Currently Available Monitoring and Surveillance Systems for Taenia spp., Echinococcus spp., Schistosoma spp., and Soil-Transmitted Helminths at the Control/Elimination Stage: A Systematic Review
Abstract
1. Introduction
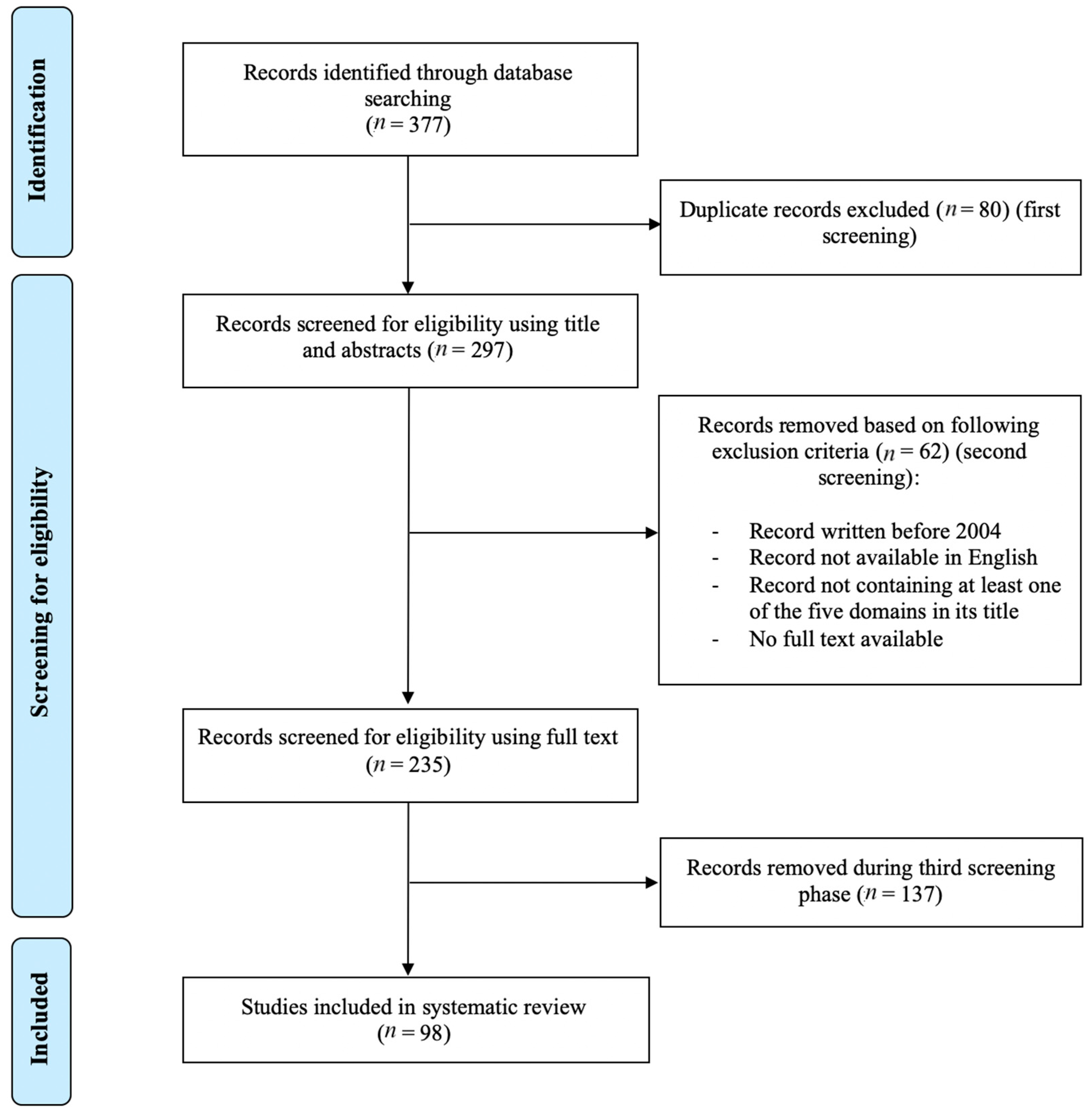
2. Methods
2.1. Review Question, Search Literature, and Literature Sources
- (Taenia solium OR Taenia saginata OR tapeworm OR taeniosis OR taeniasis OR cysticercosis) AND (surveillance OR surveil OR survey OR monitoring OR monitor).
- (Schistosoma OR schistosome OR bilharzia OR bilharziasis) AND (surveillance OR surveil OR survey OR monitoring OR monitor).
- (Echinococcus OR echinococcosis) AND (surveillance OR surveil OR survey OR monitoring OR monitor).
- (STHs OR soil-transmitted helminths OR soil-transmitted helminthiasis) AND (surveillance OR surveil OR survey OR monitoring OR monitor).
2.2. Study Selection
3. Results/Discussion
3.1. Schistosoma spp.
3.1.1. Introduction
3.1.2. Surveillance of the Snail Intermediate Host
3.1.3. Monitoring and Surveillance for Human Schistosomiasis Cases
3.1.4. Monitoring and Surveillance of Livestock
3.1.5. Monitoring and Surveillance of Schistosoma-Infected Water
3.1.6. Integrated Monitoring and Surveillance Systems for Schistosomiasis
3.1.7. Monitoring and Surveillance for Schistosoma mansoni in Egypt
3.1.8. Future Challenges and Recommendations for Schistosomiasis
3.2. Soil-Transmitted Helminths
3.2.1. Introduction
3.2.2. Monitoring and Surveillance of Soil-Transmitted Helminths
3.3. Echinococcus spp.
3.3.1. Introduction
3.3.2. Monitoring and Surveillance of Echinococcus spp.
3.4. Taenia spp.
3.4.1. Introduction
3.4.2. Monitoring and Surveillance of Taenia solium
3.4.3. Monitoring and Surveillance of Taenia saginata
3.5. Integrated Monitoring and Surveillance of Neglected Tropical Diseases
3.6. Future Challenges and Recommendations
3.6.1. The Case for Strengthening Diagnostic Capacity
3.6.2. The Emergence of Drug Resistance
3.6.3. The Introduction of Spatial Technology
3.6.4. The lack of data for Taenia spp. and Echinococcus spp.
3.7. Limitations of Systematic Literature Review
4. Conclusions and Global Lessons
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, X.N.; Bergquist, R.; Tanner, M. Elimination of tropical disease through surveillance and response. Infect. Dis. Poverty 2013, 2, 1:1–1:5. [Google Scholar] [CrossRef]
- Dorny, P.; Flisser, A.; Geerts, S.; Kyvsgaard, N.C.; McManus, D.; Nash, T.; Pawlowski, Z.; World Organisation for Animal Health (OIE). WHO/FAO/OIE Guidelines for the Surveillance, Prevention and Control of Taeniosis/Cysticercosis. Available online: https://www.who.int/taeniasis/resources/9290446560/en/ (accessed on 16 October 2019).
- Bergquist, R.; Yang, G.J.; Knopp, S.; Utzinger, J.; Tanner, M. Surveillance and response: Tools and approaches for the elimination stage of neglected tropical diseases. Act. Trop. 2015, 141, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Murrell, K.D. Zoonotic foodborne parasites and their surveillance. Rev. Sci. Tech. 2013, 32, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J. Epidemiological concepts regarding disease monitoring and surveillance. Act. Vet. Scand. 2001, 94, S1. [Google Scholar] [CrossRef] [PubMed]
- Gyorkos, T.W. Monitoring and evaluation of large-scale helminth control programmes. Act. Trop. 2003, 86, 275–282. [Google Scholar] [CrossRef]
- Tambo, E.; Ai, L.; Zhou, X.; Chen, J.H.; Hu, W.; Bergquist, R.; Guo, J.G.; Utzinger, J.; Tanner, M.; Zhou, X.N. Surveillance-response systems: The key to elimination of tropical diseases. Infect. Dis. Poverty 2014, 5, 49:1–49:12. [Google Scholar] [CrossRef]
- PRISMA-P Group; Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1:1–1:9. [Google Scholar] [CrossRef]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef]
- Sun, L.P.; Wang, W.; Zuo, Y.P.; Zhang, Z.Q.; Hong, Q.B.; Yang, G.J.; Zhu, H.R.; Liang, Y.S.; Yang, H.T. An integrated environmental improvement of marshlands: Impact on control and elimination of schistosomiasis in marshland regions along the Yangtze River, China. Infect. Dis. Poverty 2017, 6, 1:1–1:10. [Google Scholar] [CrossRef]
- Rollinson, D.; Knopp, S.; Levitz, S.; Stothard, J.R.; Tchuem Tchuenté, L.A.; Garba, A.; Mohammed, K.A.; Schur, N.; Person, B.; Colley, D.G.; et al. Time to set the agenda for schistosomiasis elimination. Act. Trop. 2013, 128, 423–440. [Google Scholar] [CrossRef]
- Bergquist, R.; Zhou, X.N.; Rollinson, D.; Reinhard-Rupp, J.; Klohe, K. Elimination of schistosomiasis: The tools required. Infect. Dis. Poverty 2017, 6, 158:1–158:9. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huang, S.Y.; Pei, F.Q.; Chen, Y.; Jiang, Q.W.; Deng, Z.H.; Zhou, Y.B. Spatial distribution and habitat suitability of Biomphalaria straminea, intermediate host of Schistosoma mansoni, in Guangdong, China. Infect. Dis. Poverty 2018, 7, 109:1–109:10. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.L.; Wang, J.R.; Wen, L.Y.; Huang, Y.Y.; Xu, X.F.; Yu, W.M. Surveillance and control of post-transmission schistosomiasis in Jiaxing prefecture, Zhejiang province, China. Act. Trop. 2005, 96, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Li, S.Z.; Wen, L.Y.; Lin, D.D.; Abe, E.M.; Zhu, R.; Du, Y.; Lv, S.; Xu, J.; Webster, B.L.; et al. The establishment and function of schistosomiasis surveillance system towards elimination in The People’s Republic of China. Adv. Parasit. 2016, 92, 117–141. [Google Scholar] [CrossRef]
- Grobusch, M.P.; Mühlberger, N.; Jelinek, T.; Bisoffi, Z.; Corachán, M.; Harms, G.; Matteelli, A.; Fry, G.; Hatz, C.; Gjørup, E.; et al. Imported schistosomiasis in Europe: Sentinel surveillance data from TropNetEurop. J. Travel Med. 2006, 10, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Angeles, J.M.M.; Leonardo, L.R.; Goto, Y.; Kirinoki, M.; Villacorte, E.A.; Hakimi, H.; Moendeg, K.J.; Lee, S.; Rivera, P.T.; Inoue, N.; et al. Water buffalo as sentinel animals for schistosomiasis surveillance. Bull. World Health Organ. 2015, 93, 511–512. [Google Scholar] [CrossRef]
- Sun, L.P.; Liang, Y.S.; Wu, H.H.; Tian, Z.X.; Dai, J.R.; Yang, K.; Hong, Q.B.; Zhou, X.N.; Yang, G.J. A Google Earth-based surveillance system for schistosomiasis japonica implemented in the lower reaches of the Yangtze River, China. Parasit. Vectors 2011, 4, 223:1–223:8. [Google Scholar] [CrossRef]
- Sun, L.P.; Wang, W.; Hong, Q.B.; Li, S.Z.; Liang, Y.S.; Yang, H.T.; Zhou, X.N. Approaches being used in the national schistosomiasis elimination programme in China: A review. Infect. Dis. Poverty 2017, 6, 55:1–55:9. [Google Scholar] [CrossRef]
- McManus, D.P.; Gordon, C.; Weerakoon, K.G.A.D. Testing of water samples for environmental DNA as a surveillance tool to assess the risk of schistosome infection in a locality. Int. J. Infect. Dis. 2018, 76, 128–129. [Google Scholar] [CrossRef]
- Zhao, G.M.; Zhao, Q.; Jiang, Q.W.; Chen, X.Y.; Wang, L.Y.; Yuan, H.C. Surveillance for schistosomiasis japonica in China from 2000 to 2003. Act. Trop. 2005, 96, 288–295. [Google Scholar] [CrossRef]
- Liang, S.; Yang, C.; Zhong, B.; Guo, J.; Li, H.; Carlton, E.J.; Freeman, M.C.; Remais, J.V. Surveillance systems for neglected tropical diseases: Global lessons from China’s evolving schistosomiasis reporting systems, 1949–2014. Emerg. Themes Epidemiol. 2014, 11, 19:1–19:14. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.H.; Ming-Gang, C.; Jiang, Z. Surveillance of schistosomiasis in five provinces of China which have reached the national criteria for elimination of the disease. Act. Trop. 2005, 96, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.J.; Li, S.Z.; Xu, J.; Yang, K.; Huang, Y.X.; Cao, Z.G.; Miu, F.; Dang, H.; Zhang, L.J.; Wang, Q.; et al. Potential schistosomiasis foci in China: A prospective study for schistosomiasis surveillance and response. Act. Trop. 2015, 141, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; Xu, J.; Li, S.Z.; Cao, Z.G.; Huang, Y.X.; Wu, C.G.; Tu, Z.W.; Zhou, X.N. Monitoring the transmission of Schistosoma japonicum in potential risk regions of China, 2008–2012. Int. J. Environ. Res. Public Health 2014, 11, 2278–2287. [Google Scholar] [CrossRef]
- Abou-El-Naga, I.F. Towards elimination of schistosomiasis after 5000 years of endemicity in Egypt. Act. Trop. 2018, 181, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Haggag, A.A.; Rabiee, A.; Abd Elaziz, K.M.; Gabrielli, A.F.; Abdel Hay, R.; Ramzy, R.M.R. Mapping of Schistosoma mansoni in the Nile Delta, Egypt: Assessment of the prevalence by the circulating cathodic antigen urine assay. Act. Trop. 2017, 167, 9–17. [Google Scholar] [CrossRef] [PubMed]
- GeoSentinel Surveillance Network; Schwartz, E.; Von Sonnenburg, F.; Reed, C.; Weld, L.H.; Nicolls, D.J.; Kozarsky, P.E. Characteristics of schistosomiasis in travelers reported to the GeoSentinel Surveillance Network 1997–2008. Am. J. Trop. Med. Hyg. 2008, 79, 729–734. [Google Scholar] [CrossRef]
- Cao, C.; Bao, Z.; Li, S.; Wei, W.; Yi, P.; Yu, Q.; Zhu, H.Q.; Xu, J.; Guo, J.G.; Feng, Z. Schistosomiasis in a migrating population in the lake region of China and its potential impact on control operation. Act. Trop. 2015, 145, 88–92. [Google Scholar] [CrossRef]
- Boissier, J.; Grech-Angelini, S.; Webster, B.L.; Allienne, J.F.; Huyse, T.; Mas-Coma, S.; Toulza, E.; Barré-Cardi, H.; Rollinson, D.; Kincaid-Smith, J.; et al. Outbreak of urogenital schistosomiasis in Corsica (France): An epidemiological case study. Lancet Infect. Dis. 2016, 16, 971–979. [Google Scholar] [CrossRef]
- Zeng, H.; Yang, X.; Meng, S.; Wang, H.; Tang, X.; Tang, W.; Zeng, S.; Jeschke, S.; Wang, Y. Awareness and knowledge of schistosomiasis infection and prevention in the “Three Gorges Dam” reservoir area: A cross-sectional study on local residents and health personnel. Act. Trop. 2011, 120, 238–244. [Google Scholar] [CrossRef]
- Shaikh, N.; Rahman-Shepherd, A.; Dar, O. Schistosomiasis in the Senegal River basin. Lancet Planet. Health 2018, 2, S27. [Google Scholar] [CrossRef]
- Carlton, E.J.; Bates, M.N.; Zhong, B.; Seto, E.Y.W.; Spear, R.C. Evaluation of mammalian and intermediate host surveillance methods for detecting schistosomiasis re-emergence in southwest China. PLoS Negl. Trop. Dis. 2011, 5, e987. [Google Scholar] [CrossRef] [PubMed]
- Jex, A.R.; Lim, Y.A.L.; Bethony, J.M.; Hotez, P.J.; Young, N.D.; Gasser, R.B. Soil-transmitted helminths of humans in southeast Asia—Towards integrated control. Adv. Parasit. 2011, 74, 231–265. [Google Scholar] [CrossRef]
- Montresor, A. World Health Organization 2012. Eliminating Soil-Transmitted Helminthiases as a Public Health Problem in Children: Progress Report 2001–2010 and Strategic Plan 2011–2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/44804/9789241503129_eng.pdf?sequence=1 (accessed on 19 October 2019).
- Montresor, A. World Health Organization 2011. In Helminth Control in School-Age Children: A Guide for Managers of Control Programmes, 2nd ed.; World Health Organization: Geneva, Switzerland, 2011; Available online: https://apps.who.int/iris/bitstream/handle/10665/44671/9789241548267_eng.pdf?sequence=1 (accessed on 19 October 2019).
- Kim, T.S.; Cho, S.H.; Huh, S.; Kong, Y.; Sohn, W.M.; Hwang, S.S.; Chai, J.Y.; Lee, S.H.; Park, Y.K.; Oh, D.K.; et al. A nationwide survey on the prevalence of intestinal parasitic infections in The Republic of Korea, 2004. Korean J. Parasitol. 2009, 47, 37–47. [Google Scholar] [CrossRef]
- Bahk, Y.Y.; Park, Y.K.; Na, B.K.; Sohn, W.M.; Hong, S.J.; Chai, J.Y.; Kim, T.S. Survey on intestinal helminthic infection status of students in two counties, Hadong-gun and Goseong-gun, Korea. Korean J. Parasitol. 2018, 56, 335–339. [Google Scholar] [CrossRef]
- Gunawardena, S.; Gunawardena, N.K.; Kahathuduwa, G.; Karunaweera, N.D.; de Silva, N.R.; Ranasinghe, U.B.; Samarasekara, S.D.; Nagodavithana, K.C.; Rao, R.U.; Rebello, M.P.; et al. Integrated school-based surveillance for soil-transmitted helminth infections and lymphatic filariasis in Gampaha District, Sri Lanka. Am. J. Trop. Med. Hyg. 2014, 90, 661–666. [Google Scholar] [CrossRef][Green Version]
- Smith, J.L.; Sturrock, H.J.W.; Assefa, L.; Nikolay, B.; Njenga, S.M.; Kihara, J.; Mwandawiro, C.S.; Brooker, S.J. Factors associated with the performance and cost-effectiveness of using lymphatic filariasis transmission assessment surveys for monitoring soil-transmitted helminths: A case study in Kenya. Am. J. Trop. Med. Hyg. 2015, 92, 342–353. [Google Scholar] [CrossRef]
- Truscott, J.E.; Dunn, J.C.; Papaiakovou, M.; Schaer, F.; Werkman, M.; Littlewood, D.T.J.; Walson, J.L.; Anderson, R.M. Calculating the prevalence of soil-transmitted helminth infection through pooling of stool samples: Choosing and optimizing the pooling strategy. PLoS Negl. Trop. Dis. 2019, 13, e0007196. [Google Scholar] [CrossRef]
- Leta, G.T.; French, M.; Dorny, P.; Vercruysse, J.; Levecke, B. Comparison of individual and pooled diagnostic examination strategies during the national mapping of soil-transmitted helminths and Schistosoma mansoni in Ethiopia. PLoS Negl. Trop. Dis. 2018, 12, e0006723. [Google Scholar] [CrossRef]
- Eckert, J.; Deplazes, P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin. Microbiol. Rev. 2004, 17, 107–135. [Google Scholar] [CrossRef]
- Phythian, C.; Jackson, B.; Bell, R.; Citer, L.; Barwell, R.; Windsor, P. Abattoir surveillance of Sarcocystis spp., Cysticercosis ovis and Echinococcus granulosus in Tasmanian slaughter sheep, 2007–2013. Aust. Vet. J. 2018, 96, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Lind, E.O.; Juremalm, M.; Christensson, D.; Widgren, S.; Hallgren, G.; Erik, A.A.; Uhlhorn, H.; Lindberg, A.; Cedersmyg, M.; Wahlström, H. First detection of Echinococcus multilocularis in Sweden, February to March 2011. Eurosurveillance 2011, 16, 696–705. [Google Scholar] [CrossRef]
- Wahlström, H.; Enemark, H.L.; Davidson, R.K.; Oksanen, A. Present status, actions taken and future considerations due to the findings of E. multilocularis in two Scandinavian countries. Vet. Parasitol. 2015, 213, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Madslien, K.; Øines, Ø.; Handeland, K.; Urdahl, A.M.; Albin-Amiot, C.; Jore, S.; Norwegian Veterinary Institute. The Surveillance Programme for Echinococcus Multilocularis in Red Foxes (Vulpes Vulpes) in Norway in 2014. Available online: https://www.vetinst.no/en/surveillance-programmes/echinococcus-in-red-foxes (accessed on 29 August 2019).
- European Food Safety Authority. Annual Assessment of Echinococcus multilocularis Surveillance Reports Submitted in 2018 in the Context of Commission Regulation (EU) No 1152/2011. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/5486 (accessed on 29 August 2019).
- Irie, T.; Mukai, T.; Yagi, K. Echinococcus multilocularis surveillance using copro-DNA and egg examination of shelter dogs from an endemic area in Hokkaido, Japan. Vector-Borne Zoonot. Dis. 2018, 18, 390–392. [Google Scholar] [CrossRef]
- Hobbs, E.C.; Mwape, K.E.; Devleesschauwer, B.; Gabriël, S.; Chembensofu, M.; Mambwe, M.; Phiri, I.K.; Masuku, M.; Zulu, G.; Colston, A.; et al. Taenia solium from a community perspective: Preliminary costing data in the Katete and Sinda districts in Eastern Zambia. Vet. Parasitol. 2018, 251, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, C.; Sotiraki, S.; Laranjo-González, M.; Dermauw, V.; Wang, Z.; Kärssin, A.; Cvetkovikj, A.; Winkler, A.S.; Abraham, A.; Bobic, B.; et al. Epidemiology of taeniosis/cysticercosis in Europe, a systematic review: Eastern Europe. Parasit. Vectors 2018, 11, 569:1–569:11. [Google Scholar] [CrossRef]
- Okello, A.L.; Thomas, L.F. Human taeniasis: Current insights into prevention and management strategies in endemic countries. Risk Manag. Health Policy 2017, 10, 107–116. [Google Scholar] [CrossRef]
- Fonseca, A.G.; Torgal, J.; de Meneghi, D.; Gabriël, S.; Coelho, A.C.; Vilhena, M. One Health-ness evaluation of cysticercosis surveillance design in Portugal. Front. Public Health 2018, 6, 74:1–74:10. [Google Scholar] [CrossRef]
- Priest, J.W.; Jenks, M.H.; Moss, D.M.; Mao, B.; Buth, S.; Wannemuehler, K.; Soeung, S.C.; Lucchi, N.W.; Udhayakumar, V.; Gregory, C.J.; et al. Integration of multiplex bead assays for parasitic diseases into a national, population-based serosurvey of women 15–39 years of age in Cambodia. PLoS Negl. Trop. Dis. 2016, 10, e0004699. [Google Scholar] [CrossRef]
- Cysticercosis Working Group in Peru; O’Neal, S.E.; Moyano, L.M.; Ayvar, V.; Rodriguez, S.; Gavidia, C.; Wilkins, P.P.; Gilman, R.H.; Garcia, H.H.; Gonzalez, A.E. Ring-screening to control endemic transmission of Taenia solium. PLoS Negl. Trop. Dis. 2014, 8, e3125. [Google Scholar] [CrossRef]
- Abuseir, S.; Epe, C.; Schnieder, T.; Klein, G.; Kühne, M. Visual diagnosis of Taenia saginata cysticercosis during meat inspection: Is it unequivocal? Parasitol. Res. 2006, 99, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Jansen, F.; Dorny, P.; Berkvens, D.; Van Hul, A.; Van den Broeck, N.; Makay, C.; Praet, N.; Eichenberger, R.M.; Deplazes, P.; Gabriël, S. High prevalence of bovine cysticercosis found during evaluation of different post-mortem detection techniques in Belgian slaughterhouses. Vet. Parasitol. 2017, 244, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dorny, P.; Praet, N. Taenia saginata in Europe. Vet. Parasitol. 2007, 149, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, C.; Morlot, C.; Demont, P.; Ducrot, C.; Calavas, D.; Callait-Cardinal, M.P.; Gay, E. Construction of standardized surveillance indicators for bovine cysticercosis. Prev. Vet. Med. 2014, 115, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Marshall, L.R.; Prakashbabu, B.C.; Ferreira, J.P.; Buzdugan, S.N.; Stärk, K.D.C.; Guitian, J. Risk factors for Taenia saginata cysticercus infection in cattle in the United Kingdom: A farm-level case-control study and assessment of the role of movement history, age and sex. Prev. Vet. Med. 2016, 135, 1–8. [Google Scholar] [CrossRef]
- Blagojevic, B.; Robertson, L.J.; Vieira-Pinto, M.; Johansen, M.V.; Laranjo-González, M.; Gabriël, S. Bovine cysticercosis in the European Union: Impact and current regulations, and an approach towards risk-based control. Food Cont. 2017, 78, 64–71. [Google Scholar] [CrossRef]
- Cassini, R.; Mulatti, P.; Zanardello, C.; Simonato, G.; Signorini, M.; Cazzin, S.; Tambalo, P.; Cobianchi, M.; Pietrobelli, M.; Capelli, G. Retrospective and spatial analysis tools for integrated surveillance of cystic echinococcosis and bovine cysticercosis in hypo-endemic areas. Geospat. Health 2014, 8, 509–515. [Google Scholar] [CrossRef]
- Fürst, T.; Ouattara, M.; Silué, K.D.; N’Goran, D.N.; Adiossan, L.G.; Bogoch, I.I.; N’Guessan, Y.; Koné, S.; Utzinger, J.; N’Goran, E.K. Scope and limits of an anamnestic questionnaire in a control-induced low-endemicity helminthiasis setting in South-Central Côte d’Ivoire. PLoS ONE 2013, 8, e64380. [Google Scholar] [CrossRef]
- Cunningham, L.J.; Odoom, J.; Pratt, D.; Boatemaa, L.; Asante-Ntim, N.; Attiku, K.; Banahene, B.; Osei-Atweneboana, M.; Verweij, J.J.; Molyneux, D.; et al. Expanding molecular diagnostics of helminthiasis: Piloting use of the GPLN platform for surveillance of soil transmitted helminthiasis and schistosomiasis in Ghana. PLoS Negl. Trop. Dis. 2018, 12, e0006129. [Google Scholar] [CrossRef]
- Spear, R.C.; Seto, E.Y.W.; Carlton, E.J.; Liang, S.; Remais, J.V.; Zhong, B.; Qiu, D. The challenge of effective surveillance in moving from low transmission to elimination of schistosomiasis in China. Int. J. Parasit. 2011, 41, 1243–1247. [Google Scholar] [CrossRef]
- Isaksson, M.; Hagström, Å.; Armua-Fernandez, M.T.; Wahlström, H.; Ågren, E.O.; Miller, A.; Holmberg, A.; Lukacs, M.; Casulli, A.; Deplazes, P.; et al. A semi-automated magnetic capture probe-based DNA extraction and real-time PCR method applied in the Swedish surveillance of Echinococcus multilocularis in red fox (Vulpes vulpes) faecal samples. Parasit. Vectors 2014, 7, 583:1–583:10. [Google Scholar] [CrossRef] [PubMed]
- French, M.D.; Rollinson, D.; Basáñez, M.G.; Mgeni, A.F.; Khamis, I.S.; Stothard, J.R. School-based control of urinary schistosomiasis on Zanzibar, Tanzania: Monitoring micro-haematuria with reagent strips as a rapid urological assessment. J. Pediatr. Urol. 2007, 3, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lu, L.; Zhang, L.; Bai, Y.; Medina, A.; Rozelle, S.; Smith, D.S.; Zhou, C.; Zang, W. More poop, more precision: Improving epidemiologic surveillance of soil-transmitted helminths with multiple fecal sampling using the Kato–Katz Technique. Am. J. Trop. Med. Hyg. 2017, 97, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Turner, H.C.; Bettis, A.A.; Dunn, J.C.; Whitton, J.M.; Hollingsworth, T.D.; Fleming, F.M.; Anderson, R.M. Economic considerations for moving beyond the Kato-Katz Technique for diagnosing intestinal parasites as we move towards elimination. Trends Parasitol. 2017, 33, 435–443. [Google Scholar] [CrossRef]
- Knopp, S.; Rinaldi, L.; Khamis, I.S.; Stothard, J.R.; Rollinson, D.; Maurelli, M.P.; Steinmann, P.; Marti, H.; Cringoli, G.; Utzinger, J. A single FLOTAC is more sensitive than triplicate Kato–Katz for the diagnosis of low-intensity soil-transmitted helminth infections. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 347–354. [Google Scholar] [CrossRef]
- Koukounari, A.; Donnelly, C.A.; Moustaki, I.; Tukahebwa, E.M.; Kabatereine, N.B.; Wilson, S.; Webster, J.P.; Deelder, A.M.; Vennervald, B.J.; van Dam, G.J. A latent Markov modelling approach to the evaluation of circulating cathodic antigen strips for schistosomiasis diagnosis pre- and post-praziquantel treatment in Uganda. PLoS Comput. Biol. 2013, 9, e1003402. [Google Scholar] [CrossRef]
- Adriko, M.; Standley, C.J.; Tinkitina, B.; Tukahebwa, E.M.; Fenwick, A.; Fleming, F.M.; Sousa-Figueiredo, J.C.; Stothard, J.R.; Kabatereine, N.B. Evaluation of circulating cathodic antigen (CCA) urine-cassette assay as a survey tool for Schistosoma mansoni in different transmission settings within Bugiri District, Uganda. Act. Trop. 2014, 136, 50–57. [Google Scholar] [CrossRef]
- Knopp, S.; Corstjens, P.L.A.M.; Koukounari, A.; Cercamondi, C.I.; Ame, S.M.; Ali, S.M.; de Dood, C.J.; Mohammed, K.A.; Utzinger, J.; Rollinson, D.; et al. Sensitivity and specificity of a urine circulating anodic antigen test for the diagnosis of Schistosoma haematobium in low endemic settings. PLoS Negl. Trop. Dis. 2015, 9, e0003752. [Google Scholar] [CrossRef]
- Mubanga, C.; Mwape, K.E.; Phiri, I.K.; Trevisan, C.; Zulu, G.; Chabala, C.; van Damme, I.; Schmidt, V.; Dorny, P.; Gabriël, S. Progress on the development of rapid diagnostic tests for foodborne neglected zoonotic helminthiases: A systematic review. Act. Trop. 2019, 194, 135–147. [Google Scholar] [CrossRef]
- Angeles, J.M.; Goto, Y.; Kirinoki, M.; Leonardo, L.; Tongol-Rivera, P.; Villacorte, E.; Inoue, N.; Chigusa, Y.; Kawazu, S. Human antibody response to thioredoxin peroxidase-1 and tandem repeat proteins as immunodiagnostic antigen candidates for Schistosoma japonicum infection. Am. J. Trop. Med. Hyg. 2011, 85, 674–679. [Google Scholar] [CrossRef]
- Zhou, X.N.; Xu, J.; Chen, H.G.; Wang, T.P.; Huang, X.B.; Lin, D.D.; Wang, Q.Z.; Tang, L.; Guo, J.G.; Wu, X.H.; et al. Tools to support policy decisions related to treatment strategies and surveillance of schistosomiasis Japonica towards elimination. PLoS Negl. Trop. Dis. 2011, 5, e1408. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Yang, H.; Feng, Y.; Yang, X.; Zhu, Y. Magnetic affinity enzyme-linked immunoassay based on recombinant 26 kDa glutathione-S-transferase for serological diagnosis of schistosomiasis japonica. Act. Trop. 2012, 124, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Morel, N.; Lassabe, G.; Elola, S.; Bondad, M.; Herrera, S.; Marí, C.; Last, J.A.; Jensen, O.; Gonzalez-Sapienza, G. A monoclonal antibody-based copro-ELISA kit for canine echinococcosis to support the PAHO effort for hydatid disease control in South America. PLoS Negl. Trop. Dis. 2013, 7, e1967. [Google Scholar] [CrossRef] [PubMed]
- Moendeg, K.J.; Angeles, J.M.M.; Goto, Y.; Leonardo, L.R.; Kirinoki, M.; Villacorte, E.A.; Rivera, P.T.; Inoue, N.; Chigusa, Y.; Kawazu, S. Development and optimization of cocktail-ELISA for a unified surveillance of zoonotic schistosomiasis in multiple host species. Parasitol. Res. 2015, 114, 1225–1228. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, R.L.; Jannotti-Passos, L.K.; Dos Santos Carvalho, O. Use of molecular methods for the rapid mass detection of Schistosoma mansoni (Platyhelminthes: Trematoda) in Biomphalaria spp. (Gastropoda: Planorbidae). J. Trop. Med. 2017, 2017, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.W.; McManus, D.P.; Lou, Z.Z.; Yang, J.F.; Yan, H.B.; Li, L.; Li, H.M.; Liu, Q.Y.; Li, C.H.; Shi, W.G.; et al. A comparison of Loop-Mediated Isothermal Amplification (LAMP) with other surveillance tools for Echinococcus granulosus diagnosis in canine definitive hosts. PLoS ONE 2014, 9, e100877. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.A.; Gray, D.J.; Gobert, G.N.; McManus, D.P. DNA amplification approaches for the diagnosis of key parasitic helminth infections of humans. Mol. Cell. Probes 2011, 25, 143–152. [Google Scholar] [CrossRef]
- Mohammed, A.S.; Daubenberger, C.; Benninghoff, M.; Knopp, S.; Schindler, T.; Rothen, J.; Lweno, O.; Mohammed, A.S.; Singo, R.; Benninghoff, M.; et al. Diagnostic accuracy of Kato–Katz, FLOTAC, Baermann, and PCR Methods for the detection of light-intensity hookworm and Strongyloides stercoralis infections in Tanzania. Am. J. Trop. Med. Hyg. 2014, 90, 535–545. [Google Scholar] [CrossRef]
- Gordon, C.A.; Acosta, L.P.; Gobert, G.N.; Olveda, R.M.; Ross, A.G.; Williams, G.M.; Gray, D.J.; Harn, D.; Li, Y.; McManus, D.P. Real-time PCR demonstrates high prevalence of Schistosoma japonicum in the philippines: Implications for surveillance and control. PLoS Negl. Trop. Dis. 2015, 9, e0003483. [Google Scholar] [CrossRef]
- Hessler, M.J.; Cyrs, A.; Krenzke, S.C.; Mahmoud, E.S.; Sikasunge, C.; Mwansa, J.; Lodh, N. Detection of duo-schistosome infection from filtered urine samples from school children in Zambia after MDA. PLoS ONE 2017, 12, e0189400. [Google Scholar] [CrossRef]
- Weerakoon, K.G.; Gordon, C.A.; Williams, G.M.; Cai, P.; Gobert, G.N.; Olveda, R.M.; Ross, A.G.; Olveda, D.U.; McManus, D.P. Droplet digital PCR diagnosis of Human schistosomiasis: Parasite cell-free DNA detection in diverse clinical samples. J. Infect. Dis. 2017, 216, 1611–1622. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Duan, Z.L.; Guan, Z.N.; Wang, Y.Y.; Lin, C.; Zhang, T.T.; Zhang, H.Q.; Qian, X.; Xia, C.M. Early detection of circulating DNA of Schistosoma japonicum in sentinel mice models. Exp. Parasitol. 2017, 176, 82–88. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Gordon, C.A.; Williams, G.M.; Li, Y.; Wang, Y.; Hu, J.; Gray, D.J.; Ross, A.G.; Harn, D.; McManus, D.P. Real-time PCR diagnosis of Schistosoma japonicum in low transmission areas of China. Infect. Dis. Poverty 2018, 7, 8:1–8:11. [Google Scholar] [CrossRef] [PubMed]
- Corstjens, P.L.A.M.; Hoekstra, P.T.; de Dood, C.J.; van Dam, G.J. Utilizing the ultrasensitive Schistosoma up-converting phosphor lateral flow circulating anodic antigen (UCP-LF CAA) assay for sample pooling-strategies. Infect. Dis. Poverty 2017, 6, 155:1–155:13. [Google Scholar] [CrossRef] [PubMed]
- Poulton, K.; Webster, B. Development of a lateral flow recombinase polymerase assay for the diagnosis of Schistosoma mansoni infections. Anal. Biochem. 2018, 546, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Hamburger, J.; Abbasi, I.; Kariuki, C.; Wanjala, A.; Mzungu, E.; Mungai, P.; King, C.H. Evaluation of Loop-Mediated Isothermal Amplification suitable for molecular monitoring of schistosome-infected snails in field laboratories. Am. J. Trop. Med. Hyg. 2013, 88, 344–351. [Google Scholar] [CrossRef]
- Fernández-Soto, P.; Gandasegui, A.J.; Sánchez, H.A.; López, A.J.; Vicente, S.B.; Muro, A. A Loop-Mediated isothermal amplification (LAMP) assay for early detection of schistosoma mansoni in stool samples: A diagnostic approach in a murine model. PLoS Negl. Trop. Dis. 2014, 8, e3126. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, W.; Qiu, J.; Chen, X.; Yang, Y.; Qiu, D.; Xiao, N.; Xiao, Y.; Heath, D. A modified coproantigen test used for surveillance of echinococcus spp. in Tibetan dogs. Vet. Parasitol. 2007, 149, 229–238. [Google Scholar] [CrossRef]
- Dunn, J.C.; Turner, H.C.; Tun, A.; Anderson, R.M. Epidemiological surveys of, and research on, soil-transmitted helminths in Southeast Asia: A systematic review. Parasit. Vectors 2016, 9, 31:1–31:13. [Google Scholar] [CrossRef]
- Hoekendijk, D.J.L.; Hill, P.C.; Sowerby, S.J. Rationale for quality assurance in fecal egg monitoring of soil-transmitted helminthiasis. Am. J. Trop. Med. Hyg. 2016, 95, 502–504. [Google Scholar] [CrossRef]
- Tchuem Tchuenté, L.A. Control of soil-transmitted helminths in sub-Saharan Africa: Diagnosis, drug efficacy concerns and challenges. Act. Trop. 2011, 120, S4–S11. [Google Scholar] [CrossRef] [PubMed]
- Halder, J.B.; Benton, J.; Julé, A.M.; Guérin, P.J.; Olliaro, P.L.; Basáñez, M.G.; Walker, M. Systematic review of studies generating individual participant data on the efficacy of drugs for treating soil-transmitted helminthiases and the case for data-sharing. PLoS Negl. Trop. Dis. 2017, 11, e0006053. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.; Mabud, T.S.; Olliaro, P.L.; Coulibaly, J.T.; King, C.H.; Raso, G.; Scherrer, A.U.; Stothard, J.R.; Sousa-Figueiredo, J.C.; Stete, K.; et al. New approaches to measuring anthelminthic drug efficacy: Parasitological responses of childhood schistosome infections to treatment with praziquantel. Parasit. Vectors 2016, 9, 41:1–41:15. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cheng, W.; Wu, X.; Huang, S.; Deng, Z.; Zeng, X.; Yuan, D.; Yang, Y.; Wu, Z.; Chen, Y.; et al. Prediction of the potential global distribution for Biomphalaria straminea, an intermediate host for Schistosoma mansoni. PLoS Negl. Trop. Dis. 2018, 12, e0006548. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; He, J.; Yang, K.; Liang, S. Applications of spatial technology in schistosomiasis control programme in The People’s Republic of China. Adv. Parasitol. 2016, 92, 143–163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Carpenter, T.E.; Chen, Y.; Clark, A.B.; Lynn, H.S.; Peng, W.; Zhou, Y.; Zhao, G.; Jiang, Q. Identifying high-risk regions for schistosomiasis in Guichi, China: A spatial analysis. Act. Trop. 2008, 107, 217–223. [Google Scholar] [CrossRef]
- Nihei, N.; Komagata, O.; Kobayashi, M.; Saitoh, Y.; Mochizuki, K.; Nakamura, S. Spatial analysis and remote sensing for monitoring systems of Oncomelania nosophora following the eradication of schistosomiasis japonica in Yamanashi Prefecture, Japan. Jpn. J. Infect. Dis. 2009, 62, 125–132. [Google Scholar] [CrossRef]
- Nihei, N.; Kajihara, N.; Kirinoki, M.; Chigusa, Y.; Matsuda, H.; Saitoh, Y.; Shimamura, R.; Kaneta, H.; Nakamura, S. Establishment of a GIS monitoring system for schistosomiasis japonica in Kofu, Japan. Ann. Trop. Med. Parasit. 2006, 100, 143–153. [Google Scholar] [CrossRef]
- Tong, Q.B.; Chen, R.; Zhang, Y.; Yang, G.J.; Kumagai, T.; Furushima-Shimogawara, R.; Lou, D.; Yang, K.; Wen, L.Y.; Lu, S.H.; et al. A new surveillance and response tool: Risk map of infected Oncomelania hupensis detected by Loop-mediated isothermal amplification (LAMP) from pooled samples. Act. Trop. 2015, 141, 170–177. [Google Scholar] [CrossRef]
- Zhang, Z.; Clark, A.B.; Bivand, R.; Chen, Y.; Carpenter, T.E.; Peng, W.; Zhou, Y.; Zhao, G.; Jiang, Q. Nonparametric spatial analysis to detect high-risk regions for schistosomiasis in Guichi, China. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 1045–1052. [Google Scholar] [CrossRef]
- Scholte, R.G.C.; Gosoniu, L.; Malone, J.B.; Chammartin, F.; Utzinger, J.; Vounatsou, P. Predictive risk mapping of schistosomiasis in Brazil using bayesian geostatistical models. Act. Trop. 2014, 132, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.F.; Lv, S.; Wang, Q.Y.; Qian, M.B.; Liu, Q.; Bergquist, R.; Zhou, X.N. Schistosomiasis japonica: Modelling as a tool to explore transmission patterns. Act. Trop. 2015, 141, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ward, M.P.; Xia, C.; Li, R.; Sun, L.; Lynn, H.; Gao, F.; Wang, Q.; Zhang, S.; Xiong, C.; et al. Monitoring schistosomiasis risk in East China over space and time using a Bayesian hierarchical modeling approach. Sci. Rep. 2016, 6, 24173:1–24173:9. [Google Scholar] [CrossRef] [PubMed]
- Schur, N.; Utzinger, J.; Vounatsou, P. Modelling age-heterogeneous Schistosoma haematobium and S. mansoni survey data via alignment factors. Parasit. Vectors 2011, 4, 142:1–142:10. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Sun, L.P.; Huang, Y.X.; Yang, G.J.; Wu, F.; Hang, D.R.; Li, W.; Zhang, J.F.; Liang, Y.S.; Zhou, X.N. A real-time platform for monitoring schistosomiasis transmission supported by google earth and a web-based geographical information system. Geospat. Health 2012, 6, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liu, L.; Xia, S.; Xu, J.F.; Bergquist, R.; Yang, G.J. Reaching the surveillance-response stage of schistosomiasis control in The People’s Republic of China. Adv. Parasitol. 2016, 92, 165–196. [Google Scholar] [CrossRef]
- Brooker, S.; Clements, A.C.A.; Bundy, D.A.P. Global epidemiology, ecology and control of soil-transmitted helminth infections. Adv. Parasit. 2006, 62, 221–261. [Google Scholar] [CrossRef]
- Lai, Y.S.; Zhou, X.N.; Utzinger, J.; Vounatsou, P. Bayesian geostatistical modelling of soil-transmitted helminth survey data in The People’s Republic of China. Parasit. Vectors 2013, 6, 359:1–359:16. [Google Scholar] [CrossRef][Green Version]
- Scholte, R.G.C.; Schur, N.; Bavia, M.E.; Carvalho, E.M.; Chammartin, F.; Utzinger, J.; Vounatsou, P. Spatial analysis and risk mapping of soil-transmitted helminth infections in Brazil, using Bayesian geostatistical models. Geospat. Health 2013, 8, 97–110. [Google Scholar] [CrossRef]
- Karagiannis-Voules, D.A.; Biedermann, P.; Ekpo, U.F.; Garba, A.; Langer, E.; Mathieu, E.; Midzi, N.; Mwinzi, P.; Polderman, A.M.; Raso, G.; et al. Spatial and temporal distribution of soil-transmitted helminth infection in sub-Saharan Africa: A systematic review and geostatistical meta-analysis. Lancet Infect. Dis. 2015, 15, 74–84. [Google Scholar] [CrossRef]
- Atkinson, J.A.; Gray, D.J.; Clements, A.C.A.; Barnes, T.S.; McManus, D.P.; Yang, Y.R. Environmental changes impacting Echinococcus transmission: Research to support predictive surveillance and control. Glob. Chang. Biol. 2013, 19, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Conraths, F.J.; Deplazes, P. Echinococcus multilocularis: Epidemiology, surveillance and state-of-the-art diagnostics from a veterinary public health perspective. Vet. Parasitol. 2015, 213, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Cadavid, A.M.; Yang, Y.R.; McManus, D.P.; Gray, D.J.; Giraudoux, P.; Barnes, T.S.; Williams, G.M.; Soares, R.J.; Hamm, A.S.; Clements, A.C.A. The landscape epidemiology of echinococcoses. Infect. Dis. Poverty 2016, 5, 13:1–13:13. [Google Scholar] [CrossRef]
- Schur, N.; Hürlimann, E.; Garba, A.; Traoré, M.S.; Ndir, O.; Ratard, R.C.; Tchuem Tchuenté, L.A.; Kristensen, T.K.; Utzinger, J.; Vounatsou, P. Geostatistical model-based estimates of schistosomiasis prevalence among individuals aged ≤20 years in West Africa. PLoS Negl. Trop. Dis. 2011, 5, e1194. [Google Scholar] [CrossRef]
- Handali, S.; Pawitan, Y. Verifying elimination programs with a special emphasis on cysticercosis endpoints and postelimination surveillance. J. Parasitol. Res. 2012, 2012, 6. [Google Scholar] [CrossRef]
- Basáñez, M.G.; McCarthy, J.S.; French, M.D.; Yang, G.J.; Walker, M.; Gambhir, M.; Prichard, R.K.; Churcher, T.S. A research agenda for helminth diseases of humans: Modelling for control and elimination. PLoS Negl. Trop. Dis. 2012, 6, e1548. [Google Scholar] [CrossRef]
- Stothard, J.R.; Campbell, S.J.; Osei-Atweneboana, M.Y.; Durant, T.; Stanton, M.C.; Biritwum, N.K.; Rollinson, D.; Ombede, D.R.E.; Tcheum-Tchuenté, L.A. Towards interruption of schistosomiasis transmission in sub-Saharan Africa: Developing an appropriate environmental surveillance framework to guide and to support ‘end game’ interventions. Infect. Dis. Poverty 2017, 6, 10:1–10:4. [Google Scholar] [CrossRef]
- Solomon, A.W.; Engels, D.; Bailey, R.L.; Blake, I.M.; Brooker, S.; Chen, J.X.; Chen, J.H.; Churcher, T.S.; Drakeley, C.J.; Edwards, T.; et al. A diagnostics platform for the integrated mapping, monitoring, and surveillance of neglected tropical diseases: Rationale and target product profiles. PLoS Negl. Trop. Dis. 2012, 6, e1746. [Google Scholar] [CrossRef]
- Baker, M.; Mathieu, E.; Fleming, F.; Deming, M.; King, J.; Garba, A.; Koroma, J.B.; Bockarie, M.; Kabore, A.; Sankara, D.P.; et al. Mapping, monitoring, and surveillance of neglected tropical diseases: Towards a policy framework. Lancet 2010, 375, 231–238. [Google Scholar] [CrossRef]
- Albonico, M.; Levecke, B.; LoVerde, P.T.; Montresor, A.; Prichard, R.; Vercruysse, J.; Webster, J.P. Monitoring the efficacy of drugs for neglected tropical diseases controlled by preventive chemotherapy. J. Glob. Antimicrob. Resist. 2015, 3, 229–236. [Google Scholar] [CrossRef]
- Singer, B.H.; de Castro, M.C. Bridges to sustainable tropical health. Proc. Natl. Acad. Sci. USA 2007, 104, 16038–16043. [Google Scholar] [CrossRef] [PubMed]
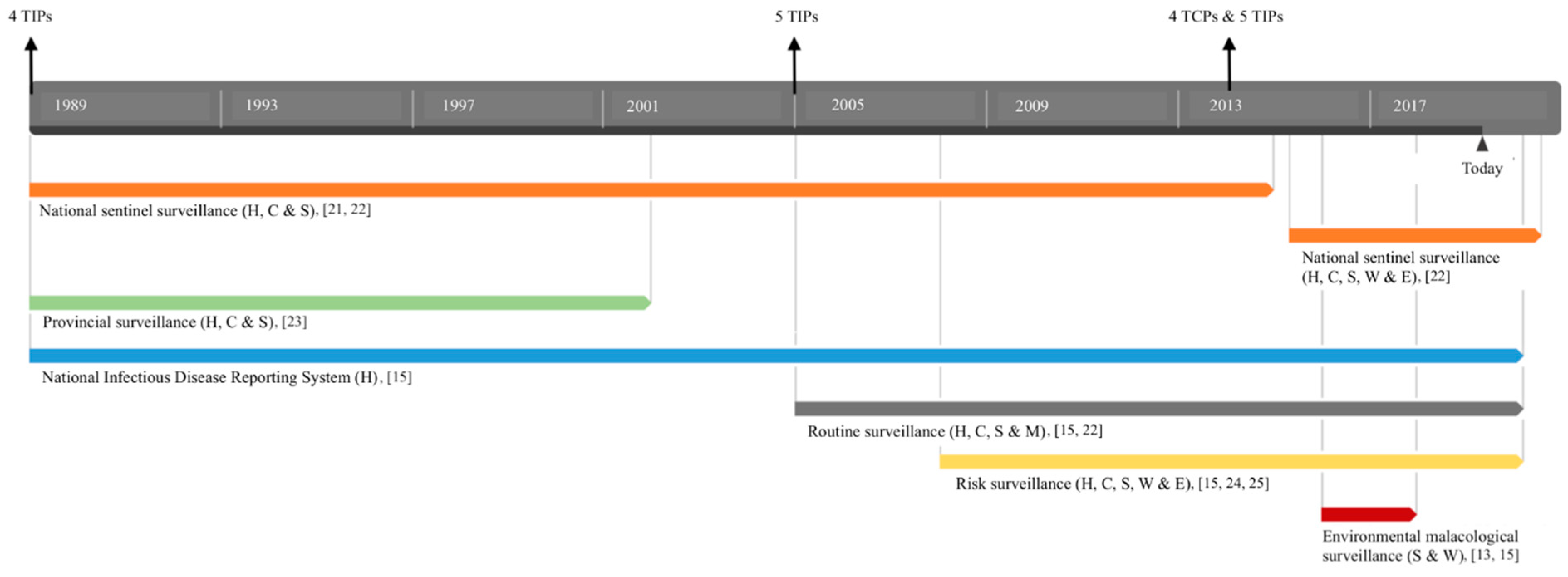
| Schistosoma Species | Monitoring & Surveillance (M&S) System | Time | Country | Reference |
|---|---|---|---|---|
| S. mansoni | Malacological survey to determine dispersal: Capture and identification of snails in a radius of 2 m at 168 selected sites, linked to data collection and analysis of water/sediment samples; landscape and climate data. | 2016–2017 | China | [13] |
| S. japonicum | Surveillance for human schistosomiasis cases: Continuous surveillance of advanced human cases in addition to immunological testing of villagers at risk and suspected patients. | 1995–2002 | China | [14] |
| All Schistosoma spp. | M&S for human schistosomiasis cases: Reporting of both acute and chronic cases of schistosomiasis diagnosed in hospitals to the National Infectious Diseases Reporting System by all levels of medical institutions. | 1989–…1 | China | [15] |
| All Schistosoma spp. | M&S for human schistosomiasis cases: Self-selected data entry of schistosomiasis cases in the TropNetEurop 2 database. | 1999–…1 | Europe | [16] |
| S. japonicum | M&S of livestock: selective treatment or isolation of the infected animal reservoir (i.e., cattle, water buffalo, goats, sheep, pigs, and dogs) to prevent further contamination of the environment. | Not yet implemented | NA | [17] |
| S. japonicum | M&S of Schistosoma-infected water using sentinel mice in cages on the water surface. | 2008–…1 | China | [18,19] |
| S. japonicum | M&S of Schistosoma-infected water: Detection of contaminated water with environmental qPCR. | 2016 | Madagascar | [20] |
| S. japonicum | National sentinel surveillance system: Surveillance of humans, cattle, and snails in 20–458 sentinel sites across the country. Treatment of positive humans and cattle, regular re-examination of advanced cases, and focal mollusciciding. Since 2015, additional water bodies and environmental surveillance (i.e., examination of wildlife feces) have been added. | 1989–…1 | China | [15,19,21,22] |
| S. japonicum | Provincial surveillance system (5 provinces): Surveillance of snails (frequency determined on absence/presence in the past 3–15 years). If snails were found, surveillance of humans and bovines, and treatment with praziquantel (PZQ) when positive. | 1985–1995 | China | [23] |
| S. japonicum | Routine surveys across the country: Case reports and surveys of human patients in addition to regular M&S of endemic villages (humans, snails, bovines, and other mammalian intermediate hosts) that achieved control and elimination. | 2005–…1 | China | [15,22] |
| S. japonicum | Risk surveillance of humans, water, free-roaming livestock, and snails based on results from previous routine surveillance. | 2008–…1 | China | [15,24,25] |
| S. mansoni | National elimination plan based on a monitoring and treatment system: Re-mapping residual schistosomiasis distribution in every governorate of the country with an additional mass treatment (with PZQ) policy based on the prevalence outcome. Additionally, application of molluscicides and treatment of water bodies regardless of the outcome. | 2017–…1 | Egypt | [26,27] |
| Method of Monitoring & Surveillance/Sampling | Time | Country | Reference |
|---|---|---|---|
| National surveys (in several counties at different time intervals): The implementation of a nationwide survey every 5–7 years in people of any age to monitor the infection rate and its relation to various characteristics (e.g., age, gender, region, and urban versus rural areas). | 1971–…1 | The Republic of Korea | [37] |
| Regional surveys: Fecal sample collection of school-aged children in a previously high- and previously low-endemic county in The Republic of Korea to monitor the current status of prevalence of STHs. | 2017 | The Republic of Korea | [38] |
| Integrated school-based monitoring and surveillance for both lymphatic filariasis and STHs by fecal examination of school-aged children. | 2006 and 2012 | Sri Lanka and Kenya | [39,40] |
| Lot quality assurance sampling: A minimalistic approach of sampling originally designed for manufacturing inspection. It entails the collection and assessment of a minimal number of samples and the addition of more samples until statistical significance has been reached. | Not yet implemented | Not applicable | [34] |
| Pooling of samples to estimate STH prevalence while minimizing the number of diagnostic procedures: 50 School-aged children of 5 schools in each district were sampled. Individual examination was compared to pooled examination (pool size = 10) and both were processed with the Kato-Katz technique. | 2017 | Ethiopia | [41,42] |
| Echinococcus Species | Monitoring and Surveillance System | Time | Country | Reference |
|---|---|---|---|---|
| E. granulosis | Routine post-mortem inspection of slaughter sheep followed by mandatory sampling and cestoidal drug treatment of shepherd dogs at the farm of origin (in case of a positive result). | 2007–2013 | Tasmania | [44] |
| E. multilocularis | Annual surveillance system that includes fox sampling (300 annually) in each municipality of the country. In case of positive detection, intensification of fox sampling (to 3000 annually), collection of rodents and fox feces for analyzation, and intensification of national Norwegian surveillance system. | 2000–2011 | Sweden, Norway | [45,46,47,48] |
| E. multilocularis | Necropsy and coprological examination in addition to preventive deworming of free roaming, stray, and companion dogs. | 2013–2017 | Japan | [49] |
| Taenia spp. | Monitoring & Surveillance System | Time | Country/Region | Reference |
|---|---|---|---|---|
| T. solium | Observatory of Taeniasis and Cysticercosis: System consisting of several core activities mainly focusing on the detection and treatment of tapeworm carriers. | 2017–…1 | Portugal | [53] |
| T. solium | The integration of multiplex bead assays for parasitic diseases into national multiple indicator surveys. | 2012 | Cambodia | [54] |
| T. solium | Ring screening and treatment system: Pig surveillance by tongue palpation. When positive, test every individual living in a 100 meter radius for taeniasis and treatment with niclosamide. | 2012–2014 | Northern Peru | [55] |
| T. saginata | Conventional, routine meat inspection of bovines over 6 weeks of age: Visual inspection and incision of predilection sites. | 1964–…1 | Europe | [56,57,58] |
| T. saginata | Risk classification of farms/cattle and slaughterhouses based on data from history cases, serological tests, and other known risk factors. Subsequently, visual inspection of low-risk cattle and rigorous meat inspection of high-risk cattle. | Not yet implemented | United Kingdom, France, and Europe | [59,60,61] |
| Parasite Species | Monitoring & Surveillance System | Time | Country/Region | Reference |
|---|---|---|---|---|
| Taenia saginata and Echinococcus granulosus | A retrospective approach of spatial analysis for the integrated surveillance of both bovine cysticercosis and cystic echinococcosis combined with further actions based on the outcome. | 2006–2010 | Veneto (northeastern Italy) | [62] |
| Schistosoma spp. and STHs | Integrated cross-sectional surveys with anamnestic questionnaire for monitoring STHs and schistosomiasis. | 2010 | Taboo (south-central Côte d’Ivoire) | [63] |
| Schistosoma spp. and STHs | Screening for STHs, schistosomiasis, and polio using the global polio laboratory network. | 2016–2017 | Ghana | [64] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saelens, G.; Gabriël, S. Currently Available Monitoring and Surveillance Systems for Taenia spp., Echinococcus spp., Schistosoma spp., and Soil-Transmitted Helminths at the Control/Elimination Stage: A Systematic Review. Pathogens 2020, 9, 47. https://doi.org/10.3390/pathogens9010047
Saelens G, Gabriël S. Currently Available Monitoring and Surveillance Systems for Taenia spp., Echinococcus spp., Schistosoma spp., and Soil-Transmitted Helminths at the Control/Elimination Stage: A Systematic Review. Pathogens. 2020; 9(1):47. https://doi.org/10.3390/pathogens9010047
Chicago/Turabian StyleSaelens, Ganna, and Sarah Gabriël. 2020. "Currently Available Monitoring and Surveillance Systems for Taenia spp., Echinococcus spp., Schistosoma spp., and Soil-Transmitted Helminths at the Control/Elimination Stage: A Systematic Review" Pathogens 9, no. 1: 47. https://doi.org/10.3390/pathogens9010047
APA StyleSaelens, G., & Gabriël, S. (2020). Currently Available Monitoring and Surveillance Systems for Taenia spp., Echinococcus spp., Schistosoma spp., and Soil-Transmitted Helminths at the Control/Elimination Stage: A Systematic Review. Pathogens, 9(1), 47. https://doi.org/10.3390/pathogens9010047





