OV16 Seroprevalence among Persons with Epilepsy in Onchocerciasis Endemic Regions: A Multi-Country Study
Abstract
1. Background
2. Methods
3. Statistical Analysis
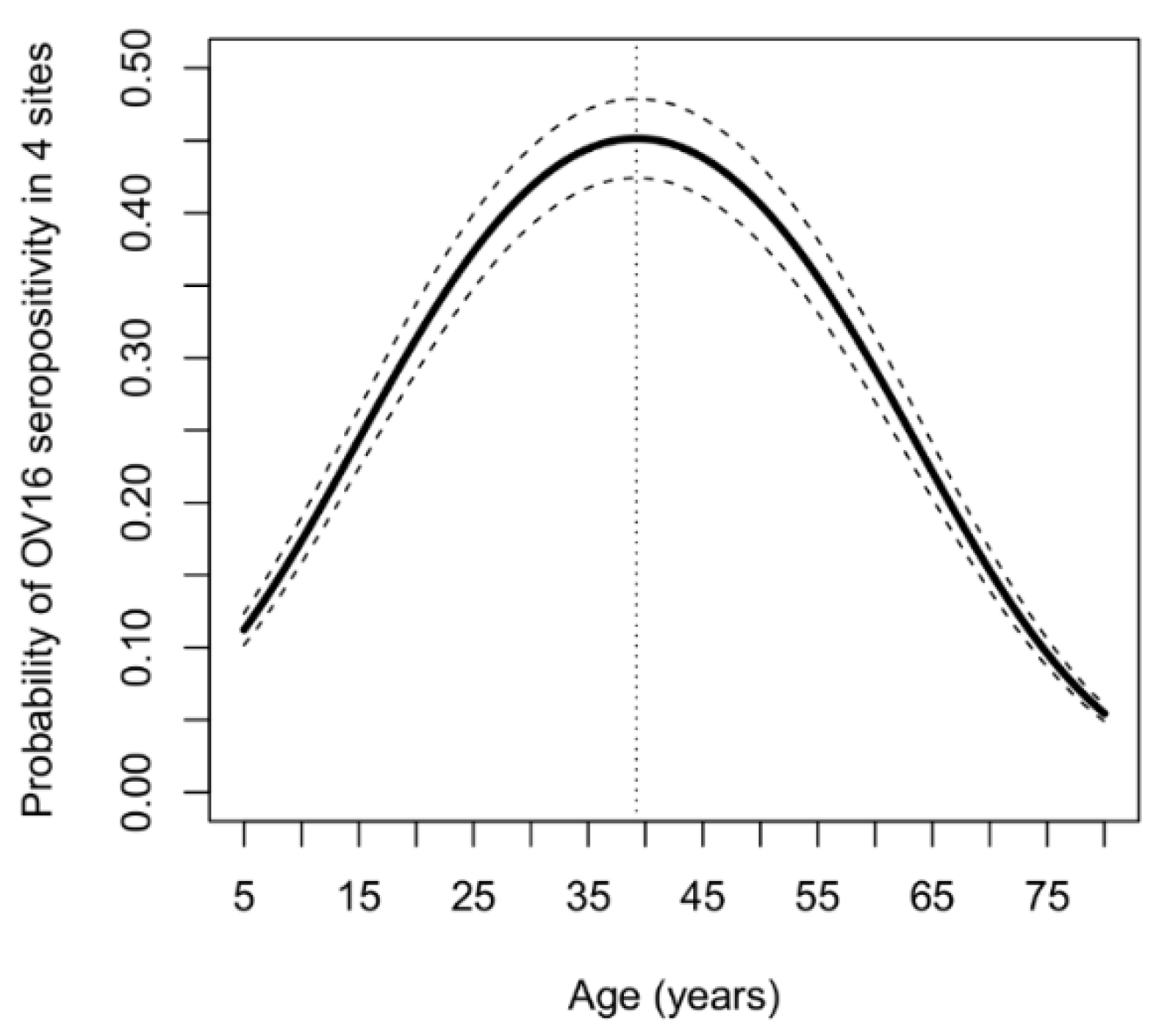
4. Results
5. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tekle, A.H.; Zouré, H.G.M.; Noma, M.; Boussinesq, M.; Coffeng, L.E.; Stolk, W.A.; Remme, J.H.F. Progress towards onchocerciasis elimination in the participating countries of the African Programme for Onchocerciasis Control: Epidemiological evaluation results. Infect. Dis. Poverty 2016, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Osei-Atweneboana, M.Y.; Awadzi, K.; Attah, S.K.; Boakye, D.A.; Gyapong, J.O.; Prichard, R.K. Phenotypic Evidence of Emerging Ivermectin Resistance in Onchocerca volvulus. PLoS Negl. Trop. Dis. 2011, 5, e998. [Google Scholar] [CrossRef] [PubMed]
- Chesnais, C.B.; Djeunga, H.N.; Njamnshi, A.K.; Lenou-Nanga, C.G.; Boullé, C.; Bissek, A.-C.Z.-K.; Kamgno, J.; Colebunders, R.; Boussinesq, M. The temporal relationship between onchocerciasis and epilepsy: A population-based cohort study. Lancet Infect. Dis. 2018, 18, 1278–1286. [Google Scholar] [CrossRef]
- Chesnais, C.B.; Bizet, C.; Campillo, J.T.; Njamnshi, W.Y.; Bopda, J.; Nwane, P.; Pion, S.D.; Njamnshi, A.K.; Boussinesq, M. A Second Population-Based Cohort Study in Cameroon Confirms the Temporal Relationship Between Onchocerciasis and Epilepsy. Open Forum Infect. Dis. 2020, 7, 206. [Google Scholar] [CrossRef]
- Gumisiriza, N.; Kaiser, C.; Asaba, G.; Onen, H.; Mubiru, F.; Kisembo, D.; Fodjo, J.N.S.; Colebunders, R. Changes in epilepsy burden after onchocerciasis elimination in a hyperendemic focus of western Uganda: A comparison of two population-based, cross-sectional studies. Lancet Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Colebunders, R.; Siewe, F.N.; Hotterbeekx, A. Onchocerciasis-Associated Epilepsy, an Additional Reason for Strengthening Onchocerciasis Elimination Programs. Trends Parasitol. 2018, 34, 208–216. [Google Scholar] [CrossRef]
- Melchers, N.V.S.V.; Mollenkopf, S.; Colebunders, R.; Edlinger, M.; Coffeng, L.E.; Irani, J.; Zola, T.; Siewe, J.N.; De Vlas, S.J.; Winkler, A.S.; et al. Burden of onchocerciasis-associated epilepsy: First estimates and research priorities. Infect. Dis. Poverty 2018, 7, 101. [Google Scholar] [CrossRef]
- Siewe, J.N.F.; Ukaga, C.N.; Nwazor, E.O.; Nwoke, M.O.; Nwokeji, M.C.; Onuoha, B.C.; Nwanjor, S.O.; Okeke, J.; Osahor, K.; Chimechefulam, L.; et al. Low prevalence of epilepsy and onchocerciasis after more than 20 years of ivermectin treatment in the Imo River Basin in Nigeria. Infect. Dis. Poverty 2019, 8, 8. [Google Scholar] [CrossRef]
- De Merxem, D.G.; Fodjo, J.N.S.; Menon, S.; Hotterbeekx, A.; Colebunders, R. Nodding syndrome research, lessons learned from the NSETHIO project. Glob. Ment. Health 2019, 6, e26. [Google Scholar] [CrossRef]
- Bhwana, D.; Mmbando, B.P.; Dekker, M.C.; Mnacho, M.; Kakorozya, A.; Matuja, W.; Makunde, W.H.; Weckhuysen, S.; Colebunders, R. Clinical presentation of epilepsy in six villages in an onchocerciasis endemic area in Mahenge, Tanzania. Epileptic Disord 2019, 21, 425–435. [Google Scholar]
- Mmbando, B.P.; Suykerbuyk, P.; Mnacho, M.; Kakorozya, A.; Matuja, W.; Hendy, A.; Greter, H.; Makunde, W.H.; Colebunders, R. High prevalence of epilepsy in two rural onchocerciasis endemic villages in the Mahenge area, Tanzania, after 20 years of community directed treatment with ivermectin. Infect. Dis. Poverty 2018, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Fodjo, J.N.S.; Tatah, G.; Tabah, E.N.; Ngarka, L.; Nfor, L.N.; Chokote, S.E.; Ntone, F.E. Epidemiology of onchocerciasis-associated epilepsy in the Mbam and Sanaga river valleys of Cameroon: Impact of more than 13 years of ivermectin. Infect. Dis. Poverty 2018, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Mandro, M.; Fodjo, J.N.S.; Mukendi, D.; Dusabimana, A.; Menon, S.; Haesendonckx, S.; Lokonda, R.; Nakato, S.; Nyisi, F.; Abhafule, G.; et al. Ivermectin as an adjuvant to anti-epileptic treatment in persons with onchocerciasis-associated epilepsy: A randomized proof-of-concept clinical trial. PLoS Negl. Trop. Dis. 2020, 14, e0007966. [Google Scholar] [CrossRef] [PubMed]
- Levick, B.; Laudisoit, A.; Tepage, F.; Ensoy-Musoro, C.; Mandro, M.; Osoro, C.B.; Suykerbuyk, P.; Kashama, J.M.; Komba, M.; Tagoto, A.; et al. High prevalence of epilepsy in onchocerciasis endemic regions in the Democratic Republic of the Congo. PLoS Negl. Trop. Dis. 2017, 11, e0005732. [Google Scholar] [CrossRef]
- Diagana, M.; Preux, P.-M.; Tuillas, M.; Hamady, A.O.; Druet-Cabanac, M. Dépistage de l’épilepsie en zones tropicales: Validation d’un questionnaire en Mauritanie. Bulletin de la Société de Pathologie Exotique 2006, 99, 103–107. [Google Scholar]
- Fodjo, J.N.S.; Dekker, M.C.J.; Idro, R.; Mandro, M.; Preux, P.-M.; Njamnshi, A.K.; Colebunders, R. Comprehensive management of epilepsy in onchocerciasis-endemic areas: Lessons learnt from community-based surveys. Infect. Dis. Poverty 2019, 8, 1–12. [Google Scholar] [CrossRef]
- Armitage, P. Tests for Linear Trends in Proportions and Frequencies. Biometrics 1955, 11, 375. [Google Scholar] [CrossRef]
- Komlan, K.; Vossberg, P.S.; Gantin, R.G.; Solim, T.; Korbmacher, F.; Banla, M.; Padjoudoum, K.; Karabou, P.; Köhler, C.; Soboslay, P.T. Onchocerca volvulus infection and serological prevalence, ocular onchocerciasis and parasite transmission in northern and central Togo after decades of Simulium damnosum s.l. vector control and mass drug administration of ivermectin. PLoS Negl. Trop. Dis. 2018, 12, e0006312. [Google Scholar] [CrossRef]
- Golden, A.; Faulx, D.; Kalnoky, M.; Stevens, E.; Yokobe, L.; Peck, R.; Karabou, P.; Banla, M.; Rao, R.; Adade, K.; et al. Analysis of age-dependent trends in Ov16 IgG4 seroprevalence to onchocerciasis. Parasites Vectors 2016, 9, 338. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, C.D.; Al-Qubati, Y.; Al-Kubati, A.-S.; Nutman, T.B.; Kubofcik, J.; Hopkins, A.; Behan-Braman, A. A Serological Survey of Human Onchocerciasis in Yemen. Am. J. Trop. Med. Hyg. 2018, 99, 1049–1052. [Google Scholar] [CrossRef]
- Lenaerts, E.; Mandro, M.; Mukendi, D.; Suykerbuyk, P.; Dolo, H.; Deogratias, W.; Nyisi, F.; Ensoy, C.; Laudisoit, A.; Hotterbeekx, A.; et al. High prevalence of epilepsy in onchocerciasis endemic health areas in Democratic Republic of the Congo. Infect. Dis. Poverty 2018, 7, 68. [Google Scholar] [CrossRef]
- Gumisiriza, N.; Mubiru, F.; Fodjo, J.N.S.; Kayitale, M.M.; Hotterbeekx, A.; Idro, R.; Makumbi, I.; Lakwo, T.; Opar, B.T.; Kaducu, J.; et al. Prevalence and incidence of nodding syndrome and other forms of epilepsy in onchocerciasis-endemic areas in northern Uganda after the implementation of onchocerciasis control measures. Infect. Dis. Poverty 2020, 9, 12. [Google Scholar] [CrossRef]
- Fodjo, J.N.S.; Mandro, M.; Mukendi, D.; Tepage, F.; Menon, S.; Nakato, S.; Nyisi, F.; Abhafule, G.; Deogratias, W.; Anyolito, A.; et al. Onchocerciasis-associated epilepsy in the Democratic Republic of Congo: Clinical description and relationship with microfilarial density. PLoS Negl. Trop. Dis. 2019, 13, 577247. [Google Scholar] [CrossRef]
- Colebunders, R.; Carter, J.Y.; Olore, P.C.; Puok, K.; Bhattacharyya, S.; Menon, S.; Abd-Elfarag, G.; Ojok, M.; Ensoy-Musoro, C.; Lako, R.; et al. High prevalence of onchocerciasis-associated epilepsy in villages in Maridi County, Republic of South Sudan: A community-based survey. Seizure 2018, 63, 93–101. [Google Scholar] [CrossRef]
- Kaur, S.; Garg, R.; Aggarwal, S.; Chawla, S.P.S.; Pal, R. Adult onset seizures: Clinical, etiological, and radiological profile. J. Fam. Med. Prim. Care 2018, 7, 191–197. [Google Scholar] [CrossRef]
- Ba-Diop, A.; Marin, B.; Druet-Cabanac, M.; Ngoungou, E.B.; Newton, C.R.; Preux, P.-M. Epidemiology, causes, and treatment of epilepsy in sub-Saharan Africa. Lancet Neurol. 2014, 13, 1029–1044. [Google Scholar] [CrossRef]
- Weil, G.J.; Steel, C.; Liftis, F.; Li, B.-W.; Mearns, G.; Lobos, E.; Nutman, T.B. A Rapid-Format Antibody Card Test for Diagnosis of Onchocerciasis. J. Infect. Dis. 2000, 182, 1796–1799. [Google Scholar] [CrossRef]
- Hotterbeekx, A.; Raimon, S.; Abd-Elfarag, G.; Carter, J.Y.; Sebit, W.; Suliman, A.; Fodjo, J.N.S.; De Witte, P.A.; Logora, M.Y.; Colebunders, R.; et al. Onchocerca volvulus is not detected in the cerebrospinal fluid of persons with onchocerciasis-associated epilepsy. Int. J. Infect. Dis. 2020, 91, 119–123. [Google Scholar] [CrossRef]
- Hotterbeekx, A.; Lammens, M.; Idro, R.; Akun, P.R.; Lukande, R.; Akena, G.; Nath, A.; Taylor, J.; Olwa, F.; Kumar-Singh, S.; et al. Neuroinflammation and Not Tauopathy Is a Predominant Pathological Signature of Nodding Syndrome. J. Neuropathol. Exp. Neurol. 2019, 78, 1049–1058. [Google Scholar] [CrossRef]
- Johnson, T.P.; Tyagi, R.; Lee, P.R.; Lee, M.-H.; Johnson, K.R.; Kowalak, J.; Elkahloun, A.; Medynets, M.; Hategan, A.; Kubofcik, J.; et al. Nodding syndrome may be an autoimmune reaction to the parasitic worm Onchocerca volvulus. Sci. Transl. Med. 2017, 9, eaaf6953. [Google Scholar] [CrossRef]
- Hotterbeekx, A.; Perneel, J.; Mandro, M.; Abhafule, G.; Fodjo, J.N.S.S.; Dusabimana, A.; Abrams, S.; Kumar-Singh, S.; Colebunders, R. Comparison of Diagnostic Tests for Onchocerca volvulus in the Democratic Republic of Congo. Pathogens 2020, 9, 435. [Google Scholar] [CrossRef]
| Characteristics | Logo, DRC (n = 391) | Mbam and Sanaga Valley, Cameroon (n = 77) | Mahenge, Tanzania (n = 187) | Kitgum and Pader, Uganda (n = 105) |
|---|---|---|---|---|
| Age in years: median (IQR) | 20.0 (14.0–29.0) | 26.0 (21.0–31.0) | 24.0 (18.0–34.5) | 20.0 (16.0–24.0) |
| Male gender: n (%) | 205 (52.4) | 34 (44.2) | 85 (47.2) | 56 (53.3) |
| Past ivermectin use: n (%) | 0 | 51 (66.2) | 89 (47.6) | 82 (78.1) |
| OV16 positive: n (%) | 149 (38.1) | 46 (59.7) | 102 (54.5) | 37 (35.2) |
| Age Group | OV16 Seroprevalence, n (%) |
|---|---|
| 10 years or younger (n = 79) | 7 (8.9) |
| 11–20 years (n = 253) | 103 (40.7) |
| 21–30 years (n = 235) | 128 (54.5) |
| 31–40 years (n = 93) | 39 (51.6) |
| 41–50 years (n = 39) | 17 (53.8) |
| 51 years or older (n = 47) | 15 (46.8) |
| Parameter | Coeff | 95% CI | p Value | |
|---|---|---|---|---|
| Age (years) | 0.1256 | 0.0780 | 0.1733 | <0.0001 |
| Age*age | −0.0016 | −0.0023 | −0.0009 | <0.0001 |
| Female gender | 0.0994 | −0.2225 | 0.4213 | 0.5451 |
| Ivermectin intake vs no intake during last CDTI round | −0.3471 | −0.9851 | 0.2908 | 0.2862 |
| Country site (CMR vs. DRC) | 0.384 | −0.2788 | 1.0468 | 0.2562 |
| Country site (TZD vs. DRC) | 0.3682 | −0.3455 | 1.0819 | 0.3120 |
| Country site (UG vs. DRC) | −0.3582 | −1.086 | 0.3696 | 0.3347 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dusabimana, A.; Bhwana, D.; Mandro, M.; Mmbando, B.P.; Siewe Fodjo, J.N.; Colebunders, R. OV16 Seroprevalence among Persons with Epilepsy in Onchocerciasis Endemic Regions: A Multi-Country Study. Pathogens 2020, 9, 847. https://doi.org/10.3390/pathogens9100847
Dusabimana A, Bhwana D, Mandro M, Mmbando BP, Siewe Fodjo JN, Colebunders R. OV16 Seroprevalence among Persons with Epilepsy in Onchocerciasis Endemic Regions: A Multi-Country Study. Pathogens. 2020; 9(10):847. https://doi.org/10.3390/pathogens9100847
Chicago/Turabian StyleDusabimana, Alfred, Dan Bhwana, Michel Mandro, Bruno P. Mmbando, Joseph N. Siewe Fodjo, and Robert Colebunders. 2020. "OV16 Seroprevalence among Persons with Epilepsy in Onchocerciasis Endemic Regions: A Multi-Country Study" Pathogens 9, no. 10: 847. https://doi.org/10.3390/pathogens9100847
APA StyleDusabimana, A., Bhwana, D., Mandro, M., Mmbando, B. P., Siewe Fodjo, J. N., & Colebunders, R. (2020). OV16 Seroprevalence among Persons with Epilepsy in Onchocerciasis Endemic Regions: A Multi-Country Study. Pathogens, 9(10), 847. https://doi.org/10.3390/pathogens9100847






