Seroprevalence and Molecular Characterization of Coxiella burnetii in Cattle in the Republic of Korea
Abstract
1. Introduction
2. Results
2.1. Detection of C. burnetii Using Molecular and Serologic Assays
2.2. Risk Factor Analysis
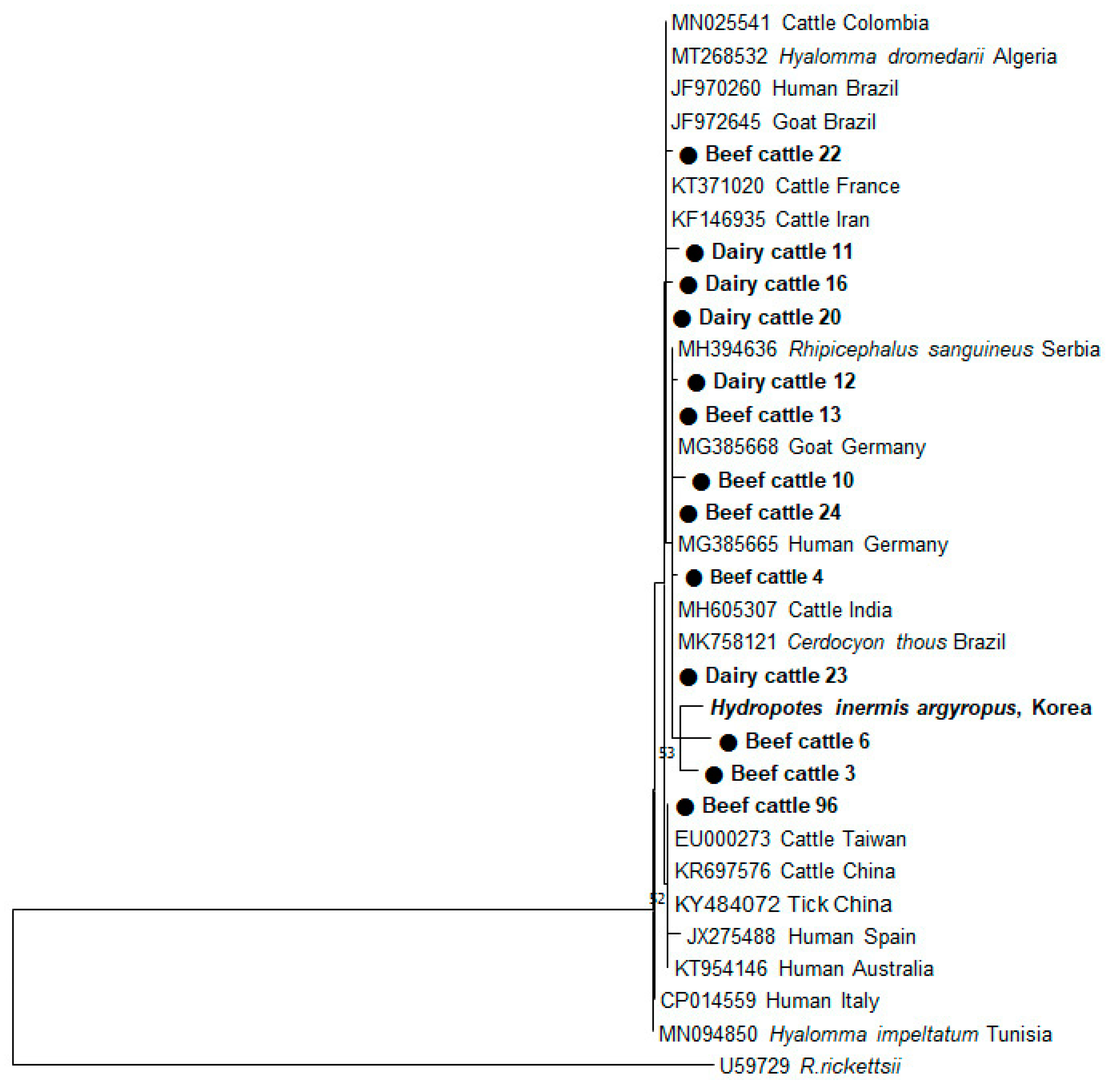
2.3. Phylogenetic Analysis ofIS1111 Sequences
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
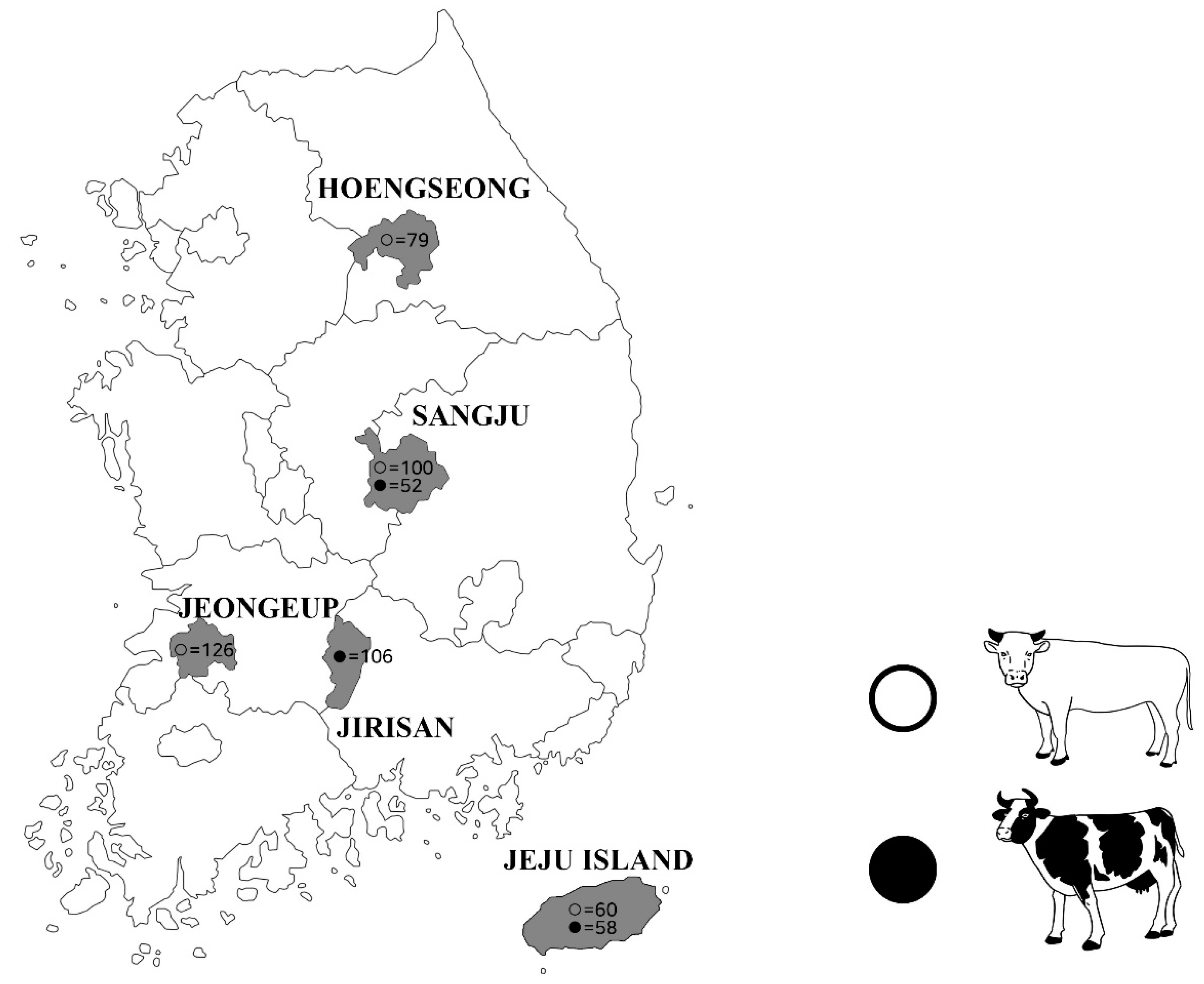
4.2. Experimental Design
4.3. Blood Sample Collection
4.4. DNA Extraction and PCR
4.5. Serological Screening of Serum Samples
4.6. Phylogenetic Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Woldehiwet, Z. Q fever (coxiellosis): Epidemiology and pathogenesis. Res. Vet. Sci. 2004, 77, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Maurin, M.; Raoult, D. Q fever. Clin. Microbiol. Rev. 1999, 12, 518–553. [Google Scholar] [CrossRef] [PubMed]
- Tissot-Dupont, H.; Raoult, D. Q fever. Infect. Dis. Clin. N. Am. 2008, 22, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Angelakis, E.; Raoult, D. Q Fever. Vet. Microbiol. 2010, 140, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Arricau-Bouvery, N.; Rodolakis, A. Is Q fever an emerging or re-emerging zoonosis? Vet. Res. 2005, 36, 327–349. [Google Scholar] [CrossRef] [PubMed]
- Chochlakis, D.; Santos, A.S.; Giadinis, N.D.; Papadopoulos, D.; Boubaris, L.; Kalaitzakis, E.; Psaroulaki, A.; Kritas, S.K.; Petridou, E.I. Genotyping of Coxiella burnetii in sheep and goat abortion samples. BMC Microbiol. 2018, 18, 204. [Google Scholar] [CrossRef] [PubMed]
- Rodolakis, A.; Berri, M.; Hechard, C.; Caudron, C.; Souriau, A.; Bodier, C.C.; Blanchard, B.; Camuset, P.; Devillechaise, P.; Natorp, J.C.; et al. Comparison of Coxiella burnetii shedding in milk of dairy bovine, caprine, and ovine herds. J. Dairy. Sci. 2007, 90, 5352–5360. [Google Scholar] [CrossRef]
- Plummer, P.J.; McClure, J.T.; Menzies, P.; Morley, P.S.; Van den Brom, R.; Van Metre, D.C. Management of Coxiella burnetii infection in livestock populations and the associated zoonotic risk: A consensus statement. J. Vet. Intern. Med. 2018, 32, 1481–1494. [Google Scholar] [CrossRef]
- Loftis, A.D.; Priestley, R.A.; Massung, R.F. Detection of Coxiella burnetii in commercially available raw milk from the United States. Foodborne Pathog. Dis. 2010, 7, 1453–1456. [Google Scholar] [CrossRef]
- Signs, K.A.; Stobierski, M.G.; Gandhi, T.N. Q fever cluster among raw milk drinkers in Michigan, 2011. Clin. Infect. Dis. 2012, 55, 1387–1389. [Google Scholar] [CrossRef]
- Dhaka, P.; Malik, S.S.; Yadav, J.P.; Kumar, M.; Baranwal, A.; Barbuddhe, S.B.; Rawool, D.B. Seroprevalence and molecular detection of coxiellosis among cattle and their human contacts in an organized dairy farm. J. Infect. Public Health. 2019, 12, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Madariaga, M.G.; Rezai, K.; Trenholme, G.M.; Weinstein, R.A. Q fever: A biological weapon in your backyard. Lancet Infect. Dis. 2003, 3, 709–721. [Google Scholar] [CrossRef]
- Szymanska-Czerwinska, M.; Jodelko, A.; Niemczuk, K. Occurrence of Coxiella burnetii in Polish dairy cattle herds based on serological and PCR tests. Comp. Immunol. Microbiol. Infect. Dis. 2019, 67, 101377. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.G.; Ouh, I.O.; Lee, S.H.; Kim, J.W.; Rhee, M.H.; Kwon, O.D.; Kim, T.H.; Kwak, D. Prevalence of Coxiella burnetii in cattle at South Korean national breeding stock farms. PLoS ONE 2017, 12, e0177478. [Google Scholar] [CrossRef]
- Menadi, S.E.; Mura, A.; Santucciu, C.; Ghalmi, F.; Hafsi, F.; Masala, G. Seroprevalence and risk factors of Coxiella burnetii infection in cattle in northeast Algeria. Trop. Anim. Health Prod. 2020, 52, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Shome, R.; Deka, R.P.; Milesh, L.; Sahay, S.; Grace, D.; Lindahl, J.F. Coxiella seroprevalence and risk factors in large ruminants in Bihar and Assam, India. Acta Trop. 2019, 194, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.Y.; Seo, M.G.; Lee, S.H.; Byun, J.W.; Oem, J.K.; Kwak, D. Molecular and serologic detection of Coxiella burnetii in native Korean goats (Capra hircus coreanae). Vet. Microbiol. 2014, 173, 152–155. [Google Scholar] [CrossRef]
- Alvarez, J.; Perez, A.; Mardones, F.O.; Perez-Sancho, M.; Garcia-Seco, T.; Pages, E.; Mirat, F.; Diaz, R.; Carpintero, J.; Dominguez, L. Epidemiological factors associated with the exposure of cattle to Coxiella burnetii in the Madrid region of Spain. Vet. J. 2012, 194, 102–107. [Google Scholar] [CrossRef]
- Adamu, S.G.; Kabir, J.; Umoh, J.U.; Raji, M.A. Seroprevalence of brucellosis and Q fever (Coxiellosis) in cattle herds in Maigana and Birnin Gwari agro-ecological zone of Kaduna State, Nigeria. Trop. Anim. Health Prod. 2018, 50, 1583–1589. [Google Scholar] [CrossRef]
- Wardrop, N.A.; Thomas, L.F.; Cook, E.A.; de Glanville, W.A.; Atkinson, P.M.; Wamae, C.N.; Fevre, E.M. The Sero-epidemiology of Coxiella burnetii in humans and cattle, Western Kenya: Evidence from a cross-sectional study. PLoS Negl. Trop. Dis. 2016, 10, e0005032. [Google Scholar] [CrossRef]
- Nokhodian, Z.; Ataei, B.; Khalili, M.; Feizi, A.; Moradi, A.; Yaran, M.; Hoseini, S.G. Detection of Coxiella burnetii and risk factors for infection in ruminants in a central county of Iran. Vet. Microbiol. 2018, 222, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Capuano, F.; Landolfi, M.C.; Monetti, D.M. Influence of three types of farm management on the seroprevalence of Q fever as assessed by an indirect immunofluorescence assay. Vet. Rec. 2001, 149, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, E.; Berktas, M.; Keles, I.; Agaoglu, Z. Seroprevalence of Q fever in cattle and sheep in the East of Turkey. Asian. J. Anim. Vet. Adv. 2009, 4, 114–121. [Google Scholar] [CrossRef]
- Klemmer, J.; Njeru, J.; Emam, A.; El-Sayed, A.; Moawad, A.A.; Henning, K.; Elbeskawy, M.A.; Sauter-Louis, C.; Straubinger, R.K.; Neubauer, H.; et al. Q fever in Egypt: Epidemiological survey of Coxiella burnetii specific antibodies in cattle, buffaloes, sheep, goats and camels. PLoS ONE 2018, 13, e0192188. [Google Scholar] [CrossRef] [PubMed]
- Scolamacchia, F.; Handel, I.G.; Fevre, E.M.; Morgan, K.L.; Tanya, V.N.; Bronsvoort, B.M. Serological patterns of brucellosis, leptospirosis and Q fever in Bos indicus cattle in Cameroon. PLoS ONE 2010, 5, e8623. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, G.; Reyna-Bello, A.; Minda-Aluisa, E.; Celi-Erazo, M.; Olmedo, L.; Garcia, H.A.; Garcia-Bereguiain, M.A.; de Waard, J.H. Serological evidence of Coxiella burnetii infection in cattle and farm workers: Is Q fever an underreported zoonotic disease in Ecuador? Infect. Drug. Resist. 2019, 12, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, V.M.; Malik, S.V.; Kaur, S.; Kumar, S.; Barbuddhe, S.B. Comparison of PCR, immunofluorescence assay, and pathogen isolation for diagnosis of Q fever in humans with spontaneous abortions. J. Clin. Microbiol. 2008, 46, 2038–2044. [Google Scholar] [CrossRef]
- Vaidya, V.M.; Malik, S.V.; Bhilegaonkar, K.N.; Rathore, R.S.; Kaur, S.; Barbuddhe, S.B. Prevalence of Q fever in domestic animals with reproductive disorders. Comp. Immunol. Microbiol. Infect. Dis. 2010, 33, 307–321. [Google Scholar] [CrossRef]
- Qiu, Y.; Nakao, R.; Namangala, B.; Sugimoto, C. First genetic detection of Coxiella burnetii in Zambian livestock. Am. J. Trop. Med. Hyg. 2013, 89, 518–519. [Google Scholar] [CrossRef]
- Boroduske, A.; Trofimova, J.; Kibilds, J.; Papule, U.; Sergejeva, M.; Rodze, I.; Grantina-Ievina, L. Coxiella burnetii (Q fever) infection in dairy cattle and associated risk factors in Latvia. Epidemiol. Infect. 2017, 145, 2011–2019. [Google Scholar] [CrossRef]
- Dobos, A.; Kreizinger, Z.; Kovacs, A.B.; Gyuranecz, M. Prevalence of Coxiella burnetii in Central and Eastern European dairy herds. Comp. Immunol. Microbiol. Infect. Dis. 2020, 72, 101489. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.G.; Ouh, I.O.; Kwak, D. Herd prevalence and genotypes of Coxiella burnetii in dairy cattle bulk tank milk in Gyeongsang provinces of South Korea. Trop. Anim. Health Prod. 2018, 50, 1399–1404. [Google Scholar] [CrossRef]
- Nokhodian, Z.; Feizi, A.; Moradi, A.; Yaran, M.; Hoseini, S.G.; Ataei, B.; Hosseini, M. Detection and risk factors of Coxiella burnetii infection in dairy cattle based on bulk tank milk samples in center of Iran. Prev. Vet. Med. 2016, 134, 139–144. [Google Scholar] [CrossRef]
- Barkema, H.W.; von Keyserlingk, M.A.; Kastelic, J.P.; Lam, T.J.; Luby, C.; Roy, J.P.; LeBlanc, S.J.; Keefe, G.P.; Kelton, D.F. Invited review: Changes in the dairy industry affecting dairy cattle health and welfare. J. Dairy Sci. 2015, 98, 7426–7445. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.S.; Yu, D.H.; Chae, J.S.; Park, B.K.; Yoo, J.G.; Park, J. Seasonal changes in hemograms and Theileria orientalis infection rates among Holstein cattle pastured in the mountains in the Republic of Korea. Prev. Vet. Med. 2016, 127, 77–83. [Google Scholar] [CrossRef]
- Han, D.G.; Ryu, J.H.; Chae, J.B.; Kim, D.W.; Kwon, C.H.; Choi, K.S. First report of Anaplasma phagocytophilum infection in Holstein cattle in the Republic of Korea. Acta Trop. 2018, 183, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.G.; Lee, S.H.; Ouh, I.O.; Lee, G.H.; Goo, Y.K.; Kim, S.; Kwon, O.D.; Kwak, D. Molecular detection and genotyping of Coxiella-like endosymbionts in ticks that infest horses in South Korea. PLoS ONE 2016, 11, e0165784. [Google Scholar] [CrossRef]
- Mediannikov, O.; Fenollar, F.; Socolovschi, C.; Diatta, G.; Bassene, H.; Molez, J.F.; Sokhna, C.; Trape, J.F.; Raoult, D. Coxiella burnetii in humans and ticks in rural Senegal. PLoS Negl. Trop. Dis. 2010, 4, e654. [Google Scholar] [CrossRef]
- Tissot-Dupont, H.; Amadei, M.A.; Nezri, M.; Raoult, D. Wind in November, Q fever in December. Emerg. Infect. Dis. 2004, 10, 1264–1269. [Google Scholar] [CrossRef]
- Shin, S.U.; Park, Y.J.; Ryu, J.H.; Jang, D.H.; Hwang, S.; Cho, H.C.; Park, J.; Han, J.I.; Choi, K.S. Identification of zoonotic tick-borne pathogens from Korean Water Deer (Hydropotes inermis argyropus). Vector Borne Zoonotic Dis. 2020, 10, 745–754. [Google Scholar] [CrossRef]
- Bellabidi, M.; Benaissa, M.H.; Bissati-Bouafia, S.; Harrat, Z.; Brahmi, K.; Kernif, T. Coxiella burnetii in camels (Camelus dromedarius) from Algeria: Seroprevalence, molecular characterization, and ticks (Acari: Ixodidae) vectors. Acta Trop. 2020, 206, 105443. [Google Scholar] [CrossRef] [PubMed]
- Parisi, A.; Fraccalvieri, R.; Cafiero, M.; Miccolupo, A.; Padalino, I.; Montagna, C.; Capuano, F.; Sottili, R. Diagnosis of Coxiella burnetii-related abortion in Italian domestic ruminants using single-tube nested PCR. Vet. Microbiol. 2006, 118, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
| Breeds | No. of Samples | PCR (Ag) | ELISA (Ab) | PCR & ELISA |
|---|---|---|---|---|
| Dairy | 216 | 16 (7.4%) | 3 (1.4%) | 2 (0.9%) |
| Housed | 158 | 1 (0.6%) | 1 (0.6%) | 0 |
| Grazing | 58 | 15 (25.9%) | 2 (3.4%) | 2 (3.4%) |
| Beef | 275 | 37 (13.5%) | 40 (14.5%) | 18 (6.5%) |
| Housed | 136 | 3 (2.2%) | 0 | 0 |
| Grazing | 139 | 34 (24.5%) | 40 (28.8%) | 18 (12.9%) |
| Total | 491 | 53 (10.8%) | 43 (8.8%) | 20 (4.1%) |
| Parameter | No. of Samples | No. of PCR Positive (%) | P-Value | No. of ELISA Positive (%) | P-Value |
|---|---|---|---|---|---|
| Beef cattle | 275 | 37 (13.5%) | 0.032 | 40 (14.5%) | 0.000 |
| Dairy cattle | 216 | 16 (7.4%) | 3 (1.4%) | ||
| Grazing | 197 | 49 (24.9%) | 0.000 | 42 (21.3%) | 0.000 |
| Housed | 294 | 4 (1.4%) | 1 (0.3%) | ||
| Total | 491 | 53 (10.8%) | 43 (8.8%) |
| Growth Type | PCR Positive | ELISA Positive | ||||
|---|---|---|---|---|---|---|
| OR | P-Value | 95% CI | OR | P-Value | 95% CI | |
| Housed (ref.) | 1.00 | – | – | 1.00 | – | – |
| Grazing | 33.34 | 0.000 | 11.77–94.80 | 114.51 | 0.000 | 15.57–842.41 |
| Variables | No. of C. burnetii Positive * | χ2 (P-Value) | OR | 95% CI | |
|---|---|---|---|---|---|
| Breed | Dairy cattle | 17/216 | − | − | − |
| Beef cattle | 59/275 | 5.82 (0.000) | 3.20 | 1.80−5.67 | |
| Growth type | Housed | 5/294 | − | − | − |
| Grazing | 71/197 | 106.324 (0.000) | 32.57 | 12.84−82.61 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, S.; Cho, H.-C.; Shin, S.-U.; Kim, H.-Y.; Park, Y.-J.; Jang, D.-H.; Kim, E.-M.; Kim, J.W.; Park, J.; Choi, K.-S. Seroprevalence and Molecular Characterization of Coxiella burnetii in Cattle in the Republic of Korea. Pathogens 2020, 9, 890. https://doi.org/10.3390/pathogens9110890
Hwang S, Cho H-C, Shin S-U, Kim H-Y, Park Y-J, Jang D-H, Kim E-M, Kim JW, Park J, Choi K-S. Seroprevalence and Molecular Characterization of Coxiella burnetii in Cattle in the Republic of Korea. Pathogens. 2020; 9(11):890. https://doi.org/10.3390/pathogens9110890
Chicago/Turabian StyleHwang, Sunwoo, Hyung-Chul Cho, Seung-Uk Shin, Ha-Young Kim, Yu-Jin Park, Dong-Hoon Jang, Eun-Mi Kim, Jong Wan Kim, Jinho Park, and Kyoung-Seong Choi. 2020. "Seroprevalence and Molecular Characterization of Coxiella burnetii in Cattle in the Republic of Korea" Pathogens 9, no. 11: 890. https://doi.org/10.3390/pathogens9110890
APA StyleHwang, S., Cho, H.-C., Shin, S.-U., Kim, H.-Y., Park, Y.-J., Jang, D.-H., Kim, E.-M., Kim, J. W., Park, J., & Choi, K.-S. (2020). Seroprevalence and Molecular Characterization of Coxiella burnetii in Cattle in the Republic of Korea. Pathogens, 9(11), 890. https://doi.org/10.3390/pathogens9110890





