First Insight into the Modulation of Noncanonical NF-κB Signaling Components by Poxviruses in Established Immune-Derived Cell Lines: An In Vitro Model of Ectromelia Virus Infection
Abstract
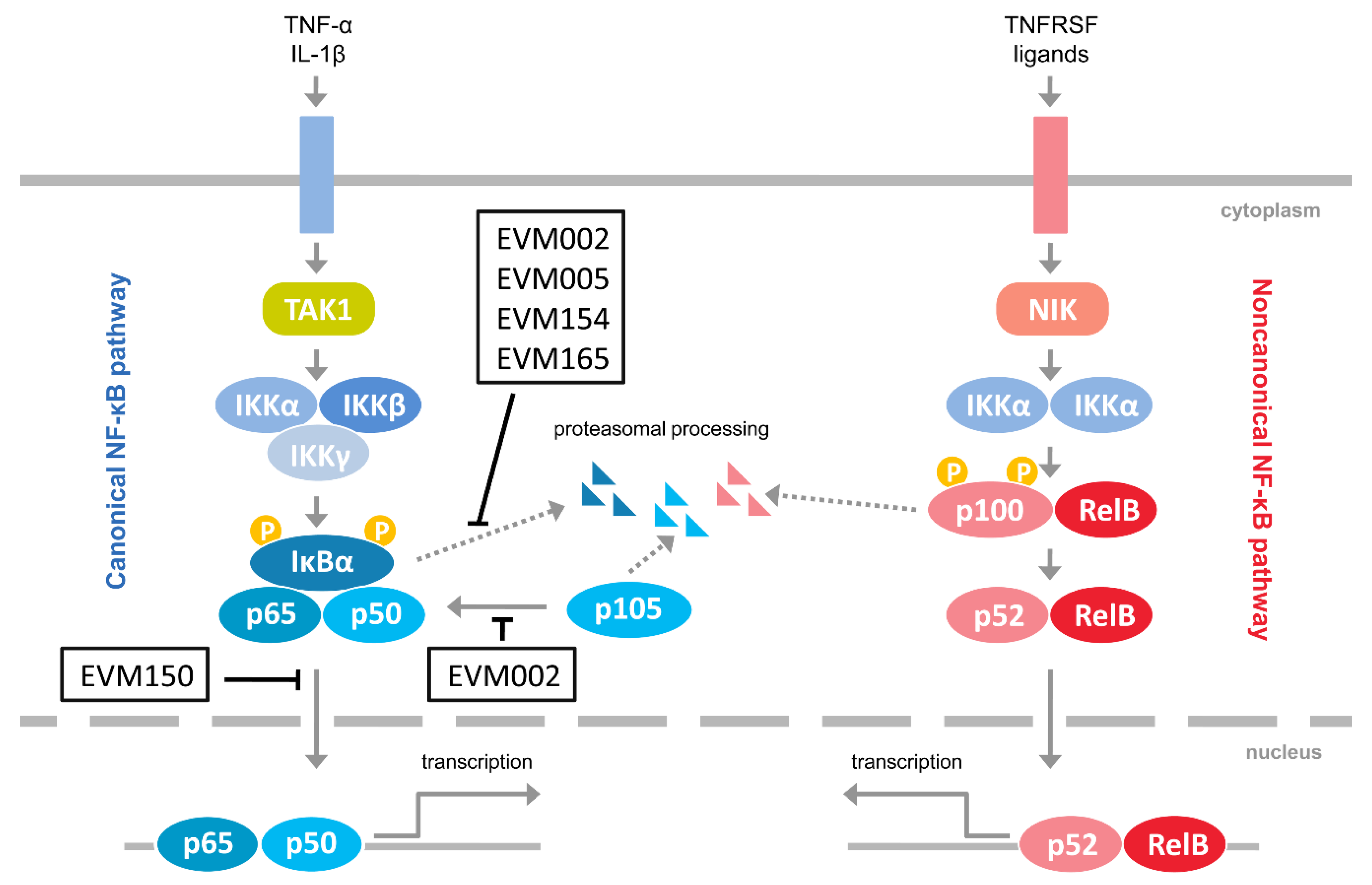
1. Introduction
2. Materials and Methods
2.1. Virus
2.2. Cell Culture, Infection, and Treatment
2.3. Immunofluorescence Staining Technique and Microscopic Analysis
2.4. Western Blot Analysis
2.5. Nuclear and Cytoplasmic Cell Fractionation and Detection of Cytoplasmic and Nuclear Proteins
2.6. DNA-Binding ELISA
2.7. RNA Isolation
2.8. Reverse Transcription and Quantitative PCR (RT-qPCR)
2.9. Statistical Analysis
3. Results
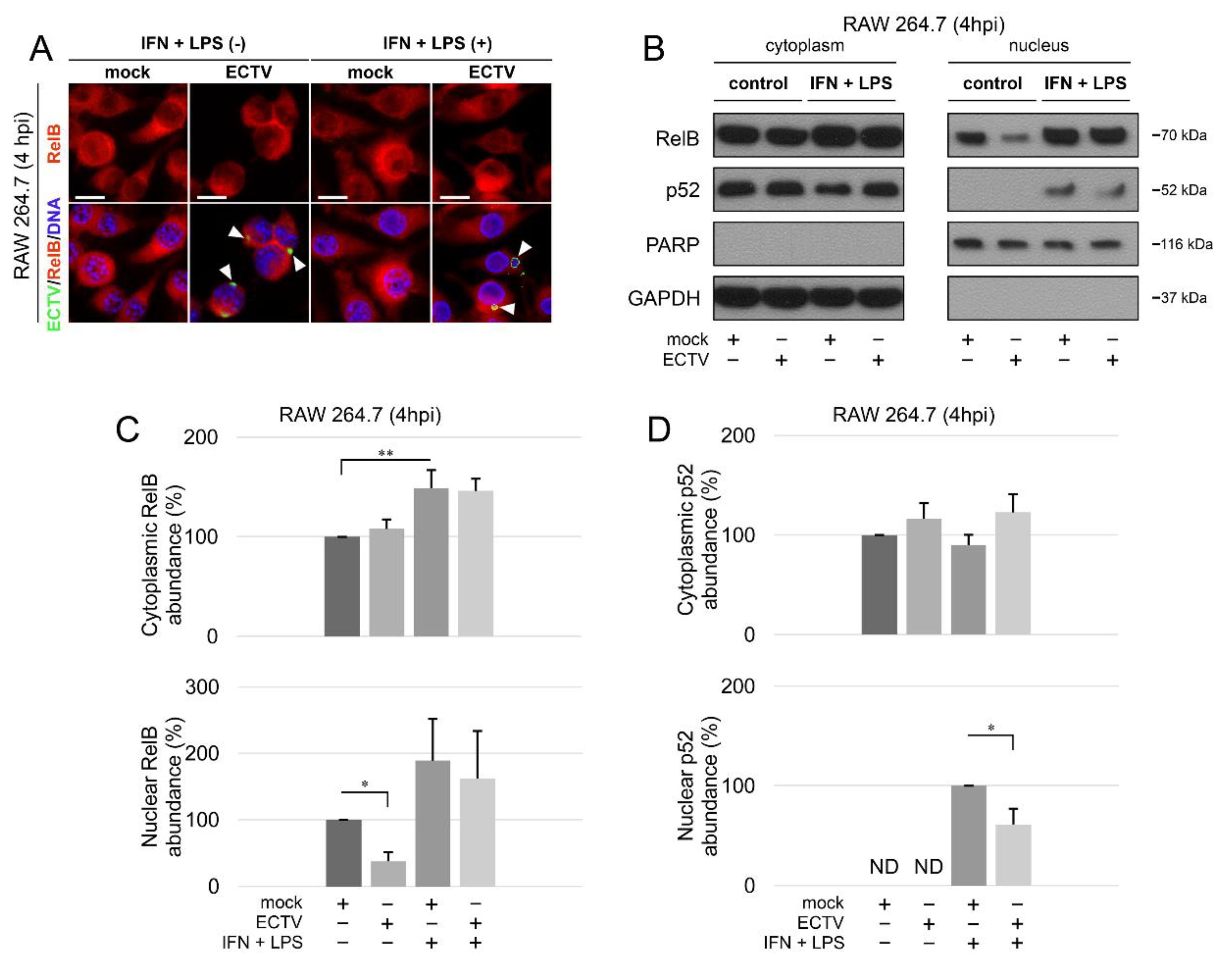
3.1. ECTV Affects RelB and p52 Nuclear Translocation in RAW 264.7 Macrophages at an Early Stage of Viral Replication
3.2. ECTV Counteracts PMA/Io-, LPS-, and IFN-Γ + LPS-Induced RelB Nuclear Translocation in JAWS II and RAW 264.7 Cells at 12-24 hpi
3.3. ECTV Inhibits Phosphorylation of RelB and p100 and Affects p100/p52 Content in JAWS II and RAW 264.7 Cells
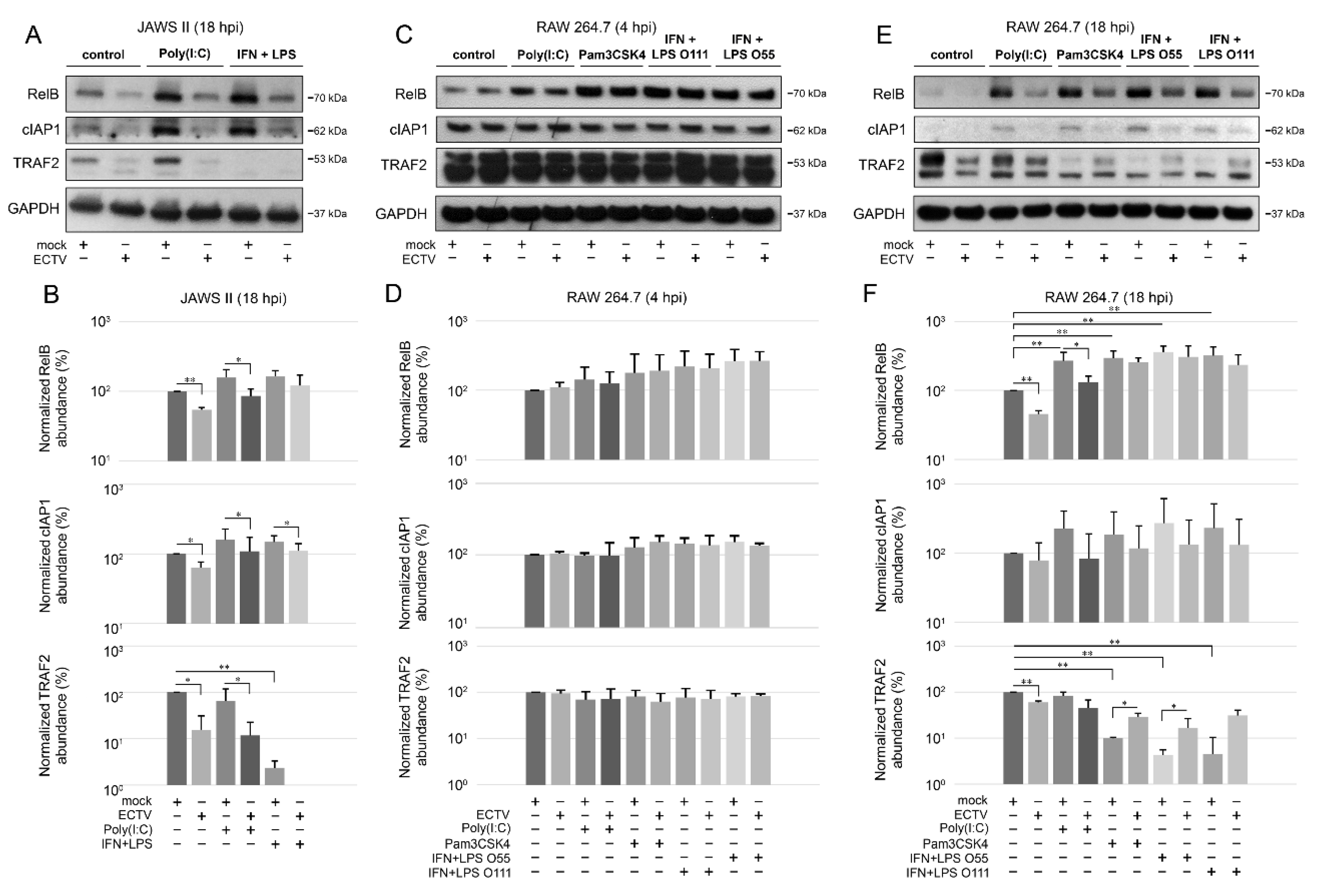
3.4. ECTV Influences the Cellular Level of Components of the Noncanonical NF-κB Signaling Pathway at Late Stages of Viral Lifecycle
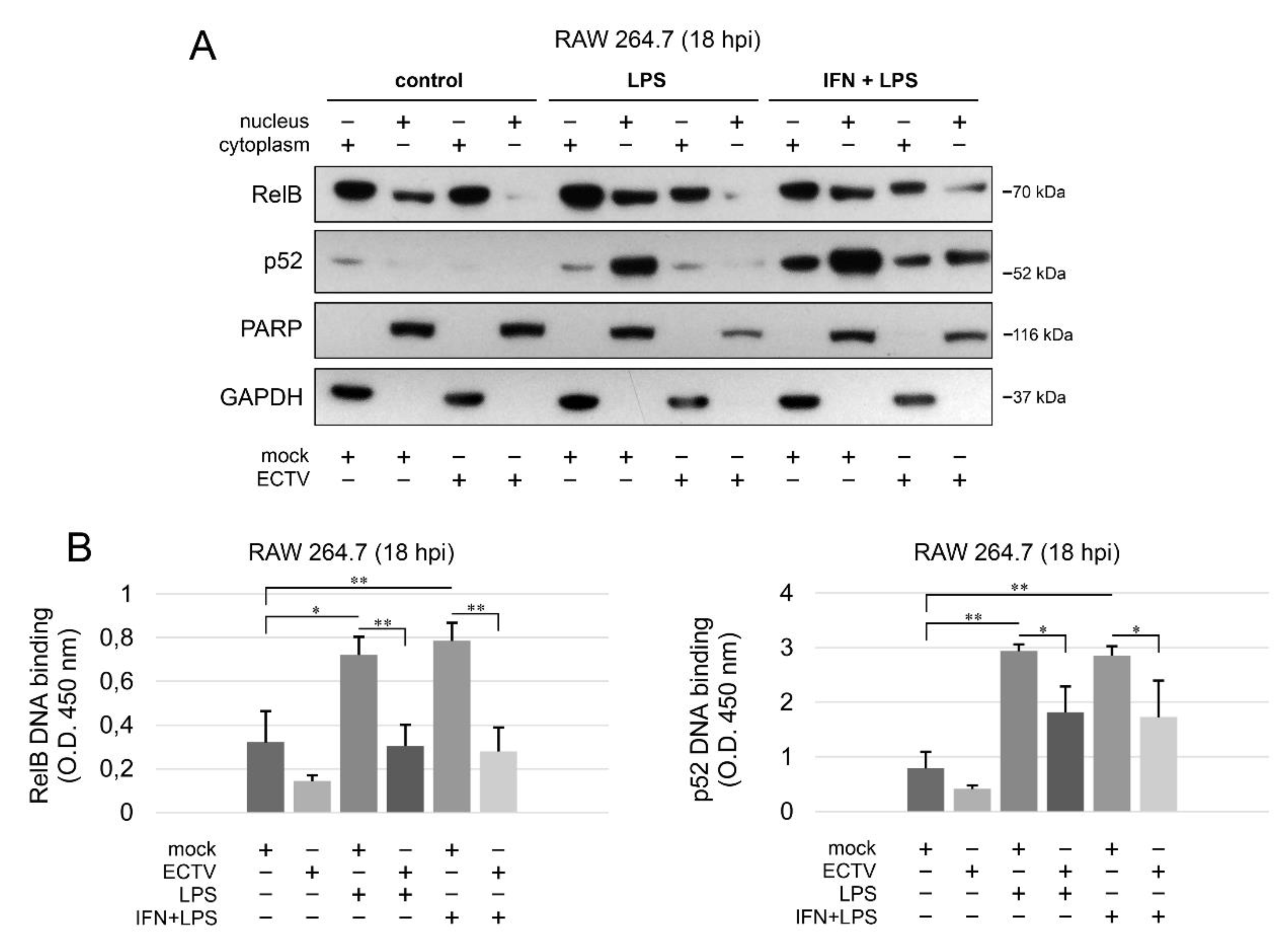
3.5. ECTV Inhibits RelB and p52 DNA-Binding Activity
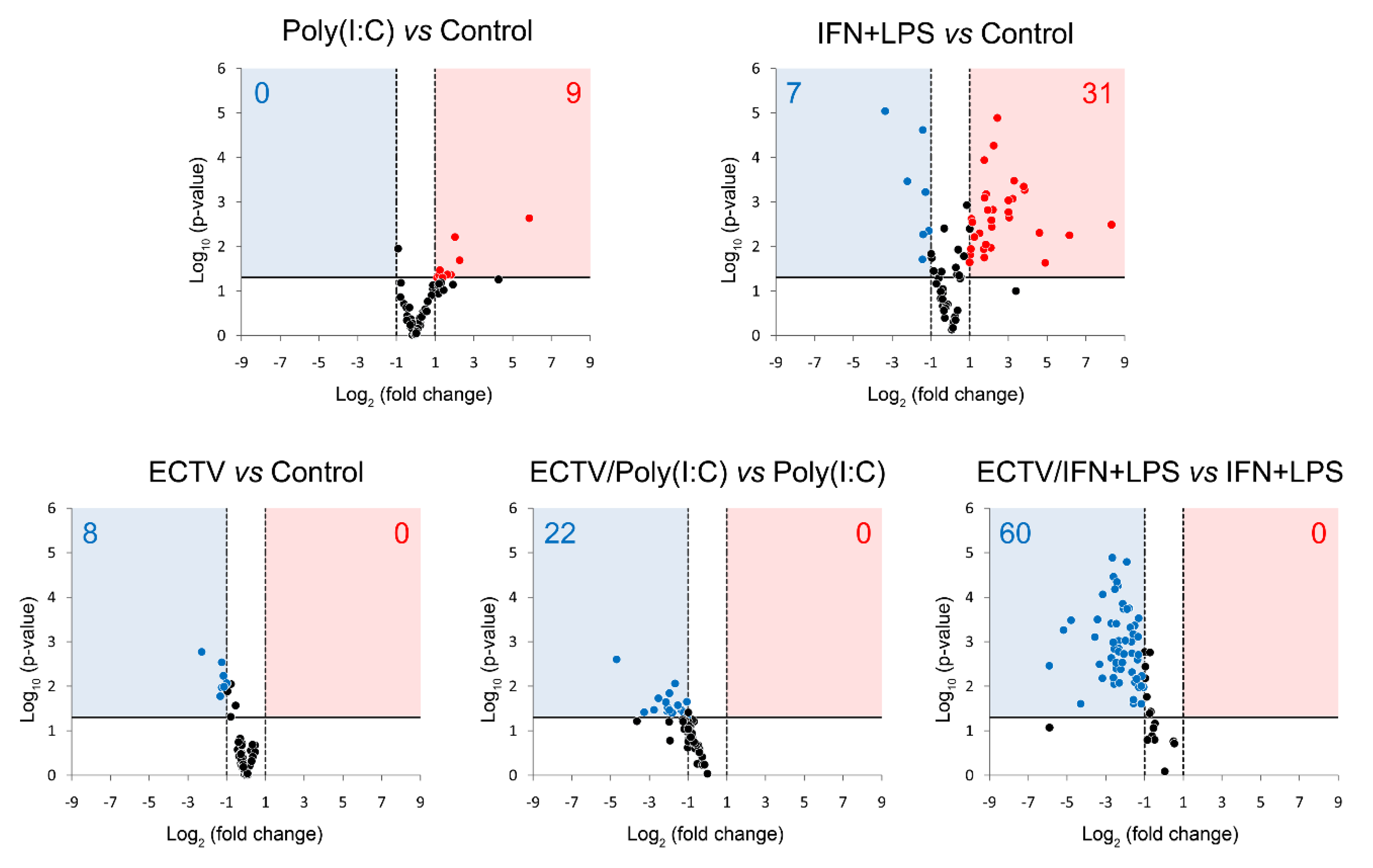
3.6. ECTV Inhibits the Expression of Genes Related to Noncanonical NF-κB Signaling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, J.; He, S.; Minassian, A.; Li, J.; Feng, P. Recent advances on viral manipulation of NF-κB signaling pathway. Curr. Opin. Virol. 2015, 15, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of double-stranded RNA and activation of NF-κΒ by Toll-like receptor 3. Nature 2001, 413, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal. Transduct. Target. Ther. 2017, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cheshire, J.L.; Baldwin, A.S., Jr. Synergistic activation of NF-κB by tumor necrosis factor α and γ interferon via enhanced IκBα degradation and de novo IκBβ degradation. Mol. Cell. Biol. 1997, 17, 6746–6754. [Google Scholar] [CrossRef]
- Sun, S.C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Kim, J.Y.; Morgan, M.; Kim, D.G.; Lee, J.Y.; Bai, L.; Lin, Y.; Liu, Z.G.; Kim, Y.S. TNFα-induced noncanonical NF-κB activation is attenuated by RIP1 through stabilization of TRAF2. J. Cell Sci. 2011, 124, 647–656. [Google Scholar] [CrossRef]
- Shih, V.F.; Davis-Turak, J.; Macal, M.; Huang, J.Q.; Ponomarenko, J.; Kearns, J.D.; Yu, T.; Fagerlund, R.; Asagiri, M.; Zuniga, E.I.; et al. Control of RelB during dendritic cell activation integrates canonical and noncanonical NF-κB pathways. Nat. Immunol. 2012, 13, 1162–1170. [Google Scholar] [CrossRef]
- Cildir, G.; Low, K.C.; Tergaonkar, V. Noncanonical NF-κB signaling in health and disease. Trends Mol. Med. 2016, 22, 414–429. [Google Scholar] [CrossRef]
- Sun, S.C. Non-canonical NF-κB signaling pathway. Cell Res. 2011, 21, 71–85. [Google Scholar] [CrossRef]
- Sun, S.C. The noncanonical NF-κB pathway. Immunol. Rev. 2012, 246, 125–140. [Google Scholar] [CrossRef]
- Jin, J.; Hu, H.; Li, H.S.; Yu, J.; Xiao, Y.; Brittain, G.C.; Zou, Q.; Cheng, X.; Mallette, F.A.; Watowich, S.S.; et al. Noncanonical NF-κB pathway controls the production of type I interferons in antiviral innate immunity. Immunity 2014, 40, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M.; Hemmi, H. Dendritic cells: Translating innate to adaptive immunity. Curr. Top. Microbiol. Immunol. 2006, 311, 17–58. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Yamamoto, M.; Taguchi, Y.; Miyauchi, M.; Akiyama, N.; Yamaguchi, N.; Gohda, J.; Akiyama, T.; Inoue, J.-T. Visualization of RelB expression and activation at the single-cell level during dendritic cell maturation in Relb-Venus knock-in mice. J. Biochem. 2015, 158, 485–495. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Millet, P.; McCall, C.; Yoza, B. RelB: An outlier in leukocyte biology. J. Leukoc. Biol. 2013, 94, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, C.; Foxwell, B.M.; Feldmann, M. RelB/p50 regulates TNF production in LPS-stimulated dendritic cells and macrophages. Cytokine 2013, 61, 736–740. [Google Scholar] [CrossRef]
- Mancino, A.; Habbeddine, M.; Johnson, E.; Luron, L.; Bebien, M.; Memet, S.; Fong, C.; Bajenoff, M.; Wu, X.; Karin, M.; et al. IκB kinase α (IKKα) activity is required for functional maturation of dendritic cells and acquired immunity to infection. EMBO J. 2013, 32, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Lind, E.F.; Ahonen, C.L.; Wasiuk, A.; Kosaka, Y.; Becher, B.; Bennett, K.A.; Noelle, R.J. Dendritic cells require the NF-κB2 pathway for cross-presentation of soluble antigens. J. Immunol. 2008, 181, 354–363. [Google Scholar] [CrossRef]
- Belladonna, M.L.; Volpi, C.; Bianchi, R.; Vacca, C.; Orabona, C.; Pallotta, M.T.; Boon, L.; Gizzi, S.; Fioretti, M.C.; Grohmann, U.; et al. Cutting edge: Autocrine TGF-β sustains default tolerogenesis by IDO-competent dendritic cells. J. Immunol. 2008, 181, 5194–5198. [Google Scholar] [CrossRef]
- Nikitina, E.; Larionova, I.; Choinzonov, E.; Kzhyshkowska, J. Monocytes and macrophages as viral targets and reservoirs. Int. J. Mol. Sci. 2018, 19, 2821. [Google Scholar] [CrossRef]
- Pollara, G.; Kwan, A.; Newton, P.J.; Handley, M.E.; Chain, B.M.; Katz, D.R. Dendritic cells in viral pathogenesis: Protective or defective? Int. J. Exp. Pathol. 2005, 86, 187–204. [Google Scholar] [CrossRef]
- Struzik, J.; Szulc-Dąbrowska, L. Manipulation of non-canonical NF-κB signaling by non-oncogenic viruses. Arch. Immunol. Ther. Exp. (Warsz.) 2019, 67, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Brady, G.; Bowie, A.G. Innate immune activation of NFκB and its antagonism by poxviruses. Cytokine Growth Factor Rev. 2014, 25, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.R.; Rahman, M.M.; Lanchbury, J.S.; Shattuck, D.; Neff, C.; Dufford, M.; van Buuren, N.; Fagan, K.; Barry, M.; Smith, S.; et al. Proteomic screening of variola virus reveals a unique NF-κB inhibitor that is highly conserved among pathogenic orthopoxviruses. Proc. Natl. Acad. Sci. USA 2009, 106, 9045–9050. [Google Scholar] [CrossRef] [PubMed]
- Burles, K.; van Buuren, N.; Barry, M. Ectromelia virus encodes a family of Ankyrin/F-box proteins that regulate NFκB. Virology 2014, 468–470, 351–362. [Google Scholar] [CrossRef]
- van Buuren, N.; Burles, K.; Schriewer, J.; Mehta, N.; Parker, S.; Buller, R.M.; Barry, M. EVM005: An ectromelia-encoded protein with dual roles in NF-κB inhibition and virulence. PLoS Pathog. 2014, 10, e1004326. [Google Scholar] [CrossRef]
- Wang, Q.; Burles, K.; Couturier, B.; Randall, C.M.; Shisler, J.; Barry, M. Ectromelia virus encodes a BTB/kelch protein, EVM150, that inhibits NF-κB signaling. J. Virol. 2014, 88, 4853–4865. [Google Scholar] [CrossRef]
- Garver, J.; Weber, L.; Vela, E.M.; Anderson, M.; Warren, R.; Merchlinsky, M.; Houchens, C.; Rogers, J.V. Ectromelia virus disease characterization in the BALB/c mouse: A surrogate model for assessment of smallpox medical countermeasures. Viruses 2016, 8, 203. [Google Scholar] [CrossRef]
- Szulc-Dąbrowska, L.; Struzik, J.; Ostrowska, A.; Guzera, M.; Toka, F.N.; Bossowska-Nowicka, M.; Gieryńska, M.M.; Winnicka, A.; Nowak, Z.; Niemiałtowski, M.G. Functional paralysis of GM-CSF-derived bone marrow cells productively infected with ectromelia virus. PLoS ONE 2017, 12, e0179166. [Google Scholar] [CrossRef]
- Struzik, J.; Szulc-Dąbrowska, L.; Papiernik, D.; Winnicka, A.; Niemiałtowski, M. Modulation of proinflammatory NF-κB signaling by ectromelia virus in RAW 264.7 murine macrophages. Arch. Virol. 2015, 160, 2301–2314. [Google Scholar] [CrossRef]
- Xu, R.H.; Wong, E.B.; Rubio, D.; Roscoe, F.; Ma, X.; Nair, S.; Remakus, S.; Schwendener, R.; John, S.; Shlomchik, M.; et al. Sequential activation of two pathogen-sensing pathways required for type I interferon expression and resistance to an acute DNA virus infection. Immunity 2015, 43, 1148–1159. [Google Scholar] [CrossRef]
- Bossowska-Nowicka, M.; Mielcarska, M.B.; Romaniewicz, M.; Kaczmarek, M.M.; Gregorczyk-Zboroch, K.P.; Struzik, J.; Grodzik, M.; Gieryńska, M.M.; Toka, F.N.; Szulc-Dąbrowska, L. Ectromelia virus suppresses expression of cathepsins and cystatins in conventional dendritic cells to efficiently execute the replication process. BMC Microbiol. 2019, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Berghaus, L.J.; Moore, J.N.; Hurley, D.J.; Vandenplas, M.L.; Fortes, B.P.; Wolfert, M.A.; Boons, G.J. Innate immune responses of primary murine macrophage-lineage cells and RAW 264.7 cells to ligands of Toll-like receptors 2, 3, and 4. Comp. Immunol. Microbiol. Infect. Dis. 2010, 33, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Hostager, B.S.; Bishop, G.A. CD40-mediated activation of the NF-κB2 pathway. Front. Immunol. 2013, 4, 376. [Google Scholar] [CrossRef]
- Deng, W.G.; Tang, S.T.; Tseng, H.P.; Wu, K.K. Melatonin suppresses macrophage cyclooxygenase-2 and inducible nitric oxide synthase expression by inhibiting p52 acetylation and binding. Blood 2006, 108, 518–524. [Google Scholar] [CrossRef]
- Bren, G.D.; Solan, N.J.; Miyoshi, H.; Pennington, K.N.; Pobst, L.J.; Paya, C.V. Transcription of the RelB gene is regulated by NF-κB. Oncogene 2001, 20, 7722–7733. [Google Scholar] [CrossRef]
- Mordmüller, B.; Krappmann, D.; Esen, M.; Wegener, E.; Scheidereit, C. Lymphotoxin and lipopolysaccharide induce NF-κB-p52 generation by a co-translational mechanism. EMBO Rep. 2003, 4, 82–87. [Google Scholar] [CrossRef]
- Roy, P.; Mukherjee, T.; Chatterjee, B.; Vijayaragavan, B.; Banoth, B.; Basak, S. Non-canonical NFκB mutations reinforce pro-survival TNF response in multiple myeloma through an autoregulatory RelB:p50 NFκB pathway. Oncogene 2017, 36, 1417–1429. [Google Scholar] [CrossRef]
- Jiang, X.; Shen, C.; Rey-Ladino, J.; Yu, H.; Brunham, R.C. Characterization of murine dendritic cell line JAWS II and primary bone marrow-derived dendritic cells in Chlamydia muridarum antigen presentation and induction of protective immunity. Infect. Immun. 2008, 76, 2392–2401. [Google Scholar] [CrossRef]
- Marienfeld, R.; Berberich-Siebelt, F.; Berberich, I.; Denk, A.; Serfling, E.; Neumann, M. Signal-specific and phosphorylation-dependent RelB degradation: A potential mechanism of NF-κB control. Oncogene 2001, 20, 8142–8147. [Google Scholar] [CrossRef]
- Xiao, G.; Fong, A.; Sun, S.C. Induction of p100 processing by NF-κB-inducing kinase involves docking IκB kinase α (IKKα) to p100 and IKKα-mediated phosphorylation. J. Biol. Chem. 2004, 279, 30099–30105. [Google Scholar] [CrossRef] [PubMed]
- Gaekwad, J.; Zhang, Y.; Zhang, W.; Reeves, J.; Wolfert, M.A.; Boons, G.J. Differential induction of innate immune responses by synthetic lipid A derivatives. J. Biol. Chem. 2010, 285, 29375–29386. [Google Scholar] [CrossRef]
- Doyle, S.L.; Shirey, K.A.; McGettrick, A.F.; Kenny, E.F.; Carpenter, S.; Caffrey, B.E.; Gargan, S.; Quinn, S.R.; Caamaño, J.H.; Moynagh, P.; et al. Nuclear factor κB2 p52 protein has a role in antiviral immunity through IκB kinase ε-dependent induction of Sp1 protein and interleukin 15. J. Biol. Chem. 2013, 288, 25066–25075. [Google Scholar] [CrossRef] [PubMed]
- Gregorczyk, K.P.; Wyżewski, Z.; Szczepanowska, J.; Toka, F.N.; Mielcarska, M.B.; Bossowska-Nowicka, M.; Gieryńska, M.; Boratyńska-Jasińska, A.; Struzik, J.; Niemiałtowski, M.G.; et al. Ectromelia virus affects mitochondrial network morphology, distribution, and physiology in murine fibroblasts and macrophage cell line. Viruses 2018, 1, 266. [Google Scholar] [CrossRef]
- Matys, V.; Fricke, E.; Geffers, R.; Gössling, E.; Haubrock, M.; Hehl, R.; Hornischer, K.; Karas, D.; Kel, A.E.; Kel-Margoulis, O.V.; et al. TRANSFAC: Transcriptional regulation, from patterns to profiles. Nucleic Acids Res. 2003, 31, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Lachmann, A.; Xu, H.; Krishnan, J.; Berger, S.I.; Mazloom, A.R.; Ma’ayan, A. ChEA: Transcription factor regulation inferred from integrating genome-wide ChIP-X experiments. Bioinformatics 2010, 26, 2438–2444. [Google Scholar] [CrossRef] [PubMed]
- Martyniszyn, L.; Szulc-Dąbrowska, L.; Boratyńska-Jasińska, A.; Struzik, J.; Winnicka, A.; Niemiałtowski, M. Crosstalk between autophagy and apoptosis in RAW 264.7 macrophages infected with ectromelia orthopoxvirus. Viral Immunol. 2013, 26, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, T.N.; Haase, C.; Michelsen, B.K. Treatment of an immortalized APC cell line with both cytokines and LPS ensures effective T-cell activation in vitro. Scand. J. Immunol. 2002, 56, 492–503. [Google Scholar] [CrossRef]
- Ak, P.; Levine, A.J. p53 and NF-κB: Different strategies for responding to stress lead to a functional antagonism. FASEB J. 2010, 24, 3643–3652. [Google Scholar] [CrossRef]
- Schneider, G.; Krämer, O.H. NFκB/p53 crosstalk-a promising new therapeutic target. Biochim. Biophys. Acta 2011, 1815, 90–103. [Google Scholar] [CrossRef]
- Mohamed, M.R.; McFadden, G. NFκB inhibitors: Strategies from poxviruses. Cell Cycle 2009, 8, 3125–3132. [Google Scholar] [CrossRef] [PubMed]
- Szulc-Dąbrowska, L.; Struzik, J.; Cymerys, J.; Winnicka, A.; Nowak, Z.; Toka, F.N.; Gieryńska, M. The in vitro inhibitory effect of ectromelia virus infection on innate and adaptive immune properties of GM-CSF-derived bone marrow cells is mouse strain-independent. Front. Microbiol. 2017, 8, 2539. [Google Scholar] [CrossRef] [PubMed]
- Khatiwada, S.; Delhon, G.; Nagendraprabhu, P.; Chaulagain, S.; Luo, S.; Diel, D.G.; Flores, E.F.; Rock, D.L. A parapoxviral virion protein inhibits NF-κB signaling early in infection. PLoS Pathog. 2017, 13, e1006561. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Murti, A.; Pfeffer, L.M. Interferon induces NF-κB-inducing kinase/tumor necrosis factor receptor-associated factor-dependent NF-κB activation to promote cell survival. J. Biol. Chem. 2005, 280, 31530–31536. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Murti, A.; Pfeffer, S.R.; Fan, M.; Du, Z.; Pfeffer, L.M. The role of TRAF2 binding to the type I interferon receptor in alternative NF-κB activation and antiviral response. J. Biol. Chem. 2008, 283, 14309–14316, Erratum in: J. Biol. Chem. 2008, 283, 36752. [Google Scholar] [CrossRef] [PubMed]
- Haga, I.R.; Pechenick Jowers, T.; Griffiths, S.J.; Haas, J.; Beard, P.M. TRAF2 facilitates vaccinia virus replication by promoting rapid virus entry. J. Virol. 2014, 88, 3664–3677. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, K.E.; Feltham, R.; Yabal, M.; Conos, S.A.; Chen, K.W.; Ziehe, S.; Graß, C.; Zhan, Y.; Nguyen, T.A.; Hall, C.; et al. XIAP loss triggers RIPK3- and caspase-8-driven IL-1β activation and cell death as a consequence of TLR-MyD88-induced cIAP1-TRAF2 degradation. Cell Rep. 2017, 20, 668–682. [Google Scholar] [CrossRef]
- Manderscheid, M.; Messmer, U.K.; Franzen, R.; Pfeilschifter, J. Regulation of inhibitor of apoptosis expression by nitric oxide and cytokines: Relation to apoptosis induction in rat mesangial cells and RAW 264.7 macrophages. J. Am. Soc. Nephrol. 2001, 12, 1151–1163. [Google Scholar]
- Dupoux, A.; Cartier, J.; Cathelin, S.; Filomenko, R.; Solary, E.; Dubrez-Daloz, L. cIAP1-dependent TRAF2 degradation regulates the differentiation of monocytes into macrophages and their response to CD40 ligand. Blood 2009, 113, 175–185. [Google Scholar] [CrossRef]
- Jin, J.; Xiao, Y.; Hu, H.; Zou, Q.; Li, Y.; Gao, Y.; Ge, W.; Cheng, X.; Sun, S.C. Proinflammatory TLR signalling is regulated by a TRAF2-dependent proteolysis mechanism in macrophages. Nat. Commun. 2015, 6, 5930. [Google Scholar] [CrossRef]
- Held, T.K.; Weihua, X.; Yuan, L.; Kalvakolanu, D.V.; Cross, A.S. Gamma interferon augments macrophage activation by lipopolysaccharide by two distinct mechanisms, at the signal transduction level and via an autocrine mechanism involving tumor necrosis factor α and interleukin-1. Infect. Immun. 1999, 67, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Dai, A.; Cao, S.; Dhungel, P.; Luan, Y.; Liu, Y.; Xie, Z.; Yang, Z. Ribosome profiling reveals translational upregulation of cellular oxidative phosphorylation mRNAs during vaccinia virus-induced host shutoff. J. Virol. 2017, 91, e01858-16. [Google Scholar] [CrossRef] [PubMed]
- Hailfinger, S.; Nogai, H.; Pelzer, C.; Jaworski, M.; Cabalzar, K.; Charton, J.E.; Guzzardi, M.; Décaillet, C.; Grau, M.; Dörken, B.; et al. Malt1-dependent RelB cleavage promotes canonical NF-κB activation in lymphocytes and lymphoma cell lines. Proc. Natl. Acad. Sci. USA 2011, 108, 14596–14601. [Google Scholar] [CrossRef]
- Neumann, M.; Klar, S.; Wilisch-Neumann, A.; Hollenbach, E.; Kavuri, S.; Leverkus, M.; Kandolf, R.; Brunner-Weinzierl, M.C.; Klingel, K. Glycogen synthase kinase-3β is a crucial mediator of signal-induced RelB degradation. Oncogene 2011, 30, 2485–2492. [Google Scholar] [CrossRef]
- Wang, V.Y.; Huang, W.; Asagiri, M.; Spann, N.; Hoffmann, A.; Glass, C.; Ghosh, G. The transcriptional specificity of NF-κB dimers is coded within the κB DNA response elements. Cell Rep. 2012, 2, 824–839. [Google Scholar] [CrossRef] [PubMed]
- Seminara, A.R.; Ruvolo, P.P.; Murad, F. LPS/IFN-γ-Induced RAW 264.7 Apoptosis is Regulated by Both Nitric Oxide–Dependent and –Independent Pathways Involving JNK and the Bcl-2 Family. Cell Cycle 2007, 6, 1772–1778. [Google Scholar] [CrossRef]
- Krausgruber, T.; Blazek, K.; Smallie, T.; Alzabin, S.; Lockstone, H.; Sahgal, N.; Hussell, T.; Feldmann, M.; Udalova, I.A. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat. Immunol. 2011, 12, 231–238. [Google Scholar] [CrossRef]
- Weiss, M.; Blazek, K.; Byrne, A.J.; Perocheau, D.P.; Udalova, I.A. IRF5 is a specific marker of inflammatory macrophages in vivo. Mediators Inflamm. 2013, 2013, 245804. [Google Scholar] [CrossRef]
- LaZear, H.M.; Lancaster, A.; Wilkins, C.; Suthar, M.S.; Huang, A.; Vick, S.C.; Clepper, L.; Thackray, L.; Brassil, M.M.; Virgin, H.W.; et al. IRF-3, IRF-5, and IRF-7 coordinately regulate the type I IFN response in myeloid dendritic cells downstream of MAVS signaling. PLoS Pathog. 2013, 9, e1003118. [Google Scholar] [CrossRef]
- Yángüez, E.; García-Culebras, A.; Frau, A.; Llompart, C.; Knobeloch, K.P.; Gutierrez-Erlandsson, S.; García-Sastre, A.; Esteban, M.; Nieto, A.; Guerra, S. ISG15 regulates peritoneal macrophages functionality against viral infection. PLoS Pathog. 2013, 9, e1003632, Erratum in: PLoS Pathog. 2016, 12, e1005969. [Google Scholar] [CrossRef]
- Royo, S.; Sainz, B., Jr.; Hernández-Jiménez, E.; Reyburn, H.; López-Collazo, E.; Guerra, S. Differential induction of apoptosis, interferon signaling, and phagocytosis in macrophages infected with a panel of attenuated and nonattenuated poxviruses. J. Virol. 2014, 88, 5511–5523, Erratum in: J. Virol. 2016, 90, 5530–5531. Erratum in: J. Virol. 2016, 90, 11281. [Google Scholar] [CrossRef] [PubMed]
- UniProtKB - Q8JLD6 (Q8JLD6_9POXV). Available online: https://www.uniprot.org/uniprot/Q8JLD6 (accessed on 8 August 2020).
- Fensterl, V.; Sen, G.C. Interferon-induced Ifit proteins: Their role in viral pathogenesis. J. Virol. 2015, 89, 2462–2468. [Google Scholar] [CrossRef] [PubMed]
- Siednienko, J.; Maratha, A.; Yang, S.; Mitkiewicz, M.; Miggin, S.M.; Moynagh, P.N. Nuclear factor κB subunits RelB and cRel negatively regulate Toll-like receptor 3-mediated β-interferon production via induction of transcriptional repressor protein YY1. J. Biol. Chem. 2011, 286, 44750–44763. [Google Scholar] [CrossRef] [PubMed]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Struzik, J.; Szulc-Dąbrowska, L.; Mielcarska, M.B.; Bossowska-Nowicka, M.; Koper, M.; Gieryńska, M. First Insight into the Modulation of Noncanonical NF-κB Signaling Components by Poxviruses in Established Immune-Derived Cell Lines: An In Vitro Model of Ectromelia Virus Infection. Pathogens 2020, 9, 814. https://doi.org/10.3390/pathogens9100814
Struzik J, Szulc-Dąbrowska L, Mielcarska MB, Bossowska-Nowicka M, Koper M, Gieryńska M. First Insight into the Modulation of Noncanonical NF-κB Signaling Components by Poxviruses in Established Immune-Derived Cell Lines: An In Vitro Model of Ectromelia Virus Infection. Pathogens. 2020; 9(10):814. https://doi.org/10.3390/pathogens9100814
Chicago/Turabian StyleStruzik, Justyna, Lidia Szulc-Dąbrowska, Matylda B. Mielcarska, Magdalena Bossowska-Nowicka, Michał Koper, and Małgorzata Gieryńska. 2020. "First Insight into the Modulation of Noncanonical NF-κB Signaling Components by Poxviruses in Established Immune-Derived Cell Lines: An In Vitro Model of Ectromelia Virus Infection" Pathogens 9, no. 10: 814. https://doi.org/10.3390/pathogens9100814
APA StyleStruzik, J., Szulc-Dąbrowska, L., Mielcarska, M. B., Bossowska-Nowicka, M., Koper, M., & Gieryńska, M. (2020). First Insight into the Modulation of Noncanonical NF-κB Signaling Components by Poxviruses in Established Immune-Derived Cell Lines: An In Vitro Model of Ectromelia Virus Infection. Pathogens, 9(10), 814. https://doi.org/10.3390/pathogens9100814



