StyleHussen, Jamal, Mahmoud Kandeel, Maged Gomaa Hemida, and Abdullah I. A. Al-Mubarak. 2020. "Antibody-Based Immunotherapeutic Strategies for COVID-19" Pathogens 9, no. 11: 917. https://doi.org/10.3390/pathogens9110917
APA StyleHussen, J., Kandeel, M., Hemida, M. G., & Al-Mubarak, A. I. A. (2020). Antibody-Based Immunotherapeutic Strategies for COVID-19. Pathogens, 9(11), 917. https://doi.org/10.3390/pathogens9110917






