Abstract
Fusarium equiseti strain FCHE and Fusarium oxysporum strain FCHJ were isolated from the roots of wilting habanero pepper (Capsicum chinense Jacq.) seedlings with root rot. Toward developing a biorational control of these serious phytopathogenic strains, ethanolic (EE) and aqueous (AE) extracts of different vegetative parts of 40 tropical native plants of the Yucatán Peninsula were screened for antifungal activity. Extracts of six out of 40 assayed plants were effective, and the most inhibitory extracts were studied further. EEs from Mosannona depressa (bark from stems and roots), Parathesis cubana (roots), and Piper neesianum (leaves) inhibited mycelial growth of both strains. Each active EE was then partitioned between hexane and acetonitrile. The acetonitrile fraction from M. depressa stem bark (MDT-b) had the lowest minimum inhibitory concentration of 1000 µg/mL against both pathogens and moderate inhibitory concentration (IC50) of 462 against F. equiseti and 472 µg/mL against F. oxysporum. After 96 h treatment with EE from M. depressa stem bark, both strains had distorted hyphae and conidia and collapsed conidia in scanning electron micrographs. Liquid chromatography–ultraviolet–high resolution mass spectrometry analysis revealed that the major component of the fraction was α-asarone. Its antifungal effect was verified using a commercial standard, which had an IC50 of 236 µg/mL against F. equiseti and >500 µg/mL against F. oxysporum. Furthermore, the P. cubana hexane fraction and P. neesianum acetonitrile fraction had antifungal activity against both Fusarium pathogens. These compounds provide new options for biorational products to control phytopathogenic fungi.
Keywords:
antifungal; α-asarone; habanero pepper; phytopathogens; Mosannona depressa; plant extracts 1. Introduction
Approximately 200 species of Fusarium are recognized as pathogens of a broad range of plants, and F. graminearum and F. oxysporum were ranked in fourth and fifth place among the top 10 scientifically or economically most important fungal pathogens [1]. In pepper (Capsicum spp.) crops, serious post-harvest losses are caused by F. oxysporum [2]. Peppers from about 35 Capsicum species are consumed, most widely from C. annuum, C. baccatum, C. frutescens, C. pubescens and C. chinense, which have been the most successfully domesticated and cultivated [3]. México reported an annual production of 3.2 million tons of pepper crops and average annual growth in production of 4.82% during 2003–2016 [4]. In particular, habanero peppers (C. chinense) are appreciated worldwide for their high content of capsaicin, the main alkaloid responsible for their hotness [5]. Capsaicin is also beneficial as a cardioprotective, anti-inflammatory, analgesic and a gastrointestinal aid and for its thermogenic properties [6]. In the chemical industry, it is useful in the production of paints and varnishes, tear gas and other compounds. In the Yucatán Peninsula, habanero peppers are part of the culinary identity as a condiment [7]. The habanero pepper from the Yucatán Peninsula Denomination of Origin (NOM-189-SCFI-2017) is presently cultivated on 1134 ha [8], and its production has been increasing steadily in recent years. However, Fusarium spp. cause production losses of at least 50% or even 100% when conditions are favorable [9]. F. oxysporum and F. equiseti, which infect the roots of habanero pepper seedlings and cause root rot and wilting in the Yucatán Peninsula, México [10], also produce mycotoxins such as fumonisins and trichothecenes in crops and feed products and represent a risk to human health [11].
Currently, the management of Fusarium species depends on the intensive use of synthetic fungicides such as a benomyl, carbendazim, thiabendazole and alliete [12]. However, such intensive use can induce resistance in the pathogen and negatively impact the environment, beneficial microorganisms and humans by acting as a skin irritant and carcinogen [13,14]. To reduce dependence on synthetic pesticides, numerous strategies, such as the rotation of crops, use of resistant cultivars and biorational products and solarization of the soils, are thus integrated into a pest management program [15,16]. Natural products derived from plants are a highly viable option as biorational antifungal products that should leave less environmental residue and be nontoxic to beneficial organisms and humans [17,18]
To discover and incorporate new antifungal agents in the control of diseases caused by Fusarium species, several groups have tested plant extracts in vitro and in vivo [19,20,21]. The high plant diversity in Mexico, with 23,314 reported species, 50% of which are endemic, has scarcely been explored for their biological and chemical properties. In the Yucatán Peninsula, the 2330 known species of vascular plants, belonging to 956 genera and 161 families, represent 6% of the Mexican flora [22,23]. Previous bioprospecting of Yucatecan native plant extracts for activity against phytopathogenic fungi has revealed good fungicidal properties of extracts from plants such as Acacia pennatula, Acalypha gaumeri and Croton chichenensis [24,25,26].
Because of the increasing demand for natural fungicides to control habanero pepper diseases, more bioprospecting programs have been needed. Therefore, here, we screened 184 extracts from 40 plant species native to the Yucatán Peninsula for activity against F. equiseti FCHE and F. oxysporum FCHJ strains from habanero pepper (Table 1), examined hyphae using scanning electron microscopy (SEM) for any morphological effects of the active extracts and analyzed the chemical profile of the active fractions obtained from active extracts using liquid chromatography–ultraviolet–high-resolution mass spectrometry (LC-UV-HRMS).

Table 1.
Plants collected from the Yucatán Peninsula to screen for activity against F. equiseti strain FHCE and F. oxysporum strain FCHJ.
2. Results
2.1. Antifungal Activity of Plant Extracts Against Fusarium spp.
Table 2 shows the results of active plant extracts on mycelial growth of F. equiseti FCHE and F. oxysporum FCHJ. Ethanolic extracts (EEs) from Mosannona depressa (bark of stem and root), Parathesis cubana (root) and Piper neesianum (leaves) at 2000 µg/mL and aqueous extracts (AE) from Cameraria latifolia (root), Calea jamaicensis (whole plant) and Heteropterys laurifolia (leaves) at 3% w/v were active against one or both Fusarium strains after 96 h. All these active extracts inhibited mycelial growth of F. equiseti, but only four EEs inhibited mycelial growth of F. oxysporum. No active AEs were detected against F. oxysporum.

Table 2.
Inhibition of mycelial growth of Fusarium equiseti strain FCHE and F. oxysporum strain FCHJ by active plant extracts from native species of the Yucatán Peninsula in microdilution assay.
Complete mycelial growth inhibition (MGI of 100%) for both phytopathogens was achieved with EEs from M. depressa bark of stems and P. cubana roots. The EE from leaves of P. neesianum was also effective (MGI of 100% against F. equiseti and 75% against F. oxysporum). The AE from C. jamaicensis also achieved 75% MGI against F. equiseti. The EAs from C. latifolia root and H. laurifolia leaves achieved MGI of only 25% against F. equiseti (Table 2). On the other hand, none of the EAs had any activity against F. oxysporum. The positive control, prochloraz (0.11%), completely inhibited the growth of the two phytopathogens, and typical mycelial growth of both plant pathogens was observed for the negative controls. The other plant extracts did not cause significant mycelial growth inhibition with respect to the negative control (Supplementary Table S1).
2.2. Minimum Inhibitory Concentration of Ethanolic Extracts, Fractions and α-Asarone
The minimum inhibitory concentration (MIC) of the four EEs that completely inhibited mycelial growth of both Fusarium strains was determined. F. equiseti was more sensitive to the extracts from M. depressa stem bark, P. cubana roots and P. neesianum leaves (MIC: 1000 µg/mL). All these active extracts were fungicidal, except for the extract from leaves of P. neesianum, which was fungistatic (Table 2). In contrast, F. oxysporum was less sensitive to the four EEs, which were fungistatic and had MICs of 2000 µg/mL. Therefore, the four EEs were partition-fractionated, and serial dilutions of each fraction (hexane, acetonitrile and a methanol-soluble precipitate) were tested for activity.
The most active fractions against F. oxysporum were the hexane (MDT-a) and acetonitrile (MDT-b) fractions from M. depressa stem bark, which were both fungistatic, and the hexane fraction from P. cubana roots (PCR-a), which was fungicidal; all had a MIC of 1000 µg/mL (Table 3). As expected, a fungicidal effect on F. equiseti was induced by half of the fractions, with a MIC of 1000 µg/mL. These fractions were the same as those that inhibited F. oxysporum: the acetonitrile fraction from P. neesianum leaves (PNH-b), precipitates of P. cubana roots (PCR-c) and P. neesianum leaves (PNH-c). The fractions obtained from the root bark and the precipitate of the stem bark of M. depressa were considered as inactive against the two pathogens because their MIC was greater than 1000 µg/mL (Table 3).

Table 3.
Minimum inhibitory concentration (MIC) of extracts and fractions from Mosannona depressa (bark of stems and roots), P. cubana (roots), P. neesianum (leaves) and α-asarone against Fusarium equiseti strain FCHE and F. oxysporum strain FCHJ.
The MIC of the commercial α-asarone standard, evaluated in parallel with the fractions, was 500 µg/mL against F. equiseti with fungistatic effect, and >500 µg/mL against F. oxysporum (Table 3).
2.3. Inhibitory Concentration (IC50 and IC95)
The α-asarone standard had the lowest IC50 and IC95 against both species, followed by the MDT-b fraction from M. depressa stem bark (Table 4). Interestingly, the IC50 and IC95 for the MDT-b fraction and α-asarone were very similar against F. oxysporum (respectively, 472 and 539 µg/mL, MDT-b, 482 and 526 µg/mL, α-asarone). Against F. equiseti, the IC50 and IC95 for the MDT-b and PNH-b fractions were both 462 and 526 µg/mL, respectively, higher than for α-asarone and similar to those for the EE from M. depressa stem bark.

Table 4.
IC50 and IC95 of active extracts and fractions from Mosannona depressa, Parathesis cubana, Piper neesianum and of the commercial standard α-asarone against mycelial growth of Fusarium equiseti strain FCHE and F. oxysporum strain FCHJ.
2.4. Effect of Active Extracts from Mosannona depressa on Morphology of Fusarium Strains
The SEM of the untreated strains (negative control) showed typical well-formed hyphae and microconidia (Figure 1A–D and Figure 2A–D). After 96 h of exposure to 2000 µg/mL EE from M. depressa stem bark, F. equiseti had distorted hyphae, globular structures along the surface of the mycelium and contorted and dehydrated conidia (Figure 1E). Conidia of the same strain were similarly affected by 2000 µg/mL EE from M. depressa root bark (Figure 1F).
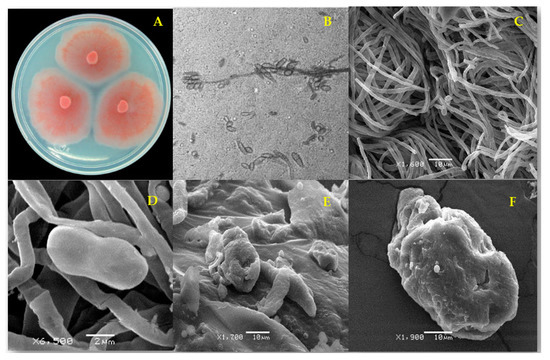
Figure 1.
Fusarium equiseti strain FCHE morphology (A) after 7 d on potato dextrose agar; (B) microconidia of F. equiseti (1000×) and (C) typical untreated mycelium and microconidia (negative control); (D) apparently normal microconidium and (E) distorted mycelium and collapsed microconidia after 96 h treatment with ethanolic extract from Mosannona depressa stem bark at 2000 µg/mL; (F) rough surface of a collapsed-looking microconidium after 96 h treatment with 2000 µg/mL ethanolic extract from M. depressa root bark.
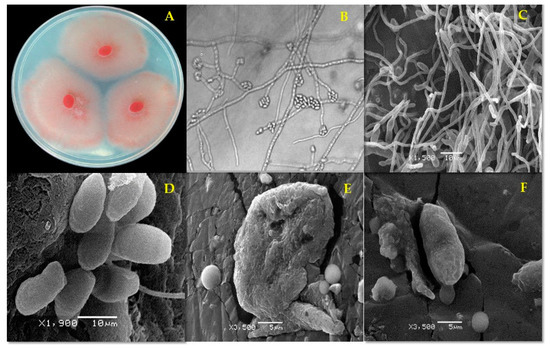
Figure 2.
Fusarium oxysporum strain FCHJ morphology (A) after 7 d on potato dextrose agar (PDA). (B) Microconidia (1000×) and (C) typical mycelium and microconidia (negative control); (D) apparently normal microconidia, (E) misshapen and collapsed microconidium after 96 h treatment with ethanolic extract from Mosannona depressa stem bark at 2000 µg/mL; (F) collapsed conidium after 96 h treatment with ethanolic extract from M. depressa root bark at 2000 µg/mL.
2.5. Identification of Active Components in Extracts from Mosannona depressa by LC-UV-HRMS
The MDT-b and MDR-b fractions from M. depressa bark from the stem and roots were analyzed by LC-UV-HRMS (Table 5). The chromatogram of the MDT-b fraction showed five components, with the most abundant eluted at a retention time of 4.27 min (peak 3, Figure 3). The HRMS of peak 3 presented a protonated molecular ion at m/z 209.1172, indicative of a molecular formula of C12H16O3 (calc. for C12H17O3+, 209.1173), and its UV spectrum exhibited maxima at 220, 260 and 320 nm. This component was identified as α-asarone based on the reference spectrum in the equipment databases and confirmed using a commercial standard (Figure 3, Table 5). The minor components at retention times of 2.33, 2.55, 4.8 and 4.89 min had structural characteristics similar to those of α-asarone, and their UV and HRMS data were compared with databases in the literature and Chapman & Hall Dictionary of Natural Products (CHDNP). The HRMS of peak 1 showed UV maxima at 230 and 290 nm, and a protonated ion at m/z 225.1120, suggesting a molecular formula of C12H16O4 (calc. for C12H17O4+, 225.1121), which was not assigned to any previously reported compound after comparison of the UV and HRMS data with databases in the literature and CHDNP. The analysis of peak 2 showed a protonated ion at m/z of 197.0808, with a molecular formula of C10H12O4 (calc. for C10H13O4+, 197.0808) and UV maxima at 238, 270 and 345 nm; thus, the compound was tentatively identified as asaraldehyde. Components 4 and 5 had the same UV maxima at 220, 240 and 290 nm and protonated ions at m/z 221.1170 and 193.0857, respectively, accounting for molecular formulae of C13H16O3 (calc. for C13H17O3+, 221.1172) for component 4 and C11H12O3 (calc. for C11H13O3+, 193.0859) for component 5. After an exhaustive comparison of their spectral data with CHDPN and databases in the literature, compound 5 was tentatively identified as isomyristicin, but compound 4 was not identified (Table 5).

Table 5.
Metabolites identified from acetonitrile fraction of Mosannona depressa stem bark (MDT-b) by liquid chromatography–ultraviolet–high-resolution mass spectrometry (LC-UV-HRMS).
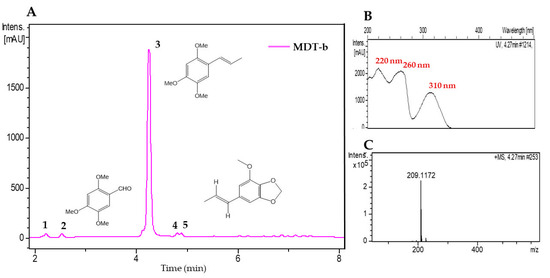
Figure 3.
(A) Liquid chromatogram (UV 210 nm) of acetonitrile fraction from stem bark of Mosannona depressa (MDT-b). 1: Not identified (C12H16O4), 2: asaraldehyde, 3: α-asarone, 4: not identified, 5: trans-isomyristicin. (B) UV spectrum of peak 3 and (C) high-resolution mass spectrum of peak 3.
Two components were detected in the medium polarity fraction (MDR-b) from M. depressa root bark (Figure 4). The most abundant was peak 2, with a retention time of 4.37 min, showing a protonated ion at m/z 239.1278, with a molecular formula of C13H18O4 (calc. for C13H19O4+, 239.1278); its UV spectrum presented maxima at 205, 215 and 280 nm. Comparison of these data with the databases led us to tentatively identify peak 2 as 1,2,3,4-tetramethoxy-5-(2-propenyl) benzene (Table 6). Data for peak 1 at a retention time of 4.25 min corresponded to α-asarone (Table 6).
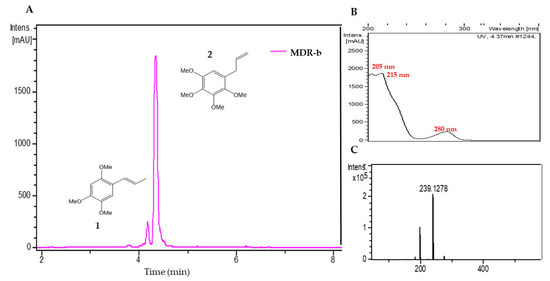
Figure 4.
(A) Liquid chromatogram (UV at 210 nm) of the acetonitrile fraction from Mosannona depressa root bark (MDR-b); 1: α-asarone 2: 1,2,3,4-tetramethoxy-5-(2-propenyl) benzene. (B) UV spectrum of peak 2 and (C) high-resolution mass spectra of peak 2.

Table 6.
Compounds identified in the acetonitrile fraction of the ethanolic extract of Mosannona depressa root bark (MDR-b) using LC-UV-HRMS.
3. Discussion
This first bioprospecting report on plant extracts with activity against fungal pathogens of habanero pepper is part of continuing efforts to discover potential bioactive compounds in the diverse flora of southeastern México. From sites not previously explored, we collected 40 plant species that our exhaustive search of the literature showed had not been tested against fungal phytopathogens, with the exception of Annona primigenia [28,29] and Mosannona depressa [30,31]. Antifungal screening of EEs and AEs from different vegetative parts of the 40 plant species led to the detection of six (15% of the total) species with activity against the Fusarium strains tested. These active extracts were from Calea jamaicensis, Cameraria latifolia, Heteropterys laurifolia, Mosannona depressa, Parathesis cubana and Piper neesianum. Interestingly, these plant species belong to different families and were collected at the same site, Jahuactal, a tropical evergreen rainforest with trees exceeding 20 m in height.
Fusarium equiseti was more sensitive than F. oxysporum to the plant extracts tested. Mycelial growth of F. oxysporum was inhibited by only four EEs, representing 4.3% of the plant extracts tested, and totally insensitive to AEs at the tested concentration (3% w/v). Several studies have indicated that AEs, even at higher concentrations, have limited effect on F. oxysporum. For example, the mycelial growth of F. oxysporum was inhibited 10–55% by extracts from leaves at 10% w/v of Ocimum sanctum [32] and stems, root and fruits of Momordica charantia [33], among others.
In contrast, in our study, four EEs completely inhibited the mycelial growth of both plant pathogens; the EEs from the bark of stems and roots of M. depressa were especially effective. Native to Mexico and Central America, this medicinal tree (syn. Annona depressa, Guatteria gaumeri, Malmea depressa and M. gaumeri) has a wide range of biological activities in humans, e.g., antifungal, antiproliferative, antiprotozoal, cytotoxic, hypoglycemic and hypocholesterolemic [34,35,36]. For agriculture applications, however, a chloroform extract from the stem bark of M. depressa was reported only as a growth inhibitor of Amaranthus hypochondriacus (IC50 = 134 µg/mL) and Echinochloa crusgalli (IC50 = 457 µg/mL), and as a fungicide against F. oxysporum (MIC = 400 µg/mL) [31]; EEs from M. depressa stem and root bark had antifungal activity against Penicillium oxalicum (MIC = 250 µg/mL) [37].
The present report is also the first on the fungicidal effect of the EEs from M. depressa against F. equiseti. The MIC of 1000 µg/mL for EEs from the bark of stems and roots of M. depressa is comparable to the effect against F. equiseti reported for ethanolic extracts of leaves from Calycopteris floribunda (MIC: 500 µg/mL) [38] and rhizomes from Acorus calamus (MIC: 1000 µg/mL) [39]. In the case of F. oxysporum, here, both EEs from the bark of stems and roots of M. depressa were fungistatic with a higher MIC of 2000 µg/mL. In a previous study, a chloroform extract of the stem bark of M. depressa was antifungal against F. oxysporum (MIC: 400 µg/mL) and Trichophyton mentagrophytes (MIC: 500 µg/mL) [31]. The lower MIC may be attributed to the polarity of the solvent used and the susceptibility and forma specialis of the pathogenic strain tested [40]. Matos et al. [41] found variation in the sensitivity to Chelidonium majus extracts among six F. oxysporum isolates, with f. sp. cubense the most sensitive.
The guided fractionation with the antifungal assay of the EEs from the bark of stems and roots of M. depressa showed that F. equiseti and F. oxysporum were more sensitive to the MDT-b fraction. LC-UV-HRMS analyses revealed a mixture of phenylpropanoids in the MDT-b fraction; the major component was α-asarone, with minor components asaraldehyde and isomyristicin, tentatively identified based on their UV and HRMS data. In the literature, we found only two phytochemical studies of an organic extract from M. depressa stem bark, which had a different metabolic profile [30,31]. Our results agree with the report by Enriquez et al. [30], who identified α-asarone as the most abundant component in a hexane extract, which also included asaraldehyde, trans-isoelemicin and trans-isomyristicin. In the study by Jimenez Arellanes et al. [31], a chloroform extract contained four tetramethoxyl derivatives [1,2,3,4-tetramethoxy-5-(2-propenyl)-benzene, 2,3,4,5-tetramethoxybenzaldehyde, 2,3,4,5-tetramethoxycinnamaldehyde, 2,3,4,5-tetramethoxycinnamyl alcohol] and trans-isomyristicin. Such differences in composition could be attributed to season, phenological stage and geographical region where plants were collected, which can greatly influence chemical biosynthesis and bioactivity. For example, essential oils from Perilla frutescens collected from 11 areas in China differed in yields and chemical composition, which were associated with antioxidant and antifungal activities [42]. When total alkaloids and the annomontine and oxopurpureine content from roots and leaves of Annona purpurea were monitored over time, the alkaloid was high during the dry season and during flowering; the strongest antifungal activity was obtained from the root extracts during the last month of the dry season [43].
In our investigation, α-asarone (syn. trans-asarone) was identified as the principal compound responsible for the antifungal effect on F. equiseti and F. oxysporum. Its IC50 (236 and 482 µg/mL, respectively) and IC95 (269 and 526 µg/mL, respectively) were lower than those of the EE from M. depressa stem bark. An antifungal effect of α-asarone at 1000 mg/L has been reported for the phytopathogens Phytophthora infestans and Pyricularia grisea with growth inhibition (GI) of 85 and 53%, respectively [44], for Botrytis cinerea, F. oxysporum and Phomopsis obscurans (GI = 57.7, 43.6 and 41.5%, respectively) at 300 µM [45] and slight activity against the yeasts Candida albicans, C. kruseii and C. parapsilasis at 100 µg/mL [46]. It also has pesticidal properties as an antifeedant against Helicovarpa zea, Helionthis virescens and Manduca sexta; it is insecticidal against Aedes aegypti and Lucila sericata, and nematocidal against Caenorhabditis elegans, Panagrellus redivivus and Nyppostrongylus brasiliensis [46,47]. Interestingly, Jimenez Arellanes et al. [31] reported that 1,2,3,4-tetramethoxy-5-(2-propenyl)-benzene was the most abundant component in the chloroform extract (0.71% from dried stem bark) and the major phytogrowth inhibitory compound in Amaranthus hychondriacus (IC50 = 43 µg/mL) and E. crusgalli (IC50 = 43 µg/mL), and it had an antifungal effect on an undocumented strain of F. oxysporum (MIC: 250 µg/mL). In the present study, this compound was not detected from the stem extracts. However, it was abundant in the MDR-b fraction from M. depressa root bark, but it had no effect on the mycelial growth of F. oxysporum strain FCHJ, and F. equiseti strain FCHE was only moderately sensitive (75% MGI at 1000 µg/mL). Based on these results, the antifungal activity of M. depressa collected in Jahuactal is considered to be primarily due to the presence of α-asarone in the extract.
As shown by SEM, EE from M. depressa stem bark at 2000 µg/mL caused prominent morphological alterations of F. oxysporum and F. equiseti. Hyphae were malformed and contorted, and microconidia had collapsed. This effect is similar to the morphological changes in conidia and hyphae of the filamentous zoopathogenic fungus Microsporum gyseum after 4 d exposure to 100 mg/mL of the β-asarone fraction [48]; further cell death of F. oxysporum induced by a mixture of asarones (α, β, γ, 3.4:94.3:1%) at 500 µg/mL was observed using epifluorescence microscopy; the rapid cell death is correlated with greater production of reactive oxygen species [49]. Studies on the mechanism of action of β-asarone showed that it interferes with ergosterol synthesis, thus the ergosterol content is lowered in the plasma membrane of Aspergillus niger ATCC 16,888 [50], confirming that the effect against F. oxysporum might be related to the inhibition of ergosterol biosynthesis, as it is in C. albicans [51]. Hence, similar to its isomer β-asarone, α-asarone in the EE from M. depressa stem bark might inhibit the mycelial growth of F. oxysporum and F. equiseti by damaging the plasma membrane and causing cell death. More studies are needed to verify the site of action of asarones and other metabolites of M. depressa on fungal pathogens.
Another promising plant species for antifungal compounds in our study was P. neesianum (Piperaceae, syn. Piper sempervirens, Arctottonia sempervirens), a tree used in traditional medicine to treat snake bites and wounds [52,53]. The EE from P. neesianum leaves and its PNH-b fraction completely inhibited the growth of F. equiseti (MIC: 1000 µg/mL) and had the same IC50 and IC95 (462 and 866 µg/mL, respectively) as the MDT-b fraction. This report is the first of an antifungal effect of P. neesianum against F. equiseti and F. oxysporum. The dichloromethane extract from leaves of P. neesianum has been reported to have various biological activities as an antioxidant (IC50 = DPPH 0.071 mg/mL) [54], anti-tyrosinase (IC50 = 6.6 µg/mL [55] and anti-urease (IC50 = 12.9 µg/mL) [56]. Essential oil from P. neesianum leaves collected in the northern region of Guatemala contained bicyclogermacrene (28%), germacrene D (11.7%) and β-caryopyllene (7.5%) as major compounds among 19 detected in a gas chromatography with flame ionization detection- mass spectrometry analysis [52].
The EE from P. cubana (Primulaceae; syn. Ardisia cubana) roots was also active against both Fusarium pathogens, and the low polarity PCR-a fraction was fungicidal (MIC: 1000 µg/mL). These findings are the first report of a biological activity for extracts from P. cubana.
The EA from C. jamaicensis (Asteraceae) was the only EA that moderately inhibited the growth of F. equiseti, suggesting that it produces a highly polar antifungal metabolite(s). This species was documented to have leishmanicidal activity and to be useful for treating colds and stomach pain [57,58], but the present report is the first on its antifungal activity. Among 125 Calea species, only C. urticifolia has been tested against fungal pathogens, but it had no activity against F. oxysporum [25,59]. Acacetin, O-methylacacetin, jamaicolides A–D and prumichromene B have been identified in aerial parts of C. jamaicensis [58].
In summary, the present investigation revealed that F. equiseti FCHE and F. oxysporum FCHJ strains isolated from habanero pepper plants were sensitive to extracts from six native plant species, and the most effective were the EEs from M. depressa, P. cubana and P. neesianum, and advanced our knowledge about the phytochemicals in the roots of M. depressa from the Yucatán Peninsula. α-Asarone was identified as the principal antifungal component in the stem bark of M. depressa. Now, we need to determine the persistence of its antifungal effect and any toxicity to the environment and beneficial macro- and microorganisms in the soil as a pure compound and in the complex ethanolic extract mixture.
Our knowledge on the pesticidal potential of the native Mexican flora has also been enriched, and on the basis of our broad screening, we will isolate and identify the compounds in the active EEs from P. cubana and P. neesianum and the AE from C. jamaicensis that contribute to the antifungal activity. Subsequently, we expect to propagate the promising species to provide material for greenhouse and field experiments. Of course, the mechanism and sites of action of the identified metabolites in the fungus need to be determined, and the metabolites tested for safety against nontarget organisms. This research also opens opportunities for future studies on the conservation and sustainable use of our regional flora in the development of biorational products for the integrated management of C. chinense and other species of Capsicum.
4. Materials and Methods
4.1. Plant Materials
Plants were collected from six locations in the Yucatán Peninsula: (1) Jahuactal, Ejido Caobas, Othón Pompeyo Blanco (18°15′34″ N, 88°57′14″ W), (2) Kaxil Kiuic, Oxkutzcab (20°06′10.8″ N; 89°33′43.2″ W), (3) Punta Laguna, Valladolid (20°38′49.4″ N, 87°38′02.2″ W), (4) Xmaben, Hopelchén (19°15′42.92″ N, 89°21′45.91″ W), (5) Punta Pulticub, Othón P. Blanco (19°04′29.96″ N, 87°33′17.15″ W) and (6) Chacchoben Limones, Othón P. Blanco (19°01′44.31″ N, 88°08′00.38″ W) of the states of Yucatán and Quintana Roo (Table 1). Each plant was separated into leaves, stems and roots for separate extractions, and whole plants (WP) of some species were extracted. Plant materials were dried in a lamp stove at 55–60 °C for 5 d and crushed in a mill (model 1520, Pagani, Azcapotzalco, México) with blades and no. 5 mm mesh. A voucher specimen for each plant species was deposited in the Roger Orellana Herbarium of the Unidad de Recursos Naturales del Centro de Investigación Científica de Yucatán and identified by experts (Table 1).
4.2. Preparation of Plant Extracts
4.2.1. Aqueous Extracts
The dried, ground plant material (1.5 g) was transferred to an Erlenmeyer flask, and 20 mL of boiling distilled water were added. After 15 min, the sample was filtered through filter paper (Whatman no. 1) and cotton to remove solid residues, then diluted with distilled water to 25 mL, to obtain an aqueous extract (AE) with a final concentration of 6% (w/v). Under aseptic conditions, the infusion was sterilized using a 0.22 μm Millipore filter (Merck-Millipore, Burlington, MA, USA), and frozen at −17.5 ± 0.5 °C until use [60].
4.2.2. Ethanolic Extracts
The dried, ground plant material was immersed in ethanol (1.5% of the total volume) and extracted three times with ethanol by sonication at 20 kHz (Cole-Parmer, Chicago, IL, USA), at room temperature for 20 min each time. The solvent was filtered and eliminated under vacuum in a rotary evaporator (IKA model RV-10, Staufen, Germany) at 40 °C to obtain the ethanolic crude extract [24]. The EEs with the greatest activity in the antifungal assay described (Section 4.4) were partitioned with hexane–acetonitrile three times (2: 1, 1: 1, 1: 1 v/v) and solvents removed as described above. In this way, a hexane fraction (A), acetonitrile fraction (B) and methanol-soluble precipitate (C) of each EE were obtained.
4.3. Fungal Cultures
Phytopathogenic strains of Fusarium equiseti (FCHE, GenBank acc. MG020433) and F. oxysporum (FCHJ, GenBank acc. MG020428) were obtained from the fungal collection of the Phytopathology Laboratory, Tecnológico Nacional de México, Instituto Tecnológico de Conkal. These strains were isolated from stem and root lesions of habanero pepper plants [10]. The strains were maintained by transferring a mycelial disc (5 mm diameter) to (a) 20% glycerol (v/v) and frozen at −80 °C, (b) sterile distilled water and (c) commercial potato dextrose agar in slant tubes (PDA, BD, Bioxon, Edo. México) and stored at 4 °C in the dark.
4.4. Antifungal Microdilution Assay of Extracts
4.4.1. Preparation of Conidial Suspension
F. equiseti and F. oxysporum strains were reactivated on PDA and incubated at 27 ± 2 °C, with 16 h light/8 h dark in a humidity chamber to induce sporulation. After 7 days, the surface of the culture was flooded with a sterile saline solution (5 mL), then gently scraped with a sterile brush to release conidia into the saline. The resulting conidial suspension was filtered through a double layer of sterile cheesecloth and adjusted to a final concentration of 1 × 105 conidia/mL for both pathogens with sterile saline solution, using a hemocytometer [61].
4.4.2. Bioassay with Aqueous Extracts
In the broth microdilution to determine the mycelial growth inhibition (MGI) of the Fusarium strains, 100 µL of each 6% AE were transferred to each microwell of a 96-well plate. As a negative control, 100 µL of the conidial suspension were used and as positive control, 5 µL of the fungicide Mirage CE 45 (prochloraz 450 g a.i./L) (Bayer CropScience, NC, USA). Finally, 100 µL of the conidial suspension were added for a final concentration of 3% w/v AE, 0.112% of prochloraz (w/v), 5 × 104 conidia/mL of Fusarium strains. All tests were performed in triplicate and microdilution plates maintained at 27 ± 2 °C, and 16 h light/8 h dark. The MG was recorded at 96 h, visually determined with a microscope at 50× using the National Committee for Clinical Laboratory Standards with slight modifications, using a 0–4 scale, where 4 is full MG (0% MGI) and 0 the absence of MG (MGI =100%) [62,63]. Data were converted to a percentage of mycelial growth inhibition (MGI) using Abbott’s formula: [(% MG in the negative control − % MG in the treatment)/% MG in the negative control)] × 100 [62].
4.4.3. Bioassay with Ethanolic Extracts
Each EE was dissolved in a mixture of dimethylsulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO, USA) with 0.5% Tween 20 to obtain a solution at 40 µg/µL EE. Then 10 µL of this EE solution were added to each microwell, containing 90 µL of RPMI liquid medium (Roswell Park Memorial Institute 1640). Mirage CE 45 (5 µL) was used as the positive control as described above; negative growth controls were RMPI (Merck Millipore Darmstadt, Germany), water (100 µL) and a blank (0.5% Tween 20 DMSO: RPMI 1:9, v/v). Each microwell then received 100 µL of the conidial suspension for a final EE concentration of 2000 µg/mL and 5% of DMSO with 0.5% Tween 20 (Merck Millipore Darmstadt, Germany) [24]. All tests were done three times, and the plates were incubated and assessed as described above.
4.4.4. Minimum Inhibitory Concentration of Active EEs and Fractions
Serial dilutions of fractions A, B and C and active EE solutions (80 µg/µL), prepared as described above, were evaluated in a microdilution assay to determine the MIC [24]. The EEs were tested at final concentrations of 2000, 1000, 500 and 250 µg/mL. The fractions were evaluated at 1000, 500 and 250 µg/mL. The commercial α-asarone standard (Sigma-Aldrich, St. Louis, MO, USA) was tested at 500, 250 and 125 µg/mL. The same controls and incubation conditions were used as described above. All determinations were made with four replicates, three times. After incubation at 96 h, the MIC was determined as the lowest concentration of the extract at which no mycelial growth was observed in the well.
After 96 h of incubation, 10 µL from each well that had no growth were transferred to PDA and incubated at 27 ± 2 °C. After 72 h, the presence of growth was cataloged as fungicidal, the absence of growth as fungistatic [64].
4.5. Effect of Ethanolic Extracts on Hyphal Morphology of Fusarium Strains
The strains of F. equiseti and F. oxysporum were grown on PDA in Petri dishes for 7 d, then 5 mm disks were removed from the growing edge of the colony. The samples were fixed in 2.5% v/v glutaraldehyde (Merck Millipore Darmstadt, Germany) and 0.2 M sodium phosphate (Sigma-Aldrich, St. Louis, MO, USA) pH 7.2 for 48 h at 4 °C and washed twice with the phosphate buffer (1 h each time). The samples were dehydrated in an ethanol series (1 h each: 30, 50, 70, 85, 96 and 100%, 2 × absolute ethanol). The samples were dried with CO2 in a Sandri-795 critical point dryer (Tousimis Research Corp., Rockville, MD, USA), then attached to a sample holder using double-sided adhesive carbon tape and coated with gold for 10 min in an ionizing chamber (Dentom Vacuum-Desk II, Moorestown, NJ, USA). The samples were observed in a JSM 6360 SEM (Jeol, Tokyo, Japan) at 20 kV.
After the fungus was exposed for 96 h to 200 µL of EE from M. depressa, the mixture was filtered through a nylon membrane (nucleic acid blotting membrane Hybond N+ 0.45 µm) (GE Healthcare Bioscience, Amersham PI, Little Chalfont, UK), and the fungal samples were fixed as described above.
4.6. Chromatographic and Spectrometric Analyses
4.6.1. Thin Layer Chromatography (TLC)
The active EEs and their fractions were analyzed by thin layer chromatography (TLC) using an aluminum support impregnated with 0.25 mm thick G-60 silica gel with fluorescent indicator F254 (Merck Millipore, Burlington, MA, USA). In parallel, the commercial standard α-asarone (Sigma-Aldrich, St. Louis, MO, USA) was applied to confirm its presence in M. depressa extracts. The plates were developed in three elution systems: hexane-acetone (8:2), CH2Cl2-AcOEt (9:1) and CH2Cl2-MeOH (85:15). After separation, the metabolites were visualized with ultraviolet light (UV254 and UV365) and phosphomolybdic acid (Sigma-Aldrich, St. Louis, MO, USA).
4.6.2. LC-UV-HRMS
The active fractions (2 µL) from M. depressa stem bark (MDT-b) and root bark (MDR-b) were analyzed by liquid chromatography–ultraviolet–high-resolution mass spectrometry (LC-UV-HRMS) using a data-dependent acquisition protocol [65]. Chromatograms and mass spectra were obtained using an LC-MS (Agilent, Santa Clara, CA, USA) coupled to a Bruker Maxis HR-QTOF mass detector (Bruker Daltonics GmbH, Bremen, Germany) at 40 °C. A Zorbax SB-C8 column (Agilent, Santa Clara, CA, USA) was used (2.1 × 30 mm) with a mobile phase of a mixture of solvent A (water–acetonitrile 90:10 with 0.01% v/v trifluoroacetic acid and 1.3 mM ammonium formate) and solvent B (water–acetonitrile 10:90 with 0.01% v/v trifluoroacetic acid and 1.3 mM ammonium formate) and a flow rate of 300 µL/min. The gradient was set for a constant flow rate of 10% B to 100% B in 6 min, 100% B for 2 min, then 10% B for 2 min. Mass spectra (150 to 2000 m/z) were acquired in positive mode. The components detected were compared with the MEDINA database of microbial metabolites and the Chapman & Hall Dictionary of Natural Products (v25.1, CRC Press, Boca Raton, FL, USA).
4.7. Statistical Analyses
For the % MGI data, a one-way analysis of variance was performed with prior transformation of the original data using the formula: y = arsin [sqrt (y/100)]. The treatment means were compared using Tukey’s multiple range test (p = 0.05). Variance analyses were performed using SAS ver. 9.4 for Windows (SAS Institute, Cary, NC, USA). IC50 and IC95 values with 95% confidence intervals were calculated for EEs and effective fractions using a probit analysis.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-0817/9/10/827/s1, Table S1: Inhibition of mycelial growth of Fusarium equiseti strain FCHE and F. oxysporum strain FCHJ by plant extracts from 40 native species of the Yucatán Peninsula in microdilution assay.
Author Contributions
M.G.-A and J.C.-A. conceived the project; P.C.-C. performed the experiments, collected and analyzed data and wrote the original draft; J.M. and F.R. carried out LC-UV-HRMS data acquisition, analysis and interpretation; V.R.-C. and M.V.-K. reviewed the manuscript; G.C. collected and identified the plants. J.C.-A and M.G.-A. supervised and provided biological material for assays. All authors participated in the interpretation of the data, reviewed and approved the final version of the submitted manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by CONACYT project PDCPN-2015-266. P.C.-C. thanks CONACYT for a doctoral student scholarship (No. 661906).
Acknowledgments
P.C.-C. thanks the Instituto Tecnológico Nacional de México, Campus Conkal. Mérida, Yucatán, José Luis Tapia Muñoz., Jesus Aviles Gómez, I. Leticia Medina Baizabal, and Felipe Barredo for greatly appreciated technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kajmann, R.; Ellis, J.; et al. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant. Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Velarde-Félix, S.; Garzón-Tiznado, J.A.; Hernández-Verdugo, S.; López-Orona, C.A.; Retes-Manjarrez, J.E. Occurrence of Fusarium oxysporum causing wilt on pepper in México. Can. J. Plant. Pathol. 2018, 40, 238–247. [Google Scholar] [CrossRef]
- Bosland, P.W.; Votava, E.J. Peppers: Vegetable and Spice Capsicums, 2nd ed.; CABI: Wallingford, Oxfordshire, UK, 2012; Volume 22, pp. 16–36. ISBN 9781845938253. [Google Scholar]
- Naves, E.R.; de Ávila Silva, L.; Sulpice, R.; Araújo, W.L.; Nunes-Nesi, A.; Pérez, L.E.; Zsögön, A. Capsaicinoids: Pungency beyond. Capsicum. Trends Plant. Sci. 2019, 24, 109–120. [Google Scholar] [CrossRef]
- Chiles y Pimientos. Available online: https://www.gob.mx/cms/uploads/attachment/file/255626/Planeaci_n_Agr_cola_Nacional_2017-2030-_parte_tres (accessed on 14 September 2020).
- Srinivasan, K. Biological activities of red pepper (Capsicum annuum) and its pungent principle capsaicin: A review. Crit. Rev. Food Sci. Nutr. 2015, 56, 1488–1500. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lau, N.; Medina-Lara, F.; Martínez-Estévez, M. El chile habanero: Su origen y usos. Ciencia 2011, 63, 70–77. [Google Scholar]
- Anuario Estadístico de la Producción Agrícola. Available online: https://nube.siap.gob.mx/cierreagricola (accessed on 14 September 2020).
- Mejía-Bautista, M.Á.; Reyes-Ramírez, A.; Cristóbal-Alejo, J.; Tun-Suárez, J.M.; Borges-Gómez, L.D.C.; Pacheco-Aguilar, J.R. Bacillus spp. en el control de la marchitez causada por Fusarium spp. en Capsicum chinense. Rev. Mex. Fitopatol. 2016, 34, 208–222. [Google Scholar] [CrossRef]
- Mis-Mut, D.M. Identificación Molecular de Trichoderma spp. con Aplicación Agrícola y su Efectividad in vitro Contra Fusarium spp. Tesis de Maestría; Instituto Tecnológico de Conkal: Mérida, Yucatán, Mexico, 2015. [Google Scholar]
- Shi, W.; Tan, Y.; Wang, S.; Gardiner, D.M.; De Saeger, A.; Liao, Y.; Wang, C.; Fan, Y.; Wang, Z.; Wu, A. Mycotoxigenic potentials of Fusarium species in various culture matrices revealed by mycotoxin profiling. Toxins 2017, 9, 6. [Google Scholar] [CrossRef]
- Bashir, M.R.; Atiq, M.; Sajid, M.; Mohsan, M.; Abbas, W.; Alam, M.W.; Bashair, M. Antifungal exploitation of fungicides against Fusarium oxysporum f. sp. capsici causing Fusarium wilt of chilli pepper in Pakistan. Environ. Sci. Pollut. Res. 2018, 25, 6797–6801. [Google Scholar] [CrossRef]
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energ. Sec. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Gaherwal, S.; Prakash, M.M.; Khasdeo, K.; Sharma, A. Impact of selected chemical and herbal pesticide on beneficial soil microorganism. Int. J. Microbiol. Res. 2015, 6, 236–239. [Google Scholar] [CrossRef]
- Matyjaszczyk, E. “Biorationals” in integrated pest management strategies. J. Plant. Dis. Prot. 2018, 125, 523–527. [Google Scholar] [CrossRef]
- Moya-Elizondo, E.A.; Jacobsen, B.J. Integrated management of Fusarium crown rot of wheat using fungicide seed treatment, cultivar resistance, and induction of systemic acquired resistance (SAR). Biol. Control. 2016, 92, 153–163. [Google Scholar] [CrossRef]
- Walia, S.; Saha, S.; Tripathi, V.; Sharma, K.K. Phytochemical biopesticides: Some recent developments. Phytochem. Rev. 2017, 16, 989–1007. [Google Scholar] [CrossRef]
- Ramírez-Mares, M.V.; Hernández-Carlos, B. Plant-derived natural products from the American continent for the control of phytopathogenic fungi: A review. J. Glob. Innov. Agric. Soc. Sci. 2015, 3, 96–118. [Google Scholar] [CrossRef]
- Peñuelas-Rubio, O.; Arellano-Gil, M.; Verdugo-Fuentes, A.A.; Chaparro-Encinas, L.A.; Hernández-Rodríguez, S.E.; Martínez-Carrillo, J.L.; Vargas-Arispuro, I.D.C. Larrea tridentata extracts as an ecological strategy against Fusarium oxysporum radicis-lycopersici in tomato plants under greenhouse conditions. Rev. Mex. Fitopatol. 2017, 35, 360–376. [Google Scholar] [CrossRef]
- Sesan, T.E.; Enache, E.; Iacomi, B.M.; Oprea, M.; Oancea, F.; Iacomi, C. In vitro antifungal activity of some plant extracts against Fusarium oxysporum in blackcurrant (Ribes nigrum L.). Acta Sci. Pol. Hortorum Cultus. 2017, 16, 163–172. [Google Scholar] [CrossRef]
- De Rodríguez, D.J.; Trejo-González, F.A.; Rodríguez-García, R.; Díaz-Jimenez, M.L.V.; Sáenz-Galindo, A.; Hernández-Castillo, F.D.; Peña-Ramos, F.M. Antifungal activity in vitro of Rhus muelleri against Fusarium oxysporum f. sp. lycopersici. Ind. Crops Prod. 2015, 75, 150–158. [Google Scholar] [CrossRef]
- Villaseñor, J.L. Checklist of the native vascular plants of México. Rev. Mex. Biodivers. 2016, 87, 559–902. [Google Scholar] [CrossRef]
- Ramírez-Morillo, I.M. La flora de la península de Yucatán: ¿Diversa? ¿Bien conocida? ¿Protegida? No, no y ¿No? Desde Herb. CICY 2019, 11, 130–137. [Google Scholar]
- Vargas-Díaz, A.A.; Gamboa Angulo, M.; Medina Baizabal, I.L.; Pérez Brito, D.; Cristóbal Alejo, J.; Ruiz Sánchez, E. Evaluation of native Yucatecan plant extracts against Alternaria chrysanthemi and antifungal spectrum of Acalypha gaumeri. Rev. Mex. Fitopatol. 2014, 32, 01–11. [Google Scholar]
- Gamboa-Angulo, M.M.; Cristóbal-Alejo, J.; Medina-Baizabal, I.L.; Chí-Romero, F.; Méndez-González, R.; Simá-Polanco, P.; May-Pat, F. Antifungal properties of selected plants from the Yucatan peninsula, Mexico. World J. Microbiol. Biotechnol. 2008, 24, 1955–1959. [Google Scholar] [CrossRef]
- Peraza-Sánchez, S.R.; Chan-Che, E.O.; Ruiz-Sánchez, E. Screening of Yucatecan plant extracts to control Colletotrichum gloeosporioides and isolation of a new pimarene from Acacia pennatula. J. Agric. Food Chem. 2005, 53, 2429–2432. [Google Scholar] [CrossRef] [PubMed]
- Digital Flora: Península de Yucatán, Herbario CICY, Unidad de Recursos Naturales. Available online: http://www.cicy.mx/sitios/flora%20digital/ficha_virtual.php?especie=820 (accessed on 21 May 2020).
- Baños, S.B.; Necha, L.L.B.; Luna, L.B.; Torres, K.B. Antifungal activity of leaf and stem extracts from various plant species on the incidence of Colletotrichum gloeosporioides of papaya and mango fruit after storage. Rev. Mex. Fitopatol. 2002, 20, 8–12. [Google Scholar]
- Mogle, U.P. Efficacy of leaf extracts against the post-harvest fungal pathogens of cowpea. Biosci. Discov. 2013, 4, 39–42. [Google Scholar]
- Enríquez, R.G.; Chávez, M.A.; Jauregui, F. Propenylbenzenes from Guatteria gaumeri. Phytochemistry 1980, 19, 2024–2025. [Google Scholar] [CrossRef]
- Jimenez Arellanes, A.; Mata, R.; Lotina-Henssen, B.; Lang, A.L.A.; Ibarra, L.V. Phytogrowth-inhibitory compounds from Malmea depressa. J. Nat. Prod. 1996, 59, 202–204. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, R.; Thakre, B. Antifungal activity of leaf extracts of Ocimum sanctum against fungal pathogens. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1210–1214. [Google Scholar] [CrossRef]
- Gupta, M.; Sharma, S.; Bhadauria, R. In vitro efficacy of Momordica charantia extracts against phytopathogenic fungi, Fusarium oxysporum. J. Biopest. 2016, 9, 8–22. [Google Scholar]
- Mejía, R. Guatteria gaumeri, Malmea depressa o Yumel, una revisión sobre su historia, sus propiedades y su uso en la homeopatía. Homeopatia Méx. 2016, 85, 28–38. [Google Scholar]
- Fort, R.S.; Barnech, T.J.M.; Dourron, J.; Colazzo, M.; Aguirre-Crespo, F.J.; Duhagon, M.A.; Álvarez, G. Isolation and structural characterization of bioactive molecules on prostate cancer from Mayan traditional medicinal plants. Pharmaceuticals 2018, 11, 78. [Google Scholar] [CrossRef]
- Husain, A.; Indani, A.; Bhutada, P. Hypercholesterolemia effectively managed with homoeopathic medicine Gautteria gaumeri (Yumel): Results from a clinical study in academic clinical set up in north India. Int. J. Adv. Med. 2017, 4, 772. [Google Scholar] [CrossRef]
- Godoy-Rodríguez, T. Actividad Antifúngica de Plantas Nativas de la Península de Yucatán Para el Control de Fitopatógenos Poscosecha de Capsicum spp. Tesis de Maestría; Centro de Investigación Científica de Yucatán: Mérida, Yucatán, Mexico, 2019. [Google Scholar]
- Saha, A.; Rahman, M.S. Antimicrobial activity of crude extract from Calycopteris floribunsa. Bangladesh J. Microbiol. 2008, 25, 137–139. [Google Scholar] [CrossRef]
- Begum, J.; Yusuf, M.; Chowdhury, J.U.; Khan, S.; Anwar, M.N. Antifungal activity of forty higher plants against phytopathogenic fungi. Bangladesh J. Microbiol. 2007, 24, 76–78. [Google Scholar] [CrossRef]
- Rinez, A.; Daami-Remadi, M.; Ladhari, A.; Omezzine, F.; Rinez, I.; Haouala, R. Antifungal activity of Datura metel L. organic and aqueous extracts on some pathogenic and antagonistic fungi. Afr. J. Microbiol. Res. 2013, 7, 1605–1612. [Google Scholar] [CrossRef]
- Matos, O.C.; Baeta, J.; Silva, M.J.; Ricardo, C.P. Sensitivity of Fusarium strains to Chelidonium majus L. extracts. J. Ethnopharmacol. 1999, 66, 151–158. [Google Scholar] [CrossRef]
- Tian, J.; Zeng, X.; Zhang, S.; Wang, Y.; Zhang, P.; Lü, A.; Peng, X. Regional variation in components and antioxidant and antifungal activities of Perilla frutescens essential oils in China. Ind. Crops Prod. 2014, 59, 69–79. [Google Scholar] [CrossRef]
- De-la-Cruz-Chacón, I.; Riley-Saldaña, C.A.; Arrollo-Gómez, S.; Sancristóbal-Domínguez, T.J.; Castro-Moreno, M.; González-Esquinca, A.R. Spatio-temporal variation of alkaloids in Annona purpurea and the associated influence on their antifungal activity. Chem. Biodiv. 2019, 16, 1–14. [Google Scholar] [CrossRef]
- Lee, H.S. Fungicidal property of active component derived from Acorus gramineus rhizome against phytopathogenic fungi. Bioresour. Technol. 2007, 98, 1324–1328. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Li, S.X.; Zhao, J.; Bernier, U.R.; Becnel, J.J.; Agramonte, N.M.; Duke, S.O.; Cantrell, C.L.; Wedge, D.E. Identification and characterization of biopesticides from Acorus tatarinowii and A. calamus. In Medicinal and Aromatic Crops: Production, Phytochemistry and Utilization; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2016; pp. 121–143. [Google Scholar] [CrossRef]
- Momin, R.A.; Nair, M.G. Pest-managing efficacy of trans-asarone isolated from Daucus carota L. seeds. J. Agric. Food Chem. 2002, 50, 4475–4478. [Google Scholar] [CrossRef]
- Perrett, S.; Whitfield, P.J. Anthelmintic and pesticidal activity of Acorus gramineus (Araceae) is associated with penylpropanoid asarones. Phytother. Res. 1995, 9, 405–409. [Google Scholar] [CrossRef]
- Phongpaichit, S.; Pujenjob, N.; Rukachaisirikul, V.; Ongsakul, M. Antimicrobial activities of the crude methanol extract of Acorus calamus Linn. Songklanakarin J. Sci. Technol. 2005, 27, 517–523. [Google Scholar]
- Dissanayake, M.L.M.C.; Ito, S.I.; Akakabe, Y. TLC bioautography guided detection and biological activity of antifungal compounds from medicinal plant Acorus calamus Linn. Asian J. Plant. Pathol. 2015, 9, 16–26. [Google Scholar] [CrossRef][Green Version]
- Venkatesan, R.; Karuppiah, P.S.; Arumugam, G.; Balamuthu, K. β-Asarone exhibits antifungal activity by inhibiting ergosterol biosynthesis in Aspergillus niger ATCC 16888. Proc. Natl. Acad. Sci. India 2019, 89, 173–184. [Google Scholar] [CrossRef]
- Rajput, S.B.; Karuppayil, S.M. β-Asarone, an active principle of Acorus calamus rhizome, inhibits morphogenesis, biofilm formation and ergosterol biosynthesis in Candida albicans. Phytomedicine 2013, 20, 139–142. [Google Scholar] [CrossRef]
- Cruz, S.; Cáceres, A.; Álvarez, L.; Apel, M.; Henríquez, A. Chemical diversity of essential oils from 15 Piper species from Guatemala. Acta Hortic. 2012, 964, 39–46. [Google Scholar] [CrossRef]
- Giovannini, P.; Howes, M.J.R. Medicinal plants used to treat snakebite in Central America: Review and assessment of scientific evidence. J. Ethnopharmacol. 2017, 199, 240–256. [Google Scholar] [CrossRef]
- Cáceres, A.; Cruz, S.M.; Gaitán, I.; Guerrero, K.; Álvarez, L.E.; Marroquín, M.N. Antioxidant activity and quantitative composition of extracts of Piper species from Guatemala with potential use in natural product industry. Acta Hortic. 2012, 964, 77–84. [Google Scholar] [CrossRef]
- Almeda, F.; Astorga, L.; Orellana, A.; Sampuel, L.; Sierra, P.; Gaitán, I.; Cáceres, A. Piper genus: Source of natural products with anti-tyrosinase activity favored in phytocosmetics. Int. J. Phytocos. Nat. Ingred. 2015, 2, 1–5. [Google Scholar] [CrossRef]
- Cáceres, A.; Almeda, F.; Astorga, L.M.; Orellana, A.C.; Sampuel, L.I.; Sierra, P.; Zelada, V.F. Anti-urease activity of native species of genus Piper from Guatemala with potential application in infection control. Int. J. Phytocos. Nat. Ingred. 2018, 5. [Google Scholar] [CrossRef]
- Calderón, Á.I.; Romero, L.I.; Ortega-Barría, E.; Solís, P.N.; Zacchino, S.; Jiménez, A.; Espinosa, A. Screening of Latin American plants for antiparasitic activities against malaria, Chagas disease, and leishmaniasis. Pharm. Biol. 2010, 48, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Ober, A.G.; Fischer, N.H.; Parodi, F. Jamaicolides AD, four sesquiterpene lactones from Calea jamaicensis. Phytochemistry 1986, 25, 877–881. [Google Scholar] [CrossRef]
- Lima, T.C.; de Jesus Souza, R.; da Silva, F.A.; Biavatti, M.W. The genus Calea L.: A review on traditional uses, phytochemistry, and biological activities. Phytother. Res. 2018, 32, 769–795. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Estrada, A.; Medina-Baizabal, I.L.; Ruiz-Sánchez, E.; Gamboa-Angulo, M. Effect of Eugenia winzerlingii extracts on Bemuse tabaci and evaluation of its nursery propagation. Phyton. Int. J. Exp. Bot. 2019, 88, 161–170. [Google Scholar] [CrossRef]
- Abou-Jawdah, Y.; Sobh, H.; Salameh, A. Antimycotic activities of selected plant flora, growing wild in Lebanon, against phytopathogenic fungi. J. Agric. Food Chem. 2002, 50, 3208–3213. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard M38-A2; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2002. [Google Scholar]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Irkin, R.; Korukluoglu, M. Control of Aspergillus niger with garlic, onion and leek extracts. Afr. J. Biotechnol. 2007, 6, 384–387. [Google Scholar]
- Martín, J.; Crespo, G.; González-Menéndez, V.; Pérez-Moreno, G.; Sánchez-Carrasco, P.; Pérez-Victoria, I.; Bills, G.F. MDN-0104, an antiplasmodial betaine lipid from Heterospora chenopodii. J. Nat. Prod. 2014, 77, 2118–2123. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).