Clinical and Laboratory Features of Three Rare Chinese V210I gCJD Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Data Collection
2.3. Routine Laboratory Tests for CJD
2.4. RT-QuIC Assays
3. Results
3.1. Clinical Data
3.2. Laboratory Tests
3.3. Family History
3.4. PRNP Sequencing
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Schmitz, M.; Dittmar, K.; Llorens, F.; Gelpi, E.; Ferrer, I.; Schulz-Schaeffer, W.J.; Zerr, I. Hereditary Human Prion Diseases: An Update. Mol. Neurobiol. 2017, 54, 4138–4149. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Dong, X.P. Epidemiological characteristics of human prion diseases. Infect. Dis. Poverty 2016, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Zhou, W.; Chen, C.; Zhang, B.Y.; Xiao, K.; Zhang, X.C.; Shen, X.J.; Li, Q.; Deng, L.Q.; Dong, J.H.; et al. The Features of Genetic Prion Diseases Based on Chinese Surveillance Program. PLoS ONE 2015, 10, e0139552. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Zhou, W.; Chen, C.; Xiao, K.; Wang, Y.; Gao, C.; Dong, X.P. Rare genetic Creutzfeldt-Jakob disease with T188K mutation: Analysis of clinical, genetic and laboratory features of30 Chinese patients. J. Neurol. Neurosurg. Psychiatry 2017, 88, 889–890. [Google Scholar] [CrossRef]
- Shi, Q.; Xiao, K.; Zhou, W.; Wang, J.; Chen, C.; Gao, C.; Dong, X.P. Fatal familial insomnia: Insight of the most common genetic prion disease in China based on the analysis of 40 patients. Neuropsychiatry 2018, 8, 739–744. [Google Scholar]
- Jeong, B.H.; Kim, Y.S. Genetic studies in human prion diseases. J. Korean Med. Sci. 2014, 29, 623–632. [Google Scholar] [CrossRef]
- Goldfarb, L.G.; Korczyn, A.; Brown, P.; Chapman, J.; Gajdusek, D.C. Mutation in codon 200 of scrapie amyloid precursor gene linked to Creutzfeldt-Jakob disease in Sephardic Jews of Libyan and non-Libyan origin. Lancet 1990, 336, 637–638. [Google Scholar] [CrossRef]
- Ladogana, A.; Puopolo, M.; Poleggi, A.; Almonti, S.; Mellina, V.; Equestre, M.; Pocchiari, M. High incidence of genetic human transmissible spongiform encephalopathies in Italy. Neurology 2005, 64, 1592–1597. [Google Scholar] [CrossRef]
- Heinemann, U.; Krasnianski, A.; Meissner, B.; Varges, D.; Kallenberg, K.; Schulz-Schaeffer, W.J.; Steinhoff, B.J.; Grasbon-Frodl, E.M.; Kretzschmar, H.A.; Zerr, I. Creutzfeldt-Jakob disease in Germany: A prospective 12-year surveillance. Brain 2007, 130, 1350–1359. [Google Scholar] [CrossRef]
- Kovacs, G.G.; Puopolo, M.; Ladogana, A.; Pocchiari, M.; Budka, H.; van Duijn, C.; Collins, S.J.; Boyd, A.; Giulivi, A.; Coulthart, M.; et al. Genetic prion disease: The EUROCJD experience. Hum. Genet. 2005, 118, 166–174. [Google Scholar] [CrossRef]
- Mouillet-Richard, S.; Teil, C.; Lenne, M.; Hugon, S.; Taleb, O.; Laplanche, J.L. Mutation at codon 210 (V210I) of the prion protein gene in a North African patient with Creutzfeldt-Jakob disease. J. Neurol. Sci. 1999, 168, 141–144. [Google Scholar] [CrossRef]
- Huang, N.; Marie, S.K.; Kok, F.; Nitrini, R. Familial Creutzfeldt-Jakob disease associated with a point mutation at codon 210 of the prion protein gene. Arq. Neuropsiquiatr. 2001, 59, 932–935. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tajima, Y.; Satoh, C.; Mito, Y.; Kitamoto, T. Creutzfeldt-Jakob disease with a codon 210 mutation: First pathological observation in a Japanese patient. Intern. Med. 2014, 53, 483–487. [Google Scholar] [CrossRef]
- Nozaki, I.; Hamaguchi, T.; Sanjo, N.; Noguchi-Shinohara, M.; Sakai, K.; Nakamura, Y.; Sato, T.; Kitamoto, T.; Mizusawa, H.; Moriwaka, F.; et al. Prospective 10-year surveillance of human prion diseases in Japan. Brain 2010, 133, 3043–3057. [Google Scholar] [CrossRef]
- Bagyinszky, E.; Van Giau, V.; Youn, Y.C.; An, S.S.A.; Kim, S. Characterization of mutations in PRNP (prion) gene and their possible roles in neurodegenerative diseases. Neuropsychiatr. Dis. Treat. 2018, 14, 2067–2085. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Chen, C.; Zhou, W.; Gao, C.; Xiao, K.; Tian, C. The Characteristics of Chinese Prion Diseases Based on 10 Years Surveillance Data from 2006 to 2015. Neuropsychiatry 2018, 8, 739–744. [Google Scholar]
- Shi, Q.; Zhou, W.; Chen, C.; Gao, C.; Xiao, K.; Wang, J.; Zhang, B.Y.; Wang, Y.; Zhang, F.; Dong, X.P. Quality evaluation for the surveillance system of human prion diseases in China based on the data from 2010 to 2016. Prion 2016, 10, 484–491. [Google Scholar] [CrossRef]
- Gao, C.; Shi, Q.; Tian, C.; Chen, C.; Han, J.; Zhou, W.; Zhang, B.-Y.; Jiang, H.-Y.; Zhang, J.; Dong, X.-P. The Epidemiological, Clinical, and Laboratory Features of Sporadic Creutzfeldt-Jakob Disease Patients in China: Surveillance Data from 2006 to 2010. PLoS ONE 2011, 6, e24231. [Google Scholar] [CrossRef][Green Version]
- Xiao, K.; Shi, Q.; Zhou, W.; Zhang, B.-Y.; Wang, Y.; Chen, C.; Ma, Y.; Gao, C.; Dong, X.-P. T188K-Familial Creutzfeldt–Jacob Disease, Predominant Among Chinese, has a Reactive Pattern in CSF RT-QuIC Different from D178N-Fatal Familial Insomnia and E200K-Familial CJD. Neurosci. Bull. 2019, 35, 519–521. [Google Scholar] [CrossRef]
- Imbriani, P.; Marfia, G.A.; Marciani, M.G.; Poleggi, A.; Pocchiari, M.; Puoti, G.; Caltagirone, C.; Pisani, A. Heidenhain variant in two patients with inherited V210I Creutzfeldt-Jakob disease. Int. J. Neurosci. 2015, 126, 381–383. [Google Scholar]
- Chen, C.; Wang, J.-C.; Shi, Q.; Zhou, W.; Zhang, X.-M.; Zhang, J.; Tian, C.; Gao, C.; Dong, X.-P. Analyses of the Survival Time and the Influencing Factors of Chinese Patients with Prion Diseases Based on the Surveillance Data from 2008–2011. PLoS ONE 2013, 8, e62553. [Google Scholar] [CrossRef] [PubMed]
- Breithaupt, M.; Romero, C.; Kallenberg, K.; Begue, C.; Sánchez-Juan, P.; Eigenbrod, S.; Kretzschmar, H.A.; Schelzke, G.; Meichtry, E.; Taratuto, A.; et al. Magnetic Resonance Imaging in E200K and V210I Mutations of the Prion Protein Gene. Alzheimer Dis. Assoc. Disord. 2013, 27, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.-P.; Shi, Q.; Xiao, K.; Wang, J.; Zhou, W.; Chen, C.; Dong, X.-P. The genetic Creutzfeldt-Jakob disease with E200K mutation: Analysis of clinical, genetic and laboratory features of 30 Chinese patients. Sci. Rep. 2019, 9, 1836. [Google Scholar] [CrossRef]
- Hoque, M.Z.; Kitamoto, T.; Furukawa, H.; Muramoto, T.; Tateishi, J. Mutation in the prion protein gene at codon 232 in Japanese patients with Creutzfeldt-Jakob disease: A clinicopathological, immunohistochemical and transmission study. Acta Neuropathol. 1996, 92, 441–446. [Google Scholar] [CrossRef]
- Itoh, Y.; Yamada, M.; Hayakawa, M.; Shozawa, T.; Tanaka, J.-I.; Matsushita, M.; Kitamoto, T.; Tateishi, J.; Otomo, E. A variant of Gerstmann-Sträussler-Scheinker disease carrying codon 105 mutation with codon 129 polymorphism of the prion protein gene: A clinicopathological study. J. Neurol. Sci. 1994, 127, 77–86. [Google Scholar] [CrossRef]
- Yamada, M.; Itoh, Y.; Fujigasaki, H.; Naruse, S.; Kaneko, K.; Kitamoto, T.; Tateishi, J.; Otomo, E.; Hayakawa, M.; Tanaka, J.; et al. A missense mutation at codon 105 with codon 129 polymorphism of the prion protein gene in a new variant of Gerstmann-Straussler-Scheinker disease. Neurology 1993, 43, 2723–2724. [Google Scholar] [CrossRef]
- Kim, M.-O.; Takada, L.T.; Wong, K.; Forner, S.A.; Geschwind, M.D. Genetic PrP Prion Diseases. Cold Spring Harb. Perspect. Boil. 2017, 10, a033134. [Google Scholar] [CrossRef]
- Mastrianni, J.A.; Capellari, S.; Telling, G.C.; Han, D.; Bosque, P.; Prusiner, S.B.; DeArmond, S.J. Inherited prion disease caused by the V210I mutation: Transmission to transgenic mice. Neurology 2001, 57, 2198–2205. [Google Scholar] [CrossRef]
- Biljan, I.; Ilc, G.; Giachin, G.; Raspadori, A.; Zhukov, I.; Plavec, J.; Legname, G. Toward the Molecular Basis of Inherited Prion Diseases: NMR Structure of the Human Prion Protein with V210I Mutation. J. Mol. Boil. 2011, 412, 660–673. [Google Scholar] [CrossRef]
- Biljan, I.; Ilc, G.; Giachin, G.; Plavec, J.; Legname, G. Structural Rearrangements at Physiological pH: Nuclear Magnetic Resonance Insights from the V210I Human Prion Protein Mutant. Biochemistry 2012, 51, 7465–7474. [Google Scholar] [CrossRef]
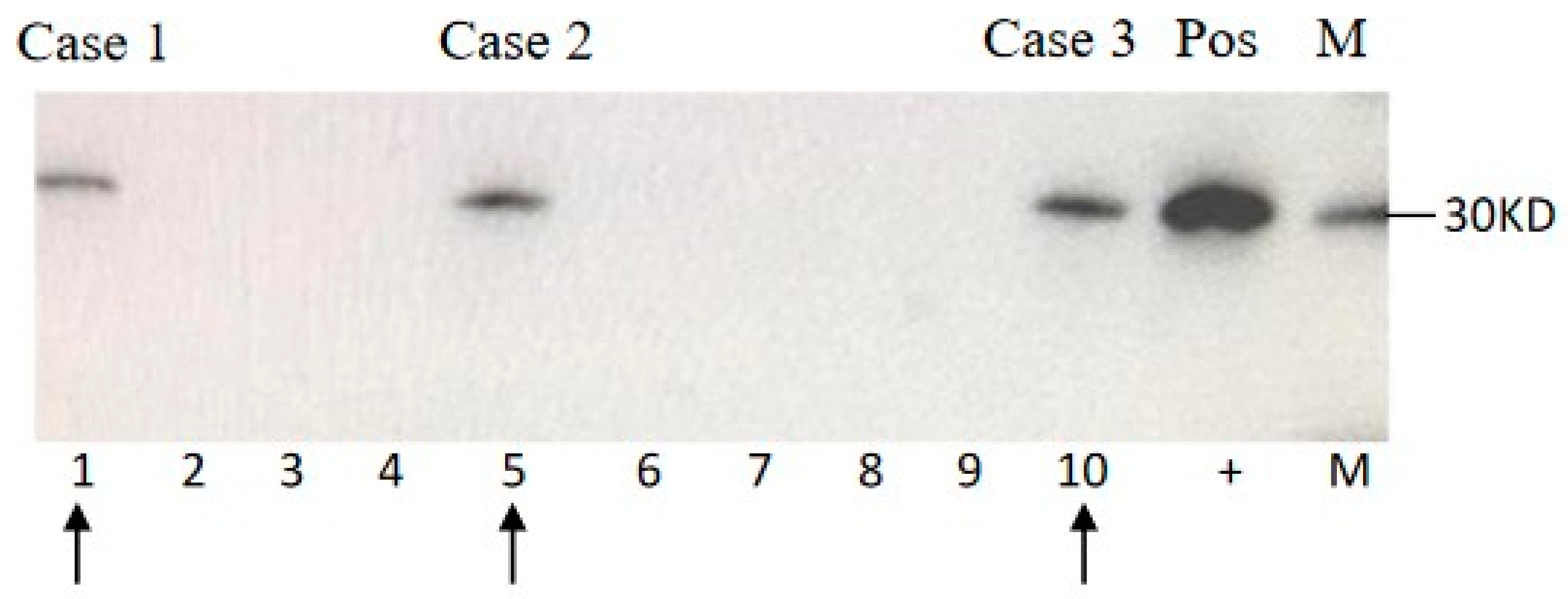
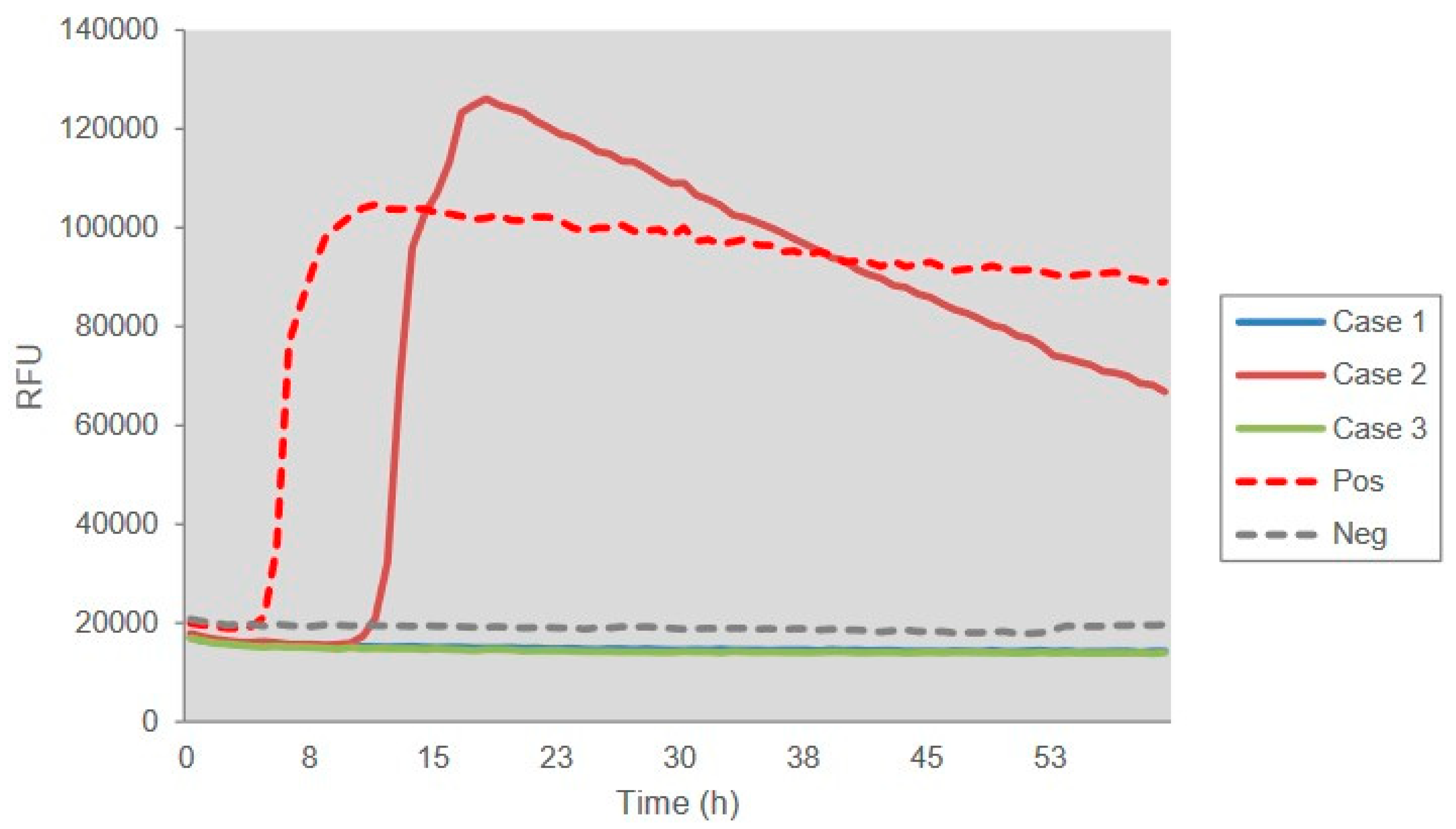

| Gender, on Set Age | Dementia 1 | Other Major CJD-Associated Problems 2 | EEG | CSF | MRI | Polymorphism | Duration | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | VI | PSWCs | 14-3-3 | RT-QuIC | Ribbon-like signal | High signals in caudate/putamen | Codon 129 | Codon 219 | Interval from onset to mutism | Situation | |||
| Case 1 | M, 69 y | + | + | + | + | + | untypical | + | - | + | + | M/M | E/E | app. 30 days | Died 75 days after onset |
| Case 2 | M, 64 y | + | + | + | + | + | typical | + | + | + | - | M/M | E/E | app. 50 days | Alive with mutism |
| Case 3 | M, 59 y | + | - | + | + | + | typical | + | - | + | + | M/M | E/E | app. 40 days | Died 9 months after onset |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, K.; Zhou, W.; Gao, L.-P.; Wu, Y.-Z.; Wang, Y.; Chen, C.; Gao, C.; Shi, Q.; Dong, X.-P. Clinical and Laboratory Features of Three Rare Chinese V210I gCJD Patients. Pathogens 2020, 9, 800. https://doi.org/10.3390/pathogens9100800
Xiao K, Zhou W, Gao L-P, Wu Y-Z, Wang Y, Chen C, Gao C, Shi Q, Dong X-P. Clinical and Laboratory Features of Three Rare Chinese V210I gCJD Patients. Pathogens. 2020; 9(10):800. https://doi.org/10.3390/pathogens9100800
Chicago/Turabian StyleXiao, Kang, Wei Zhou, Li-Ping Gao, Yue-Zhang Wu, Yuan Wang, Cao Chen, Chen Gao, Qi Shi, and Xiao-Ping Dong. 2020. "Clinical and Laboratory Features of Three Rare Chinese V210I gCJD Patients" Pathogens 9, no. 10: 800. https://doi.org/10.3390/pathogens9100800
APA StyleXiao, K., Zhou, W., Gao, L.-P., Wu, Y.-Z., Wang, Y., Chen, C., Gao, C., Shi, Q., & Dong, X.-P. (2020). Clinical and Laboratory Features of Three Rare Chinese V210I gCJD Patients. Pathogens, 9(10), 800. https://doi.org/10.3390/pathogens9100800






