Comparative Proteomic Analysis of Serum from Pigs Experimentally Infected with Trichinella spiralis, Trichinella britovi, and Trichinella pseudospiralis
Abstract
:1. Introduction
2. Results
2.1. Distribution and Intensity of T. spiralis, T. britovi, and T. pseudospiralis Larvae Infection in Muscles of Pigs Experimentally Infected with T. spiralis, T. britovi, and T. pseudospiralis
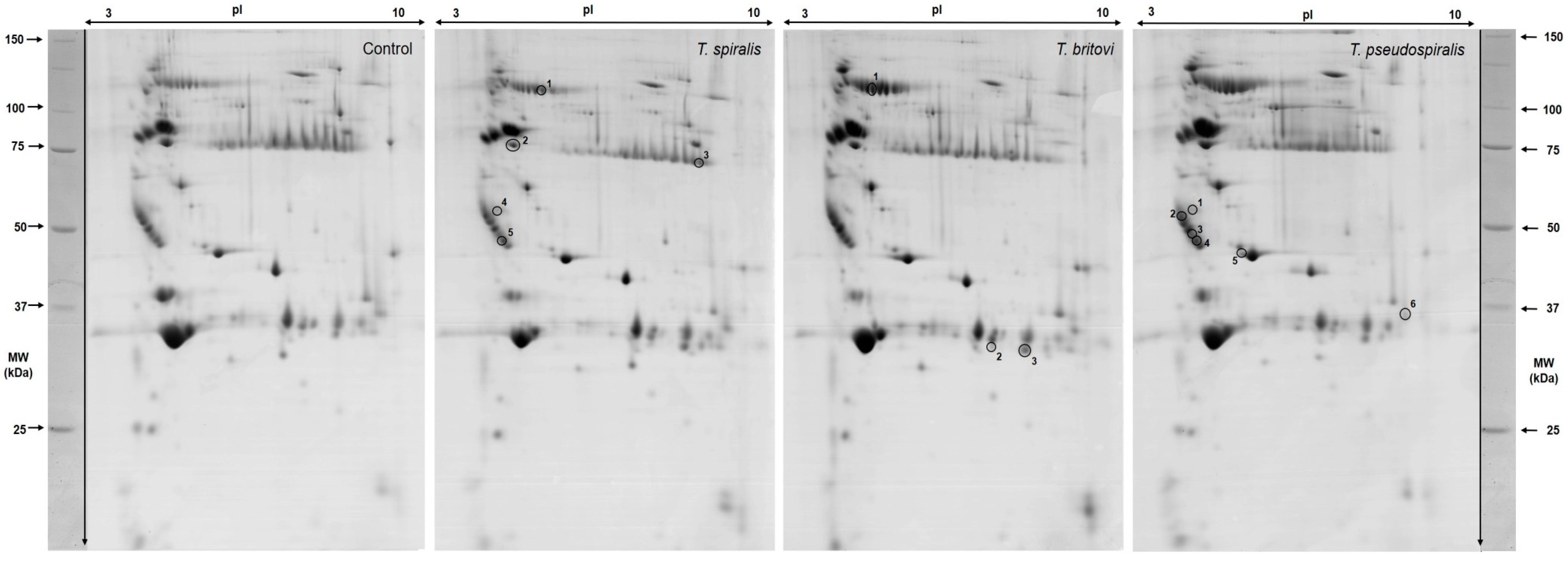
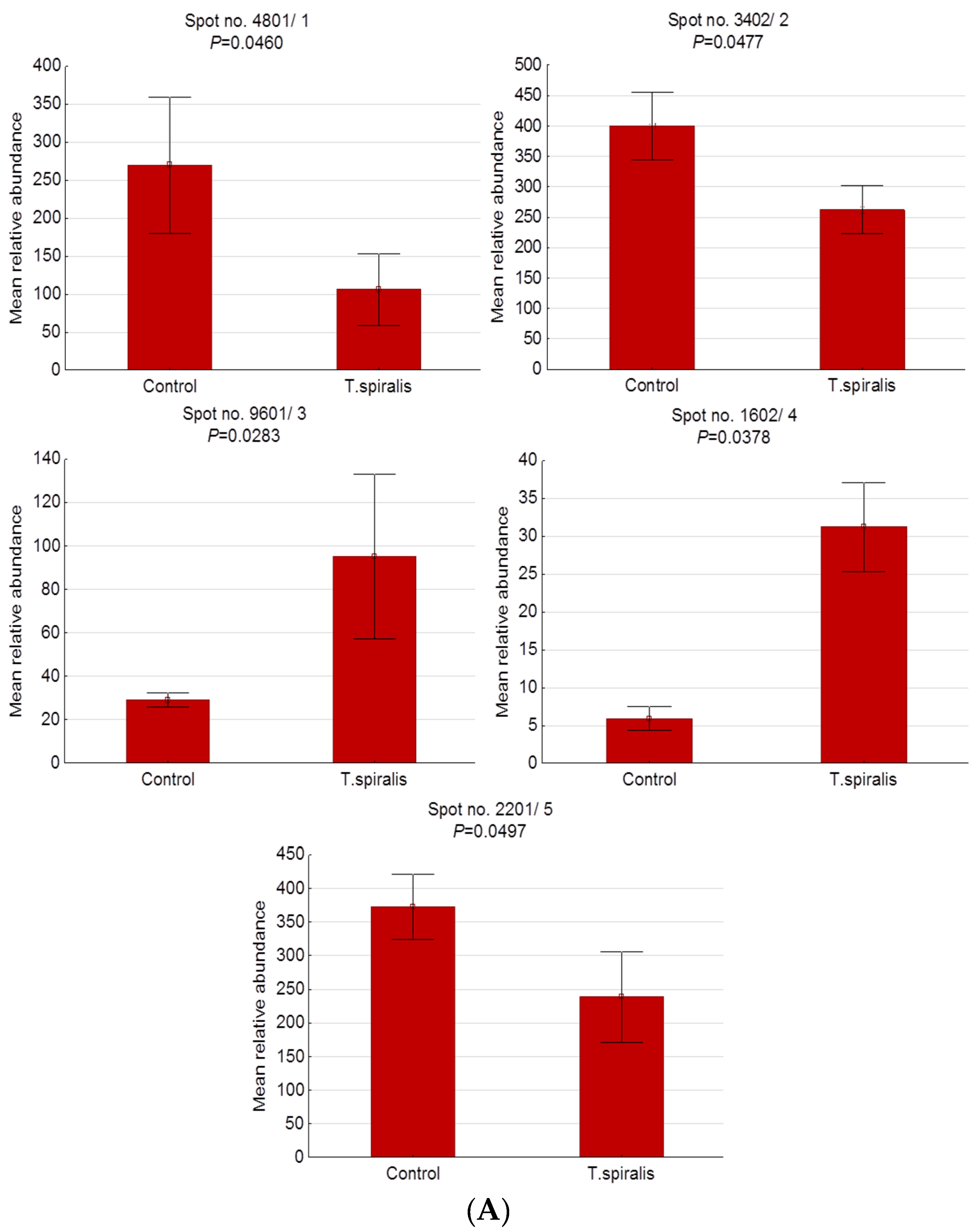
2.2. Differentially Expressed Serum Proteins in Pigs Experimentally Infected with T. spiralis, T. britovi, and T. pseudospiralis on Day 13 Postinfection
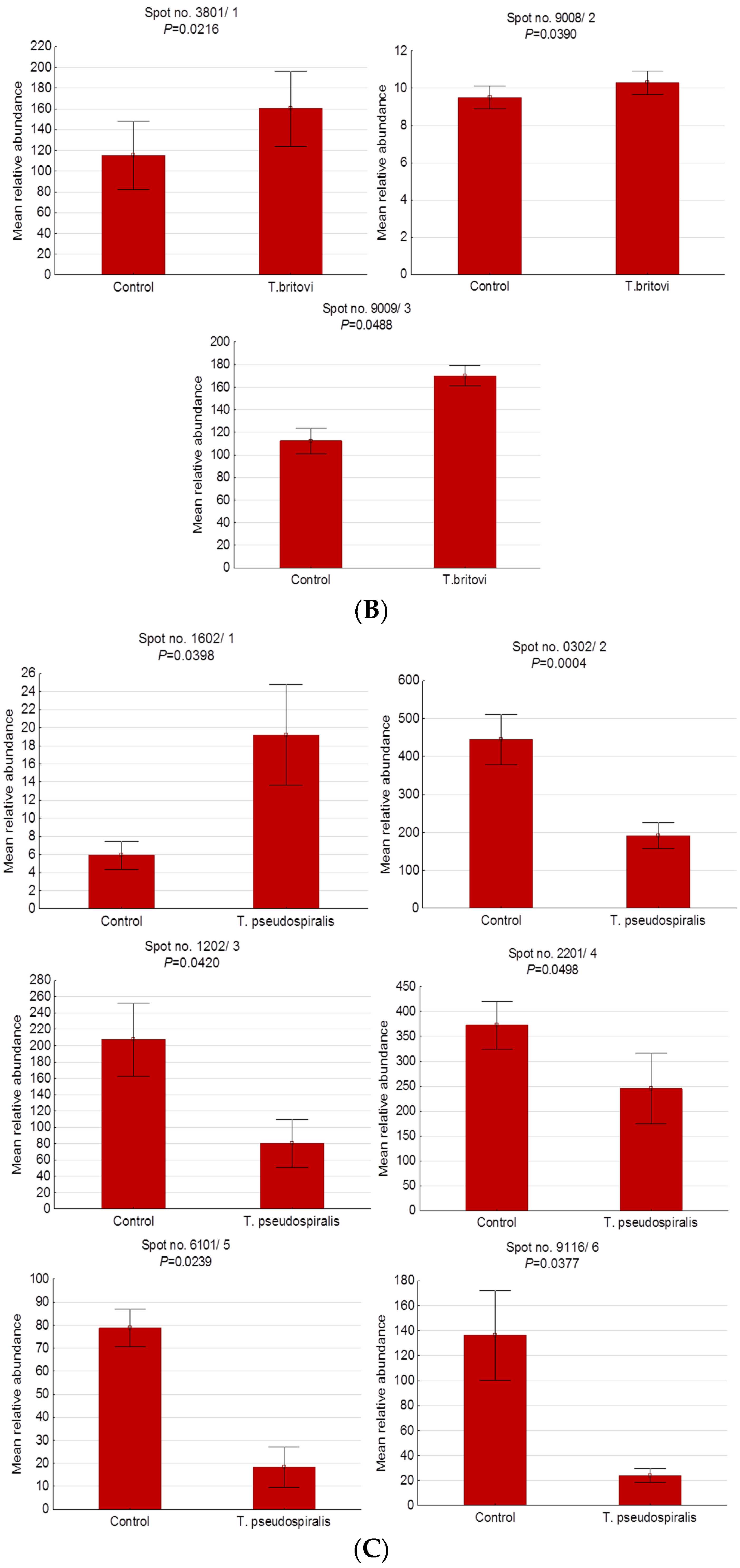
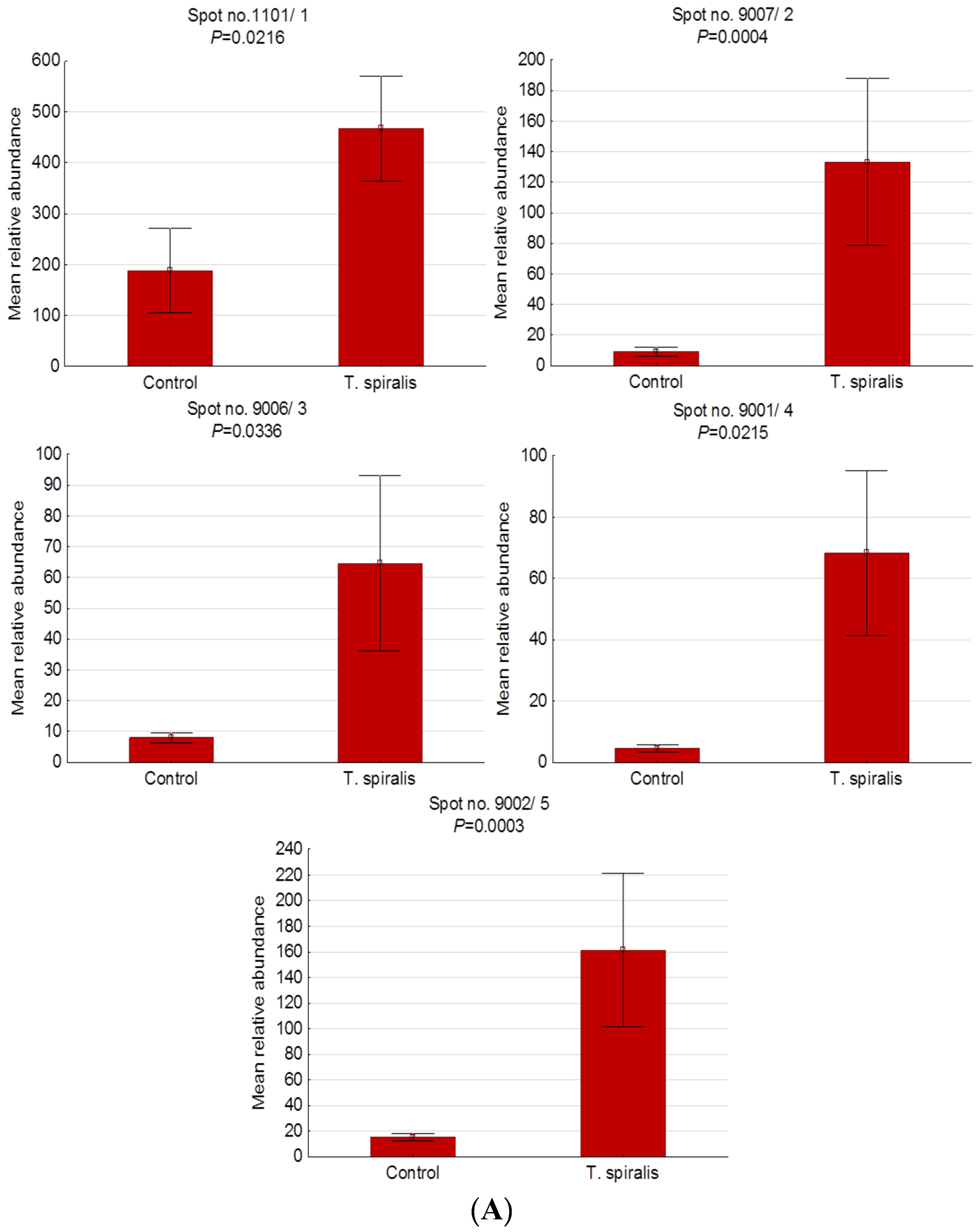
2.3. Differentially Expressed Serum Proteins of Pigs Experimentally Infected with T. spiralis, T. britovi, and T. pseudospiralis on Day 60 Postinfection
3. Discussion
3.1. Preinfective Stage of T. spiralis, T. britovi, and T. pseudospiralis Larvae in Pig Striated Muscles (13 Day Postinfection)
3.2. Fully Infective Stage of T. spiralis, T. britovi, and T. pseudospiralis Larvae in Pig Striated Muscles (60 Day Postinfection)
4. Materials and Methods
4.1. Ethics Statement
4.2. Parasites
4.3. Pig Infection
4.4. Blood Recovery
4.5. Larval Recovery and Counting
4.6. Swine Serum Sample Preparation for 2-Dimensional Gel Electrophoresis (2-DE)
4.7. 2-DE
4.8. Image Analysis
4.9. Mass Spectrometry and Bioinformatics Data Analysis
4.9.1. In-Gel Digestion of Proteins
4.9.2. Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI TOF MS) Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gottstein, B.; Pozio, E.; Nöckler, K. Epidemiology, diagnosis, treatment, and control of trichinellosis. Clin. Microbiol. Rev. 2009, 22, 127–145. [Google Scholar] [CrossRef] [Green Version]
- Kocięcka, W.; Boczoń, K.; Pozio, E.; van Knapen, F. Trichinella. In International Handbook of Foodborne Pathogen, 1st ed.; Miliotis, M.D., Bier, J.W., Eds.; Marcel Dekker: New York, NY, USA, 2003; pp. 637–658. [Google Scholar]
- Wu, Z.; Sofronic-Milosavljevic, L.J.; Nagano, I.; Takahashi, Y. Trichinella spiralis: Nurse cell formation with emphasis on analogy to muscle cell repair. Parasit. Vectors 2008, 1, 27. [Google Scholar] [CrossRef] [Green Version]
- Fröscher, W.; Gullotta, F.; Saathoff, M.; Tackmann, W. Chronic trichinosis. Clinical, bioptic, serological and electromyographic observations. Eur. Neurol. 1988, 28, 221–226. [Google Scholar] [CrossRef]
- Pozio, E.; Zarlenga, D.S. Recent advances on the taxonomy, systematics and epidemiology of Trichinella. Int. J. Parasitol. 2005, 35, 1191–1204. [Google Scholar] [CrossRef] [PubMed]
- Krivokapich, S.J.; Pozio, E.; Gatti, G.M.; Prous, C.L.; Ribicich, M.; Marucci, G.; La Rosa, G.; Confalonieri, V. Trichinella patagoniensis n. sp. (Nematoda), a new encapsulated species infecting carnivorous mammals in South America. Int. J. Parasitol. 2012, 42, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Murrell, K.D.; Pozio, E. Worldwide occurrence and impact of human trichinellosis, 1986–2009. Emerg. Infect. Dis. 2011, 17, 2194–2202. [Google Scholar] [CrossRef] [PubMed]
- Pozio, E.; Rinaldi, L.; Marucci, G.; Musella, V.; Galati, F.; Cringoli, G.; Boireau, P.; La Rosa, G. Hosts and habitats of Trichinella spiralis and Trichinella britovi in Europe. Int. J. Parasitol. 2009, 39, 71–79. [Google Scholar] [CrossRef]
- Hurníková, Z.; Snábel, V.; Pozio, E.; Reiterová, K.; Hrcková, G.; Halásová, D.; Dubinský, P. First record of Trichinella pseudospiralis in the Slovak Republic found in domestic focus. Vet. Parasitol. 2005, 128, 91–98. [Google Scholar] [CrossRef]
- Beck, R.; Beck, A.; Lucinger, S.; Florijancić, T.; Bosković, I.; Marinculić, A. Trichinella pseudospiralis in pig from Croatia. Vet. Parasitol. 2009, 159, 304–307. [Google Scholar] [CrossRef]
- Santrac, V.; Nedic, D.N.; Maric, J.; Nikolic, S.; Stevanovic, O.; Vasilev, S.; Cvetkovic, J.; Sofronic-Milosavljevic, L. The first report of Trichinella pseudospiralis presence in domestic swine and T. britovi in wild boar in Bosnia and Herzegovina. Acta Parasitol. 2015, 60, 471–475. [Google Scholar] [CrossRef]
- Zamora, M.J.; Alvarez, M.; Olmedo, J.; Blanco, M.C.; Pozio, E. Trichinella pseudospiralis in the Iberian peninsula. Vet. Parasitol. 2015, 210, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Kapel, C.M.; Webster, P.; Lind, P.; Pozio, E.; Henriksen, S.A.; Murrell, K.D.; Nansen, P. Trichinella spiralis, T. britovi, and T. nativa: Infectivity, larval distribution in muscle, and antibody response after experimental infection of pigs. Parasitol. Res. 1998, 84, 264–271. [Google Scholar] [PubMed]
- Kapel, C.M.; Gamble, H.R. Infectivity, persistence, and antibody response to domestic and sylvatic Trichinella spp. in experimentally infected pigs. Int. J. Parasitol. 2000, 30, 215–221. [Google Scholar] [CrossRef]
- Nöckler, K.; Serrano, F.J.; Boireau, P.; Kapel, C.M.; Pozio, E. Experimental studies in pigs on Trichinella detection in different diagnostic matrices. Vet. Parasitol. 2005, 132, 85–90. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2015/1375 of 10 August 2015 Laying Down Specific Rules on Official Controls for Trichinella in Meat. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32015R1375&qid=1574157105616&from=PL (accessed on 20 May 2019).
- Miller, I.; Wait, R.; Sipos, W.; Gemeiner, M. A proteomic reference map for pig serum proteins as a prerequisite for diagnostic applications. Res. Vet. Sci. 2009, 86, 362–367. [Google Scholar] [CrossRef]
- Ożgo, M.; Lepczyński, A.; Herosimczyk, A. Two-dimensional gel-based serum protein profile of growing piglets. Turk. J. Biol. 2015, 39, 320–327. [Google Scholar] [CrossRef] [Green Version]
- Srikanth, K.; Lee, E.; Kwon, A.; Shin, J.; Chung, H. A comparative proteomic analysis of blood serum for developmental stages in pigs. Anim. Genet. 2017, 48, 531–543. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Z.; Jiang, J.; Wu, D.; Liu, X.; Xie, Z.; Chen, E.; Zhu, D.; Ye, C.; Zhang, X.; et al. Proteomic Signature of Acute Liver Failure: From Discovery and Verification in a Pig Model to Confirmation in Humans. Mol. Cell. Proteom. 2017, 16, 1188–1199. [Google Scholar] [CrossRef] [Green Version]
- Marco-Ramell, A.; Pato, R.; Peña, R.; Saco, Y.; Manteca, X.; Ruiz de la Torre, J.L.; Bassols, A. Identification of serum stress biomarkers in pigs housed at different stocking densities. Vet. J. 2011, 190, e66–e71. [Google Scholar] [CrossRef]
- Yin, C.; Liu, W.; Liu, Z.; Huang, Y.; Ci, L.; Zhao, R.; Yang, X. Identification of potential serum biomarkers in pigs at early stage after Lipopolysaccharide injection. Res. Vet. Sci. 2017, 111, 140–146. [Google Scholar] [CrossRef]
- Sun, J.F.; Shi, Z.X.; Guo, H.C.; Li, S.; Tu, C.C. Proteomic analysis of swine serum following highly virulent classical swine fever virus infection. Virol. J. 2011, 8, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, W.; Jia, J.; Zhang, B.; Mi, S.; Zhang, L.; Xie, X.; Guo, H.; Shi, J.; Tu, C. Serum metabolomic profiling of piglets infected with virulent classical swine fever virus. Front. Microbiol. 2017, 8, 731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zhang, K.; Zheng, H.; Shang, Y.; Guo, J.; Tian, H.; Lu, G.; Jin, Y.; He, J.; Cai, X.; et al. Proteomics analysis of porcine serum proteins by LC-MS/MS after foot-and-mouth disease virus (FMDV) infection. J. Vet. Med. Sci. 2011, 73, 1569–1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genini, S.; Paternoster, T.; Costa, A.; Botti, S.; Luini, M.V.; Caprera, A.; Giuffra, E. Identification of serum proteomic biomarkers for early porcine reproductive and respiratory syndrome (PRRS) infection. Proteome Sci. 2012, 10, 48. [Google Scholar] [CrossRef] [Green Version]
- Bień, J.; Näreaho, A.; Varmanen, P.; Goździk, K.; Moskwa, B.; Cabaj, W.; Nyman, T.A.; Savijoki, K. Comparative analysis of excretory-secretory antigens of Trichinella spiralis and Trichinella britovi muscle larvae by two-dimensional difference gel electrophoresis and immunoblotting. Proteome Sci. 2012, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Liu, R.D.; Wang, L.; Zhang, X.; Jiang, P.; Liu, M.Y.; Wang, Z.Q. Proteomic analysis of surface proteins of Trichinella spiralis muscle larvae by two-dimensional gel electrophoresis and mass spectrometry. Parasit. Vectors 2013, 6, 355. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.Q.; Hu, D.D.; Cui, J. Proteomic analysis of Trichinella spiralis muscle larval excretory-secretory proteins recognized by early infection sera. Biomed. Res. Int. 2013, 2013, 139745. [Google Scholar]
- Liu, R.D.; Cui, J.; Wang, L.; Long, S.R.; Zhang, X.; Liu, M.Y.; Wang, Z.Q. Identification of surface proteins of Trichinella spiralis muscle larvae using immunoproteomics. Trop. Biomed. 2014, 31, 579–591. [Google Scholar]
- Wang, L.; Cui, J.; Hu, D.D.; Liu, R.D.; Wang, Z.Q. Identification of early diagnostic antigens from major excretory-secretory proteins of Trichinella spiralis muscle larvae using immunoproteomics. Parasit. Vectors 2014, 7, 40. [Google Scholar] [CrossRef]
- Bien, J.; Cabaj, W.; Moskwa, B. Proteomic analysis of potential immunoreactive proteins from muscle larvae and adult worms of Trichinella spiralis in experimentally infected pigs. Folia Parasitol. 2015, 62, 022. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Pan, W.; Sun, X.; Zhao, X.; Yuan, G.; Sun, Q.; Huang, J.; Zhu, X. Immunoproteomic profile of Trichinella spiralis adult worm proteins recognized by early infection sera. Parasit. Vectors 2015, 8, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.D.; Qi, X.; Sun, G.G.; Jiang, P.; Zhang, X.; Wang, L.A.; Liu, X.L.; Wang, Z.Q.; Cui, J. Proteomic analysis of Trichinella spiralis adult worm excretory-secretory proteins recognized by early infection sera. Vet. Parasitol. 2016, 231, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Forbes, L.B.; Gajadhar, A.A. A validated Trichinella digestion assay and an associated sampling and quality assurance system for use in testing pork and horse meat. J. Food Prot. 1999, 62, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Prost, E.K.; Nowakowski, Z. Detectability of Trichinella spiralis in muscles by pooled-sample-digestion-method. Fleischwirtschaft 1990, 70, 593–595. [Google Scholar]
- Kotula, A.W.; Murrell, K.D.; Acosta-Stein, L.; Lamb, L. Distribution of Trichinella spiralis larvae in selected muscles and organs of experimentally infected swine. J. Anim. Sci. 1984, 58, 94–98. [Google Scholar] [CrossRef] [Green Version]
- Gamble, H.R. Detection of trichinellosis in pigs by artificial digestion and enzyme immunoassay. J. Food Prot. 1996, 59, 295–298. [Google Scholar] [CrossRef]
- Serrano, F.J.; Pérez-Martín, J.E.; Reina, D.; Navarrete, I.; Kapel, C.M. Influence of infection intensity on predilection sites in swine trichinellosis. J. Helminthol. 1999, 73, 251–254. [Google Scholar] [CrossRef]
- Kapel, C.M.; Webster, P.; Gamble, H.R. Muscle distribution of sylvatic and domestic Trichinella larvae in production animals and wildlife. Vet. Parasitol. 2005, 132, 101–105. [Google Scholar] [CrossRef]
- de Silva, H.; Harmony, J.A.; Stuart, W.D.; Gil, C.M.; Robbins, J. Apolipoprotein J: Structure and tissue distribution. Biochemistry 1990, 29, 5380–5389. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Corbo, M.; Duigou, G.; Gabbai, A.A.; Hays, A.P. Expression of a cell death marker (Clusterin) in muscle target fibers. Arq. Neuropsiquiatr. 1993, 51, 371–376. [Google Scholar] [CrossRef] [Green Version]
- Kloučková, J.; Lacinová, Z.; Kaválková, P.; Trachta, P.; Kasalický, M.; Haluzíková, D.; Mráz, M.; Haluzík, M. Plasma concentrations and subcutaneous adipose tissue mRNA expression of clusterin in obesity and type 2 diabetes mellitus: The effect of short-term hyperinsulinemia, very-low-calorie diet and bariatric surgery. Physiol. Res. 2016, 65, 481–492. [Google Scholar] [PubMed]
- Stuart, W.D.; Krol, B.; Jenkins, S.H.; Harmony, J.A. Structure and stability of apolipoprotein J-containing high-density lipoproteins. Biochemistry 1992, 31, 8552–8559. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.; Leanderson, P.; Tagesson, C.; Lindahl, M. Lipoproteomics I: Mapping of proteins in low-density lipoprotein using two-dimensional gel electrophoresis and mass spectrometry. Proteomics 2005, 5, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.Y.; Chen, S.F.; Lai, M.D.; Chang, T.T.; Chen, T.L.; Li, P.Y.; Shieh, D.B.; Young, K.C. Comparative proteomic profiling of plasma very-low-density and low-density lipoproteins. Clin. Chim. Acta 2010, 411, 336–344. [Google Scholar] [CrossRef]
- Zhang, H.; Kim, J.K.; Edwards, C.A.; Xu, Z.; Taichman, R.; Wang, C.Y. Clusterin inhibits apoptosis by interacting with activated Bax. Nat. Cell. Biol. 2005, 7, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Trougakos, I.P.; Lourda, M.; Antonelou, M.H.; Kletsas, D.; Gorgoulis, V.G.; Papassideri, I.S.; Zou, Y.; Margaritis, L.H.; Boothman, D.A.; Gonos, E.S. Intracellular clusterin inhibits mitochondrial apoptosis by suppressing p53-activating stress signals and stabilizing the cytosolic Ku70-Bax protein complex. Clin. Cancer Res. 2009, 15, 48–59. [Google Scholar] [CrossRef] [Green Version]
- Baralla, A.; Sotgiu, E.; Deiana, M.; Pasella, S.; Pinna, S.; Mannu, A.; Canu, E.; Sotgiu, G.; Ganau, A.; Zinellu, A.; et al. Plasma clusterin and lipid profile: A link with aging and cardiovascular diseases in a population with a consistent number of centenarians. PLoS ONE 2015, 10, e0128029. [Google Scholar] [CrossRef] [Green Version]
- Heo, J.Y.; Kim, J.E.; Dan, Y.; Kim, Y.W.; Kim, J.Y.; Cho, K.H.; Bae, Y.K.; Im, S.S.; Liu, K.H.; Song, I.H.; et al. Clusterin deficiency induces lipid accumulation and tissue damage in kidney. J. Endocrinol. 2018, 237, 175–191. [Google Scholar] [CrossRef] [Green Version]
- Tschopp, J.; French, L.E. Clusterin: Modulation of complement function. Clin. Exp. Immunol. 1994, 97 (Suppl. 2), 11–14. [Google Scholar] [CrossRef]
- Poon, S.; Treweek, T.M.; Wilson, M.R.; Easterbrook-Smith, S.B.; Carver, J.A. Clusterin is an extracellular chaperone that specifically interacts with slowly aggregating proteins on their off-folding pathway. FEBS Lett. 2002, 513, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Trougakos, I.P.; Poulakou, M.; Stathatos, M.; Chalikia, A.; Melidonis, A.; Gonos, E.S. Serum levels of the senescence biomarker clusterin/apolipoprotein J increase significantly in diabetes type II and during development of coronary heart disease or at myocardial infarction. Exp. Gerontol. 2002, 37, 1175–1187. [Google Scholar] [CrossRef]
- Buquicchio, R.; Foti, C.; Loconsole, F.; Polimeno, L.; Ventura, M.T. Clusterin serum level: How does it affect psoriatic patients? J. Biol. Regul. Homeost. Agents 2017, 31, 785–789. [Google Scholar] [PubMed]
- Kim, J.H.; Lee, H.Y.; Ban, G.Y.; Shin, Y.S.; Park, H.S.; Ye, Y.M. Serum Clusterin as a Prognostic Marker of Chronic Spontaneous Urticaria. Medicine 2016, 95, e3688. [Google Scholar] [CrossRef] [PubMed]
- Kalenka, A.; Feldmann, R.E., Jr.; Otero, K.; Maurer, M.H.; Waschke, K.F.; Fiedler, F. Changes in the serum proteome of patients with sepsis and septic shock. Anesth. Analg. 2006, 103, 1522–1526. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Piñeiro, A.M.; de la Cadena, M.P.; López-Saco, A.; Rodríguez-Berrocal, F.J. Differential expression of serum clusterin isoforms in colorectal cancer. Mol. Cell. Proteom. 2006, 5, 1647–1657. [Google Scholar] [CrossRef] [Green Version]
- Miyake, H.; Muramaki, M.; Furukawa, J.; Kurahashi, T.; Fujisawa, M. Serum level of clusterin and its density in men with prostate cancer as novel biomarkers reflecting disease extension. Urology 2010, 75, 454–459. [Google Scholar] [CrossRef]
- Kropáčková, T.; Šléglová, O.; Růžičková, O.; Vencovský, J.; Pavelka, K.; Šenolt, L. Lower serum clusterin levels in patients with erosive hand osteoarthritis are associated with more pain. BMC Musculoskelet. Disord. 2018, 19, 264. [Google Scholar] [CrossRef] [Green Version]
- Newkirk, M.M.; Apostolakos, P.; Neville, C.; Fortin, P.R. Systemic lupus erythematosus, a disease associated with low levels of clusterin/apoJ, an antiinflammatory protein. J. Rheumatol. 1999, 26, 597–603. [Google Scholar]
- Høgåsen, K.; Mollnes, T.E.; Brandtzaeg, P. Low levels of vitronectin and clusterin in acute meningococcal disease are closely associated with formation of the terminal-complement complex and the vitronectin-thrombin-antithrombin complex. Infect. Immun. 1994, 62, 4874–4880. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, Y.H.; Mai, S.J.; He, L.J.; Liao, Y.J.; Deng, H.X.; Guan, X.Y.; Zeng, Y.X.; Kung, H.F.; Xie, D. Evaluation of serum clusterin as a surveillance tool for human hepatocellular carcinoma with hepatitis B virus related cirrhosis. J. Gastroenterol. Hepatol. 2010, 25, 1123–1128. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Ying, W.T.; Mao, Y.S.; He, H.Z.; Liu, Y.; Wang, H.X.; Liu, F.; Wang, K.; Zhang, D.C.; Wang, Y.; et al. Loss of clusterin both in serum and tissue correlates with the tumorigenesis of esophageal squamous cell carcinoma via proteomics approaches. World J. Gastroenterol. 2003, 9, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Nagano, I.; Takahashi, Y. Candidate genes responsible for common and different pathology of infected muscle tissues between Trichinella spiralis and T. pseudospiralis infection. Parasitol. Int. 2008, 57, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Taye, B.; Yeo, D.; Lee, R.T.C.; Tan, B.H.; Sugrue, R.J.; Maurer-Stroh, S. Inter-Species Host Gene Expression Differences in Response to Human and Avian Influenza A Virus Strains. Int. J. Mol. Sci. 2017, 18, 2295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mido, S.; Fath, E.M.; Farid, A.S.; Nonaka, N.; Oku, Y.; Horii, Y. Trichinella spiralis: Infection changes serum paraoxonase-1 levels, lipid profile, and oxidative status in rats. Exp. Parasitol. 2012, 131, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Koltai, T. Clusterin: A key player in cancer chemoresistance and its inhibition. Onco Targets Ther. 2014, 7, 447–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boonmars, T.; Wu, Z.; Nagano, I.; Takahashi, Y. Expression of apoptosis-related factors in muscles infected with Trichinella spiralis. Parasitology 2004, 128, 323–332. [Google Scholar] [CrossRef]
- Boonmars, T.; Wu, Z.; Nagano, I.; Takahashi, Y. What is the role of p53 during the cyst formation of Trichinella spiralis? A comparable study between knockout mice and wild type mice. Parasitology 2005, 131, 705–712. [Google Scholar] [CrossRef]
- Maclean, P.S.; Tait, R.C. Hereditary and acquired antithrombin deficiency: Epidemiology, pathogenesis and treatment options. Drugs 2007, 67, 1429–1440. [Google Scholar] [CrossRef]
- Büller, H.R.; ten Cate, J.W. Acquired antithrombin III deficiency: Laboratory diagnosis, incidence, clinical implications, and treatment with antithrombin III concentrate. Am. J. Med. 1989, 87, 44–48. [Google Scholar] [CrossRef]
- Niessen, R.W.; Lamping, R.J.; Jansen, P.M.; Prins, M.H.; Peters, M.; Taylor, F.B., Jr.; de Vijlder, J.J.; ten Cate, J.W.; Hack, C.E.; Sturk, A. Antithrombin acts as a negative acute phase protein as established with studies on HepG2 cells and in baboons. Thromb. Haemost. 1997, 78, 1088–1092. [Google Scholar] [CrossRef]
- Capó, V.; Despommier, D.D. Clinical aspects of infection with Trichinella spp. Clin. Microbiol. Rev. 1996, 9, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Kocięcka, W. Trichinellosis: Human disease, diagnosis and treatment. Vet. Parasitol. 2000, 93, 365–383. [Google Scholar] [CrossRef]
- Bruschi, F.; Murrell, K.D. New aspects of human trichinellosis: The impact of new Trichinella species. Postgrad. Med. J. 2002, 78, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.W.; Pattern, B.M. Trichinosis associated with superior sagittal sinus thrombosis. Ann. Neurol. 1982, 11, 216–217. [Google Scholar] [CrossRef] [PubMed]
- Tint, D.; Cocuz, M.E.; Ortan, O.F.; Niculescu, M.D.; Radoi, M. Cardiac involvement in trichinellosis: A case of left ventricular thrombosis. Am. J. Trop. Med. Hyg. 2009, 81, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Dalcin, D.; Zarlenga, D.S.; Larter, N.C.; Hoberg, E.; Boucher, D.A.; Merrifield, S.; Lau, R.; Ralevski, F.; Cheema, K.; Schwartz, K.L.; et al. Trichinella Nativa Outbreak with Rare Thrombotic Complications Associated With Meat From a Black Bear Hunted in Northern Ontario. Clin. Infect. Dis. 2017, 64, 1367–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ursell, P.C.; Habib, A.; Babchick, O.; Rottolo, R.; Despommier, D.; Fenoglio, J.J. Myocarditis caused by Trichinella spiralis. Arch. Pathol. Lab. Med. 1984, 108, 4–5. [Google Scholar]
- Mahley, R.W. Apolipoprotein E: Cholesterol transport protein with expanding role in cell biology. Science 1988, 240, 622–630. [Google Scholar] [CrossRef]
- Zhang, H.L.; Wu, J.; Zhu, J. The immune-modulatory role of apolipoprotein E with emphasis on multiple sclerosis and experimental autoimmune encephalomyelitis. Clin. Dev. Immunol. 2010, 2010, 186813. [Google Scholar] [CrossRef] [Green Version]
- Farid, A.S.; Fath, E.M.; Mido, S.; Nonaka, N.; Horii, Y. Paraoxonase-1 activity is related to Trichinella spiralis-induced hepatitis in rats. Eur. J. Clin. Investig. 2017, 47, 250–261. [Google Scholar] [CrossRef]
- Perales, J.; Angel Lasunción, M.; Cano, A.; Martín-Scapa, M.A.; Matíes, M.; Herrera, E. Changes in the lipid profile in chronic hepatopathies. Med. Clin. (Barc.) 1994, 102, 364–368. [Google Scholar] [PubMed]
- Spósito, A.C.; Vinagre, C.G.; Pandullo, F.L.; Mies, S.; Raia, S.; Ramires, J.A. Apolipoprotein and lipid abnormalities in chronic liver failure. Braz. J. Med. Biol. Res. 1997, 30, 1287–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brand, K.; Mackman, N.; Curtiss, L.K. Interferon-gamma inhibits macrophage apolipoprotein E production by posttranslational mechanisms. J. Clin. Investig. 1993, 91, 2031–2039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braesch-Andersen, S.; Paulie, S.; Smedman, C.; Mia, S.; Kumagai-Braesch, M. ApoE production in human monocytes and its regulation by inflammatory cytokines. PLoS ONE 2013, 8, e79908. [Google Scholar] [CrossRef] [Green Version]
- Stadnyk, A.W.; Kearsey, J.A. Pattern of proinflammatory cytokine mRNA expression during Trichinella spiralis infection of the rat. Infect. Immun. 1996, 64, 5138–5143. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Nagano, I.; Boonmars, T.; Takahashi, Y. Tumor necrosis factor receptor-mediated apoptosis in Trichinella spiralis-infected muscle cells. Parasitology 2005, 131, 373–381. [Google Scholar] [CrossRef]
- Farid, A.S.; Fath, E.M.; Mido, S.; Nonaka, N.; Horii, Y. Hepatoprotective immune response during Trichinella spiralis infection in mice. J. Vet. Med. Sci. 2019, 81, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Espiritu, D.J.; Mazzone, T. Oxidative stress regulates adipocyte apolipoprotein e and suppresses its expression in obesity. Diabetes 2008, 57, 2992–2998. [Google Scholar] [CrossRef] [Green Version]
- Smith, N.C.; Bryant, C. Free radical generation during primary infections with Nippostrongylus brasiliensis. Parasite Immunol. 1989, 11, 147–160. [Google Scholar] [CrossRef]
- Selkirk, M.E.; Smith, V.P.; Thomas, G.R.; Gounaris, K. Resistance of filarial nematode parasites to oxidative stress. Int. J. Parasitol. 1998, 28, 1315–1332. [Google Scholar] [CrossRef]
- Derda, M.; Wandurska-Nowak, E.; Hadaś, E. Changes in the level of antioxidants in the blood from mice infected with Trichinella spiralis. Parasitol. Res. 2004, 93, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Derda, M.; Boczoń, K.; Wandurska-Nowak, E.; Wojt, W. Changes in the activity of glutathione-S-transferase in muscles and sera from mice infected with Trichinella spiralis after treatment with albendazole and levamisole. Parasitol. Res. 2003, 89, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Gabrashanska, M.; Teodorova, S.E.; Petkova, S.; Mihov, L.; Anisimova, M.; Ivanov, D. Selenium supplementation at low doses contributes to the antioxidant status in Trichinella spiralis-infected rats. Parasitol. Res. 2010, 106, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, P.N. Studies with radiolabelled serum amyloid P component provide evidence for turnover and regression of amyloid deposits in vivo. Clin. Sci. 1994, 87, 289–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pepys, M.B.; Booth, D.R.; Hutchinson, W.L.; Gallimore, J.R.; Collins, P.M.; Hohenester, E. Amyloid P component. A critical review. Int. J. Exp. Clin. Investig. 1997, 4, 274–295. [Google Scholar] [CrossRef]
- de Haas, C.J. New insights into the role of serum amyloid P component, a novel lipopolysaccharide-binding protein. FEMS Immunol. Med. Microbiol. 1999, 26, 197–202. [Google Scholar] [CrossRef]
- Herbert, J.; Hutchinson, W.L.; Carr, J.; Ives, J.; Jakob-Roetne, R.; Yamamura, K.; Suzuki, M.; Pepys, M.B. Influenza virus infection is not affected by serum amyloid P component. Mol. Med. 2002, 8, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Noursadeghi, M.; Bickerstaff, M.C.; Gallimore, J.R.; Herbert, J.; Cohen, J.; Pepys, M.B. Role of serum amyloid P component in bacterial infection: Protection of the host or protection of the pathogen. Proc. Natl. Acad. Sci. USA 2000, 97, 14584–14589. [Google Scholar] [CrossRef] [Green Version]
- de Haas, C.J.; van der Tol, M.E.; Van Kessel, K.P.; Verhoef, J.; Van Strijp, J.A. A synthetic lipopolysaccharide-binding peptide based on amino acids 27–39 of serum amyloid P component inhibits lipopolysaccharide-induced responses in human blood. J. Immunol. 1998, 161, 3607–3615. [Google Scholar]
- Behrens, N.E.; Lipke, P.N.; Pilling, D.; Gomer, R.H.; Klotz, S.A. Serum Amyloid P Component Binds Fungal Surface Amyloid and Decreases Human Macrophage Phagocytosis and Secretion of Inflammatory Cytokines. MBio 2019, 10, e00218-19. [Google Scholar] [CrossRef] [Green Version]
- Pepys, M.B.; Baltz, M.; Gomer, K.; Davies, A.J.; Doenhoff, M. Serum amyloid P-component is an acute-phase reactant in the mouse. Nature 1979, 278, 259–261. [Google Scholar] [CrossRef]
- Lamontagne, L.R.; Gauldie, J.; Befus, A.D.; McAdam, K.P.; Baltz, M.L.; Pepys, M.B. The acute phase response in parasite infection. Nippostrongylus brasiliensis in the mouse. Immunology 1984, 52, 733–741. [Google Scholar] [PubMed]
- Pepys, M.B.; Dash, A.C.; Markham, R.E.; Thomas, H.C.; Williams, B.D.; Petrie, A. Comparative clinical study of protein SAP (amyloid P component) and C-reactive protein in serum. Clin. Exp. Immunol. 1978, 32, 119–124. [Google Scholar] [PubMed]
- Levo, Y.; Shalit, M.; Tur-Kaspa, R. Serum amyloid P-component as a marker of liver disease. Am. J. Gastroenterol. 1982, 77, 427–430. [Google Scholar] [PubMed]
- Anderson, D.M.; Arredondo, J.; Hahn, K.; Valente, G.; Martin, J.F.; Wilson-Rawls, J.; Rawls, A. Mohawk is a novel homeobox gene expressed in the developing mouse embryo. Dev. Dyn. 2006, 235, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Beres, B.J.; Wilson-Rawls, J.; Rawls, A. The homeobox gene Mohawk represses transcription by recruiting the sin3A/HDAC co-repressor complex. Dev. Dyn. 2009, 238, 572–580. [Google Scholar] [CrossRef]
- Liu, H.; Liu, W.; Maltby, K.M.; Lan, Y.; Jiang, R. Identification and developmental expression analysis of a novel homeobox gene closely linked to the mouse Twirler mutation. Gene Expr. Patterns 2006, 6, 632–636. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, J.K.; Bruneau, B.G. Irxl1, a divergent Iroquois homeobox family transcription factor gene. Gene Expr. Patterns 2007, 7, 51–56. [Google Scholar] [CrossRef]
- Ito, Y.; Toriuchi, N.; Yoshitaka, T.; Ueno-Kudoh, H.; Sato, T.; Yokoyama, S.; Nishida, K.; Akimoto, T.; Takahashi, M.; Miyaki, S.; et al. The Mohawk homeobox gene is a critical regulator of tendon differentiation. Proc. Natl. Acad. Sci. USA 2010, 107, 10538–10542. [Google Scholar] [CrossRef] [Green Version]
- Chuang, H.N.; Hsiao, K.M.; Chang, H.Y.; Wu, C.C.; Pan, H. The homeobox transcription factor Irxl1 negatively regulates MyoD expression and myoblast differentiation. FEBS J. 2014, 281, 2990–3003. [Google Scholar] [CrossRef]
- Husi, H.; Skipworth, R.J.; Cronshaw, A.; Fearon, K.C.; Ross, J.A. Proteomic identification of potential cancer markers in human urine using subtractive analysis. Int. J. Oncol. 2016, 48, 1921–1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpintero, R.; Piñeiro, M.; Andrés, M.; Iturralde, M.; Alava, M.A.; Heegaard, P.M.; Jobert, J.L.; Madec, F.; Lampreave, F. The concentration of apolipoprotein A-I decreases during experimentally induced acute-phase processes in pigs. Infect. Immun. 2005, 73, 3184–3187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epand, R.M.; Stafford, A.; Leon, B.; Lock, P.E.; Tytler, E.M.; Segrest, J.P.; Anantharamaiah, G.M. HDL and apolipoprotein A-I protect erythrocytes against the generation of procoagulant activity. Arterioscler. Thromb. 1994, 14, 1775–1783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, J.A.; Deguchi, H.; Banka, C.L.; Witztum, J.L.; Griffin, J.H. Re-evaluation of the anticoagulant properties of high-density lipoprotein-brief report. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 570–572. [Google Scholar] [CrossRef] [Green Version]
- Bradwell, A.R. Serum free light chain measurements move to center stage. Clin. Chem. 2005, 51, 805–807. [Google Scholar] [CrossRef] [Green Version]
- Thio, M.; Blokhuis, B.R.; Nijkamp, F.P.; Redegeld, F.A. Free immunoglobulin light chains: A novel target in the therapy of inflammatory diseases. Trends. Pharmacol. Sci. 2008, 29, 170–174. [Google Scholar] [CrossRef]
- Gottenberg, J.E.; Aucouturier, F.; Goetz, J.; Sordet, C.; Jahn, I.; Busson, M.; Cayuela, J.M.; Sibilia, J.; Mariette, X. Serum immunoglobulin free light chain assessment in rheumatoid arthritis and primary Sjogren’s syndrome. Ann. Rheum. Dis. 2007, 66, 23–27. [Google Scholar] [CrossRef] [Green Version]
- Powe, D.G.; Groot Kormelink, T.; Sisson, M.; Blokhuis, B.J.; Kramer, M.F.; Jones, N.S.; Redegeld, F.A. Evidence for the involvement of free light chain immunoglobulins in allergic and nonallergic rhinitis. J. Allergy Clin. Immunol. 2010, 125, 139–145. [Google Scholar] [CrossRef]
- Rijnierse, A.; Redegeld, F.A.; Blokhuis, B.R.; Van der Heijden, M.W.; Te Velde, A.A.; Pronk, I.; Hommes, D.W.; Nijkamp, F.P.; Koster, A.S.; Kraneveld, A.D. Ig-free light chains play a crucial role in murine mast cell-dependent colitis and are associated with human inflammatory bowel diseases. J. Immunol. 2010, 185, 653–659. [Google Scholar] [CrossRef]
- Kayserova, J.; Capkova, S.; Skalicka, A.; Vernerova, E.; Polouckova, A.; Malinova, V.; Bartunkova, J.; Sediva, A. Serum immunoglobulin free light chains in severe forms of atopic dermatitis. Scand. J. Immunol. 2010, 71, 312–316. [Google Scholar] [CrossRef]
- Bibas, M.; Lorenzini, P.; Cozzi-Lepri, A.; Calcagno, A.; di Giambenedetto, S.; Costantini, A.; Castagna, A.; Manfrin, V.; Monforte, A.D.; Antinori, A.; et al. Polyclonal serum-free light chains elevation in HIV-infected patients. AIDS 2012, 26, 2107–2110. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.S.; Cabral, M.S.; Jesus, L.S.; Paraná, R.; Atta, A.M.; Sousa Atta, M.L. Serum levels of immunoglobulin free light chains in patients with chronic hepatitis C presenting cryoglobulinemia. Braz. J. Infect. Dis. 2014, 18, 638–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, B.J.; Huizinga, E.G.; Raaijmakers, H.C.; Roos, A.; Daha, M.R.; Nilsson-Ekdahl, K.; Nilsson, B.; Gros, P. Structures of complement component C3 provide insights into the function and evolution of immunity. Nature 2005, 437, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.W.; Kuo, Y.M. The surfaces of the parasitic nematodes Trichinella spiralis and Toxocara canis differ in the binding of post-C3 components of human complement by the alternative pathway. Parasite Immunol. 1988, 10, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Kim, C.W.; Ghebrehiwet, B. Trichinella spiralis: Activation of complement by infective larvae, adults, and newborn larvae. Exp. Parasitol. 1992, 74, 290–299. [Google Scholar] [CrossRef]
- Stankiewicz, M.; Sokolska, G.; Pilarczyk, A.; Sadowska, D.; Jeska, E.L. Lack of correlation between in vitro and in vivo activation of complement in mice by infective Trichinella spiralis larvae. J. Parasitol. 1989, 75, 647–649. [Google Scholar] [CrossRef]
- Näreaho, A.; Saari, S.; Meri, S.; Sukura, A. Complement membrane attack complex formation and infectivity of Trichinella spiralis and T. nativa in rats. Vet. Parasitol. 2009, 159, 263–267. [Google Scholar] [CrossRef]
- Zhao, L.; Shao, S.; Chen, Y.; Sun, X.; Sun, R.; Huang, J.; Zhan, B.; Zhu, X. Trichinella spiralis Calreticulin Binds Human Complement C1q As an Immune Evasion Strategy. Front. Immunol. 2017, 8, 636. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Morales, M.A.; Ludovisi, A.; Amati, M.; Cherchi, S.; Tonanzi, D.; Pozio, E. Differentiation of Trichinella species (Trichinella spiralis/Trichinella britovi versus Trichinella pseudospiralis) using western blot. Parasit. Vectors 2018, 11, 631. [Google Scholar] [CrossRef] [Green Version]
- Bruschi, F.; Marucci, G.; Pozio, E.; Masetti, M. Evaluation of inflammatory responses against muscle larvae of different Trichinella species by an image analysis system. Vet. Parasitol. 2009, 159, 258–262. [Google Scholar] [CrossRef]
- Zarlenga, D.S.; Chute, M.B.; Martin, A.; Kapel, C.M. A multiplex PCR for unequivocal differentiation of all encapsulated and non-encapsulated genotypes of Trichinella. Int. J. Parasitol. 1999, 29, 1859–1867. [Google Scholar] [CrossRef]
- Pink, M.; Verma, N.; Rettenmeier, A.W.; Schmitz-Spanke, S. CBB staining protocol with higher sensitivity and mass spectrometric compatibility. Electrophoresis 2010, 31, 593–598. [Google Scholar] [CrossRef] [PubMed]


| Experimental Group | Numbers of Trichinella Larvae/g Muscle (lpg) | ||||||
|---|---|---|---|---|---|---|---|
| Diaphragm (Pillars) | Tongue | P-Value | Diaphragm Pillars and Tongue (Together) | ||||
| Mean | SD | Mean | SD | Mean | Min–Max | ||
| T. spiralis (n = 6) | 89.52 a | 60.90 | 93.07 a | 40.63 | 0.9078 | 91.29 | 42.67–177.30 |
| T. britovi (n = 6) | 41.46 a | 20.28 | 37.75 a | 19.58 | 0.7536 | 39.60 | 22.26–77.01 |
| T. pseudospiralis (n = 6) | 34.20 a | 32.43 | 19.77 a | 21.68 | 0.3861 | 26.98 | 0.22–65.27 |
| Control (n = 6) | 0.00 | 0.00 | 0.00 | 0.00 | - | 0.00 | - |
| Spot No. | Accession No. UniProt/NCBI | Protein Name | CC | Fold Change | SC 1 (%)/ MS 2 | PM 3 | Theo. pI/Mw 4 | Exp. pI/Mw 5 |
|---|---|---|---|---|---|---|---|---|
| T. spiralis-infected group (T1) vs. Control | ||||||||
| 4801/1 | BAM66301 | IgM heavy-chain constant region, partial | E | −2.55 | 60/195 | 15 | 5.74/49.94 | 5.20/83.90 |
| 3402/2 | NP_001123430 | Antithrombin-III precursor | E | −1.52 | 49/184 | 20 | 5.84/52.87 | 4.40/56.10 |
| 9601/3 | AAA51295 | Immunoglobulin gamma-chain | E | 3.28 | 25/93 | 7 | 6.71/51.89 | 8.90/50.90 |
| 1602/4 | - | Unidentified protein | - | 5.29 | - | - | - | 4.10/39.90 |
| 2201/5 | Q29549 | Clusterin | E | −1.56 | 13/73 | 5 | 5.62/52.31 | 4.20/35.00 |
| T. britovi-infected group (T3) vs. Control | ||||||||
| 3801/1 | BAM66301 | IgM heavy-chain constant region, partial | E | 1.39 | 58/200 | 13 | 5.74/49.94 | 4.80/84.00 |
| 9008/2 | - | Unidentified protein | - | 1.08 | - | - | - | 7.60/23.90 |
| 9009/3 | XP_001507016 | Homeobox protein Mohawk, partial | N | 1.51 | 20/80 | 8 | 9.88/37.06 | 8.40/23.90 |
| T. pseudospiralis-infected group (T4) vs. Control | ||||||||
| 1602/1 | - | Unidentified protein | - | 3.25 | - | - | - | 4.10/39.90 |
| 0302/2 | Q29549 | Clusterin | E | −2.31 | 21/67 | 7 | 5.62/52.31 | 3.80/40.10 |
| 1202/3 | - | Unidentified protein | - | −2.59 | - | - | - | 4.10/35.70 |
| 2201/4 | Q29549 | Clusterin | E | −1.52 | 13/73 | 5 | 5.62/52.31 | 4.20/35.00 |
| 6101/5 | NP_999473 | Apolipoprotein E precursor | CP | −4.28 | 39/117 | 12 | 5.62/36.63 | 5.40/33.00 |
| 9116/6 | NP_999052 | Serum amyloid P-component precursor | E | −5.68 | 38/110 | 8 | 8.74/25.74 | 9.30/25.50 |
| Spot No. | Accession No. UniProt/NCBI | Protein Name | CC | Fold Change | SC 1 (%)/ MS 2 | PM 3 | Theo. pI/Mw 4 | Exp. pI/Mw 5 |
|---|---|---|---|---|---|---|---|---|
| T. spiralis-infected group (T1) vs. Control | ||||||||
| 1101/1 | P01846 | Ig lambda chain C region OS | E | 2.48 | 68/73 | 4 | 6.75/11.17 | 4.60/28.60 |
| 9007/2 | - | Unidentified protein | - | 14.32 | - | - | - | 9.60/26.60 |
| 9006/3 | - | Unidentified protein | - | 8.08 | - | - | - | 9.40/24.80 |
| 9001/4 | - | Unidentified protein | - | 14.87 | - | - | - | 9.50/13.60 |
| 9002/5 | - | Unidentified protein | - | 10.47 | - | - | - | 9.50/12.90 |
| T. britovi-infected group (T3) vs. Control | ||||||||
| 5101/1 | NP_999473 | Apolipoprotein E precursor | CP | −1.64 | 44/194 | 15 | 5.62/36.63 | 6.30/32.90 |
| 1101/2 | P01846 | Ig lambda chain C region OS | E | 2.21 | 68/73 | 4 | 6.75/11.17 | 4.60/28.60 |
| 3004/3 | - | Unidentified protein | - | 3.29 | - | - | - | 5.00/26.00 |
| 3005/4 | - | Unidentified protein | - | 10.40 | - | - | - | 4.80/21.70 |
| 9007/5 | - | Unidentified protein | - | 17.13 | - | - | - | 9.60/26.60 |
| 9002/6 | - | Unidentified protein | - | 28.03 | - | - | - | 9.50/12.90 |
| T. pseudospiralis-infected group (T4) vs. Control | ||||||||
| 4301/1 | XP_020936478 | Complement C3 isoform X1 | E | 1.53 | 11/83 | 13 | 5.99/19.30 | 5.20/43.30 |
| 3001/2 | AAA30992 | Apolipoprotein A-I | E | −1.87 | 51/219 | 18 | 5.38/30.31 | 4.90/23.50 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gondek, M.; Herosimczyk, A.; Knysz, P.; Ożgo, M.; Lepczyński, A.; Szkucik, K. Comparative Proteomic Analysis of Serum from Pigs Experimentally Infected with Trichinella spiralis, Trichinella britovi, and Trichinella pseudospiralis. Pathogens 2020, 9, 55. https://doi.org/10.3390/pathogens9010055
Gondek M, Herosimczyk A, Knysz P, Ożgo M, Lepczyński A, Szkucik K. Comparative Proteomic Analysis of Serum from Pigs Experimentally Infected with Trichinella spiralis, Trichinella britovi, and Trichinella pseudospiralis. Pathogens. 2020; 9(1):55. https://doi.org/10.3390/pathogens9010055
Chicago/Turabian StyleGondek, Michał, Agnieszka Herosimczyk, Przemysław Knysz, Małgorzata Ożgo, Adam Lepczyński, and Krzysztof Szkucik. 2020. "Comparative Proteomic Analysis of Serum from Pigs Experimentally Infected with Trichinella spiralis, Trichinella britovi, and Trichinella pseudospiralis" Pathogens 9, no. 1: 55. https://doi.org/10.3390/pathogens9010055
APA StyleGondek, M., Herosimczyk, A., Knysz, P., Ożgo, M., Lepczyński, A., & Szkucik, K. (2020). Comparative Proteomic Analysis of Serum from Pigs Experimentally Infected with Trichinella spiralis, Trichinella britovi, and Trichinella pseudospiralis. Pathogens, 9(1), 55. https://doi.org/10.3390/pathogens9010055





