Toxoplasma gondii Recombinant antigen AMA1: Diagnostic Utility of Protein Fragments for the Detection of IgG and IgM Antibodies
Abstract
1. Introduction
2. Results
2.1. Expression and Purification of the Recombinant AMA1 Antigens
2.2. Immunoreactivities of the Recombinant AMA1 Antigens with Mouse Sera
2.3. IgG ELISA Using Human Serum Samples
2.4. IgM ELISA Using Human Serum Samples
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Mice
4.3. Parasite
4.4. Preparation of T. gondii Native Lysate Antigen (TLA)
4.5. T. gondii Infection of Mice
4.6. IgG and IgM ELISA of Mouse Serum Samples
4.7. T. gondii RNA Isolation and cDNA Synthesis
4.8. Construction of the Recombinant Plasmid
4.9. Expression and Purification of the Chimeric Antigens and Recombinant Proteins
4.10. Human Serum Samples
4.11. IgG and IgM ELISA–Human Serum Samples
4.12. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Pappas, G.; Roussos, N.; Falagas, M.E. Toxoplasmosis snapshots: Global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int. J. Parasitol. 2009, 39, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Torgerson, P.R.; Mastroiacovo, P. The global burden of congenital toxoplasmosis: A systematic review. Bull. World Health Organ. 2013, 91, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Holliman, R.E. Congenital toxoplasmosis: Prevention, screening and treatment. J. Hosp. Infect. 1995, 30, 179–190. [Google Scholar] [CrossRef]
- Schmidt, M.; Sonneville, R.; Schnell, D.; Bige, N.; Hamidfar, R.; Mongardon, N.; Castelain, V.; Razazi, K.; Marty, A.; Vincent, F.; et al. Clinical Features and Outcomes in Patients With Disseminated Toxoplasmosis Admitted to Intensive Care: A Multicenter Study. Clin. Infect. Dis. 2013, 57, 1535–1541. [Google Scholar] [CrossRef]
- Buxton, D. Protozoan infections (Toxoplasma gondii, Neospora caninum and Sarcocystis spp.) in sheep and goats: Recent advances. Vet. Res. 1998, 29, 289–310. [Google Scholar]
- Holec-Gasior, L. Toxoplasma gondii recombinant antigens as tools for serodiagnosis of human toxoplasmosis: Current status of studies. Clin. Vaccine Immunol. 2013, 20, 1343–1351. [Google Scholar] [CrossRef]
- Rostami, A.; Karanis, P.; Fallahi, S. Advances in serological, imaging techniques and molecular diagnosis of Toxoplasma gondii infection. Infection 2018, 46, 303–315. [Google Scholar] [CrossRef]
- Kotresha, D.; Noordin, R. Recombinant proteins in the diagnosis of toxoplasmosis. APMIS 2010, 118, 529–542. [Google Scholar] [CrossRef]
- Ferra, B.; Holec-Gąsior, L.; Kur, J. A new Toxoplasma gondii chimeric antigen containing fragments of SAG2, GRA1, and ROP1 proteins—Impact of immunodominant sequences size on its diagnostic usefulness. Parasitol. Res. 2015, 114, 3291–3299. [Google Scholar] [CrossRef][Green Version]
- Ferra, B.; Holec-Gasior, L.; Kur, J. Serodiagnosis of Toxoplasma gondii infection in farm animals (horses, swine, and sheep) by enzyme-linked immunosorbent assay using chimeric antigens. Parasitol. Int. 2015, 64, 288–294. [Google Scholar] [CrossRef]
- Holec-Gasior, L.; Drapała, D.; Lautenbach, D.; Kur, J. Toxoplasma gondii: Usefulness of ROP1 recombinant antigen in an immunoglobulin G avidity assay for diagnosis of acute toxoplasmosis in humans. Polish J. Microbiol. 2010, 59, 307–310. [Google Scholar] [CrossRef]
- Holec-Gąsior, L.; Ferra, B.; Drapała, D.; Lautenbach, D.; Kur, J. A new MIC1-MAG1 recombinant chimeric antigen can be used instead of the Toxoplasma gondii lysate antigen in serodiagnosis of human toxoplasmosis. Clin. Vaccine Immunol. 2012, 19, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Drapała, D.; Holec-Gasior, L.; Kur, J.; Ferra, B.; Hiszczyńska-Sawicka, E.; Lautenbach, D. A new human IgG avidity test, using mixtures of recombinant antigens (rROP1, rSAG2, rGRA6), for the diagnosis of difficult-to-identify phases of toxoplasmosis. Diagn. Microbiol. Infect. Dis. 2014, 79, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.G.; Peretti, L.E.; García, V.S.; Peverengo, L.; González, V.D.G.; Gugliotta, L.M.; Dalla Fontana, M.L.; Lagier, C.M.; Marcipar, I.S. P35 and P22 Toxoplasma gondii antigens abbreviate regions to diagnose acquired toxoplasmosis during pregnancy: Toward single-sample assays. Clin. Chem. Lab. Med. 2017, 55, 595–604. [Google Scholar] [CrossRef]
- Pietkiewicz, H.; Hiszczyńska-Sawicka, E.; Kur, J.; Petersen, E.; Nielsen, H.V.; Paul, M.; Stankiewicz, M.; Myjak, P. Usefulness of Toxoplasma gondii recombinant antigens (GRA1, GRA7 and SAG1) in an immunoglobulin G avidity test for the serodiagnosis of toxoplasmosis. Parasitol. Res. 2007, 100, 333–337. [Google Scholar] [CrossRef]
- Beghetto, E.; Buffolano, W.; Spadoni, A.; Del Pezzo, M.; Di Cristina, M.; Minenkova, O.; Petersen, E.; Felici, F.; Gargano, N. Use of an immunoglobulin G avidity assay based on recombinant antigens for diagnosis of primary Toxoplasma gondii infection during pregnancy. J. Clin. Microbiol. 2003, 41, 5414–5418. [Google Scholar] [CrossRef]
- Ferra, B.T.; Holec-Gąsior, L.; Gatkowska, J.; Dziadek, B.; Dzitko, K.; Grąźlewska, W.; Lautenbach, D. The first study on the usefulness of recombinant tetravalent chimeric proteins containing fragments of SAG2, GRA1, ROP1 and AMA1 antigens in the detection of specific anti-Toxoplasma gondii antibodies in mouse and human sera. PLoS ONE 2019, 14, e0217866. [Google Scholar] [CrossRef]
- Carruthers, V.B.; Sibley, L.D. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur. J. Cell Biol. 1997, 73, 114–123. [Google Scholar]
- Joiner, K.A.; Roos, D.S. Secretory traffic in the eukaryotic parasite Toxoplasma gondii: Less is more. J. Cell Biol. 2002, 157, 557–563. [Google Scholar] [CrossRef]
- Ngô, H.M.; Hoppe, H.C.; Joiner, K.A. Differential sorting and post-secretory targeting of proteins in parasitic invasion. Trends Cell Biol. 2000, 10, 67–72. [Google Scholar] [CrossRef]
- Liu, Q.; Li, F.-C.; Zhou, C.-X.; Zhu, X.-Q. Research advances in interactions related to Toxoplasma gondii microneme proteins. Exp. Parasitol. 2017, 176, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Poukchanski, A.; Fritz, H.M.; Tonkin, M.L.; Treeck, M.; Boulanger, M.J.; Boothroyd, J.C. Toxoplasma gondii Sporozoites Invade Host Cells Using Two Novel Paralogues of RON2 and AMA1. PLoS ONE 2013, 8, e70637. [Google Scholar] [CrossRef]
- Carruthers, V.B.; Giddings, O.K.; Sibley, L.D. Secretion of micronemal proteins is associated with toxoplasma invasion of host cells. Cell. Microbiol. 1999, 1, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Tomley, F.M.; Soldati, D.S. Mix and match modules: Structure and function of microneme proteins in apicomplexan parasites. Trends Parasitol. 2001, 17, 81–88. [Google Scholar] [CrossRef]
- Kappe, S.; Bruderer, T.; Gantt, S.; Fujioka, H.; Nussenzweig, V.; Ménard, R. Conservation of a gliding motility and cell invasion machinery in Apicomplexan parasites. J. Cell Biol. 1999, 147, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, V.B. Host cell invasion by the opportunistic pathogen Toxoplasma gondii. Acta Trop. 2002, 81, 111–122. [Google Scholar] [CrossRef]
- Huynh, M.; Rabenau, K.E.; Harper, J.M.; Beatty, W.L.; Sibley, L.D.; Carruthers, V.B. Rapid invasion of host cells by Toxoplasma requires secretion of the MIC2–M2AP adhesive protein complex. EMBO J. 2003, 22, 2082–2090. [Google Scholar] [CrossRef]
- Jewett, T.J.; Sibley, L.D. The toxoplasma proteins MIC2 and M2AP form a hexameric complex necessary for intracellular survival. J. Biol. Chem. 2004, 279, 9362–9369. [Google Scholar] [CrossRef]
- Cérède, O.; Dubremetz, J.F.; Soête, M.; Deslée, D.; Vial, H.; Bout, D.; Lebrun, M. Synergistic role of micronemal proteins in Toxoplasma gondii virulence. J. Exp. Med. 2005, 201, 453–463. [Google Scholar] [CrossRef]
- Mital, J.; Meissner, M.; Soldati, D.; Ward, G.E. Conditional Expression of Toxoplasma gondii Apical Membrane Antigen-1 (TgAMA1) Demonstrates That TgAMA1 Plays a Critical Role in Host Cell Invasion. Mol. Biol. Cell 2005, 16, 4341–4349. [Google Scholar] [CrossRef]
- Sawmynaden, K.; Saouros, S.; Friedrich, N.; Marchant, J.; Simpson, P.; Bleijlevens, B.; Blackman, M.J.; Soldati-Favre, D.; Matthews, S. Structural insights into microneme protein assembly reveal a new mode of EGF domain recognition. EMBO Rep. 2008, 9, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Harvey, K.L.; Yap, A.; Gilson, P.R.; Cowman, A.F.; Crabb, B.S. Insights and controversies into the role of the key apicomplexan invasion ligand, Apical Membrane Antigen 1. Int. J. Parasitol. 2014, 44, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Vetrivel, U.; Muralikumar, S.; Mahalakshmi, B.; Lily Therese, K.; Madhavan, H.N.; Alameen, M.; Thirumudi, I. Multilevel Precision-Based Rational Design of Chemical Inhibitors Targeting the Hydrophobic Cleft of Toxoplasma gondii Apical Membrane Antigen 1 (AMA1). Genom. Inform. 2016, 14, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.M.; Ferguson, D.J.P.; Blackman, M.J.; Soldati-Favre, D. Intramembrane cleavage of AMA1 triggers Toxoplasma to switch from an invasive to a replicative mode. Science 2011, 331, 473–477. [Google Scholar] [CrossRef]
- Beghetto, E.; Spadoni, A.; Buffolano, W.; Del Pezzo, M.; Minenkova, O.; Pavoni, E.; Pucci, A.; Cortese, R.; Felici, F.; Gargano, N. Molecular dissection of the human B-cell response against Toxoplasma gondii infection by lambda display of cDNA libraries. Int. J. Parasitol. 2003, 33, 163–173. [Google Scholar] [CrossRef]
- Beghetto, E.; Nielsen, H.V.; Del Porto, P.; Buffolano, W.; Guglietta, S.; Felici, F.; Petersen, E.; Gargano, N. A Combination of Antigenic Regions of Toxoplasma gondii Microneme Proteins Induces Protective Immunity against Oral Infection with Parasite Cysts. J. Infect. Dis. 2005, 191, 637–645. [Google Scholar] [CrossRef]
- Buffolano, W.; Beghetto, E.; Del Pezzo, M.; Spadoni, A.; Di Cristina, M.; Petersen, E.; Gargano, N. Use of recombinant antigens for early postnatal diagnosis of congenital toxoplasmosis. J. Clin. Microbiol. 2005, 43, 5916–5924. [Google Scholar] [CrossRef]
- Holec, L.; Gąsior, A.; Brillowska-Dąbrowska, A.; Kur, J. Toxoplasma gondii: Enzyme-linked immunosorbent assay using different fragments of recombinant microneme protein 1 (MIC1) for detection of immunoglobulin G antibodies. Exp. Parasitol. 2008, 119, 1–6. [Google Scholar] [CrossRef]
- Montoya, J.G. Laboratory Diagnosis of Toxoplasma gondii Infection and Toxoplasmosis. J. Infect. Dis. 2002, 185, S73–S82. [Google Scholar] [CrossRef]
- Montoya, J.G.; Remington, J.S. Clinical Practice: Management of Toxoplasma gondii Infection during Pregnancy. Clin. Infect. Dis. 2008, 47, 554–566. [Google Scholar] [CrossRef]
- Robert-Gangneux, F.; Darde, M.-L. Epidemiology of and Diagnostic Strategies for Toxoplasmosis. Clin. Microbiol. Rev. 2012, 25, 264–296. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.; Tonkin, M.L.; Grujic, O.; Boulanger, M.J. Structural characterization of apical membrane antigen 1 (AMA1) from Toxoplasma gondii. J. Biol. Chem. 2010, 285, 15644–15652. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, S.; Deng, B.; Del Rio, R.; Buchholz, K.R.; Treeck, M.; Urban, S.; Boothroyd, J.; Lam, Y.-W.; Ward, G.E. Not a Simple Tether: Binding of Toxoplasma gondii AMA1 to RON2 during Invasion Protects AMA1 from Rhomboid-Mediated Cleavage and Leads to Dephosphorylation of Its Cytosolic Tail. MBio 2016, 7, e00754-16. [Google Scholar] [CrossRef] [PubMed]
- Parussini, F.; Tang, Q.; Moin, S.M.; Mital, J.; Urban, S.; Ward, G.E. Intramembrane proteolysis of Toxoplasma apical membrane antigen 1 facilitates host-cell invasion but is dispensable for replication. Proc. Natl. Acad. Sci. USA 2012, 109, 7463–7468. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, V.; Boothroyd, J.C. Pulling together: An integrated model of Toxoplasma cell invasion. Curr. Opin. Microbiol. 2007, 10, 83–89. [Google Scholar] [CrossRef]
- Giovannini, D.; Späth, S.; Lacroix, C.; Perazzi, A.; Bargieri, D.; Lagal, V.; Lebugle, C.; Combe, A.; Thiberge, S.; Baldacci, P.; et al. Independent Roles of Apical Membrane Antigen 1 and Rhoptry Neck Proteins during Host Cell Invasion by Apicomplexa. Cell Host Microbe 2011, 10, 591–602. [Google Scholar] [CrossRef]
- Bargieri, D.Y.; Andenmatten, N.; Lagal, V.; Thiberge, S.; Whitelaw, J.A.; Tardieux, I.; Meissner, M.; Ménard, R. Apical membrane antigen 1 mediates apicomplexan parasite attachment but is dispensable for host cell invasion. Nat. Commun. 2013, 4, 2552. [Google Scholar] [CrossRef]
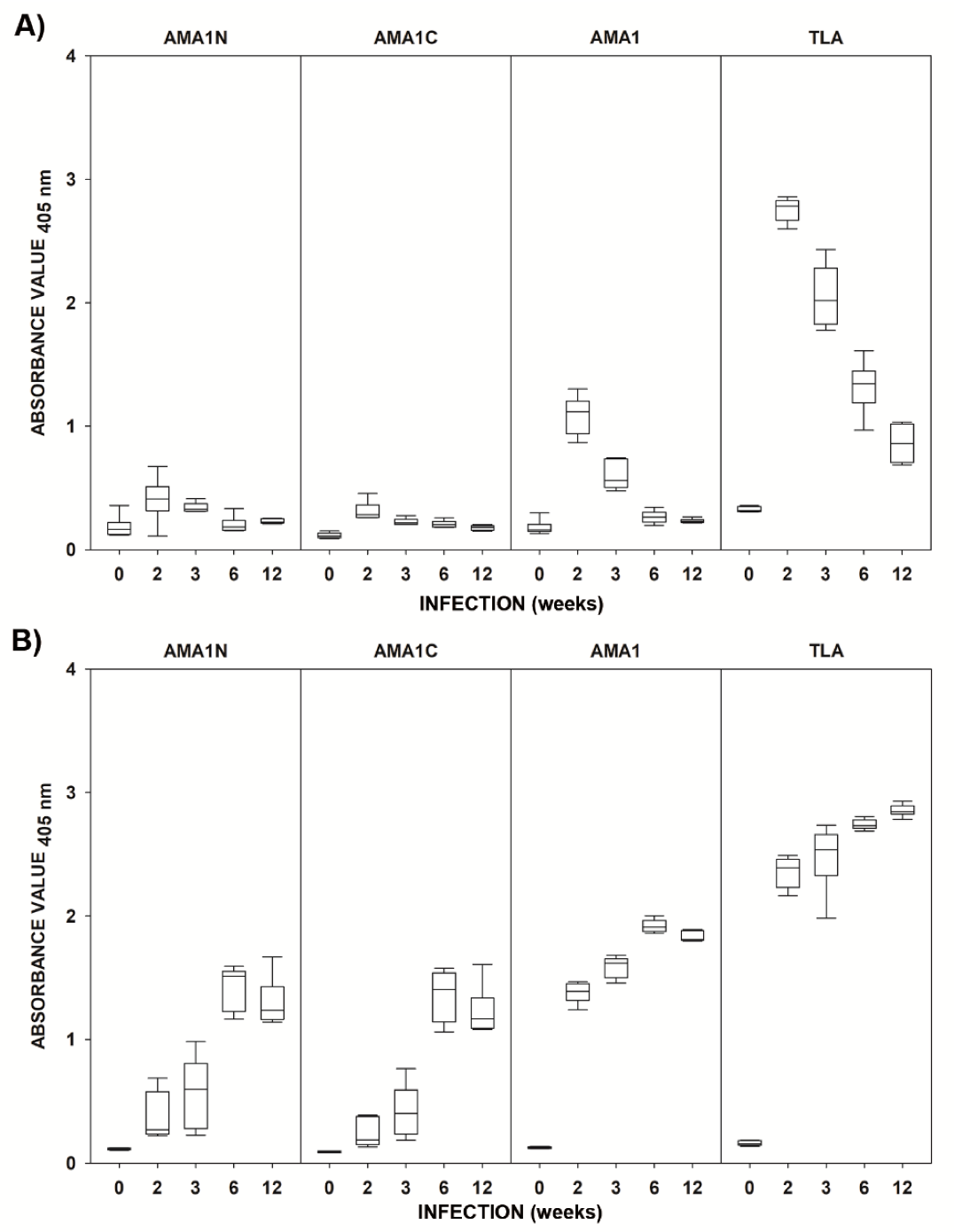
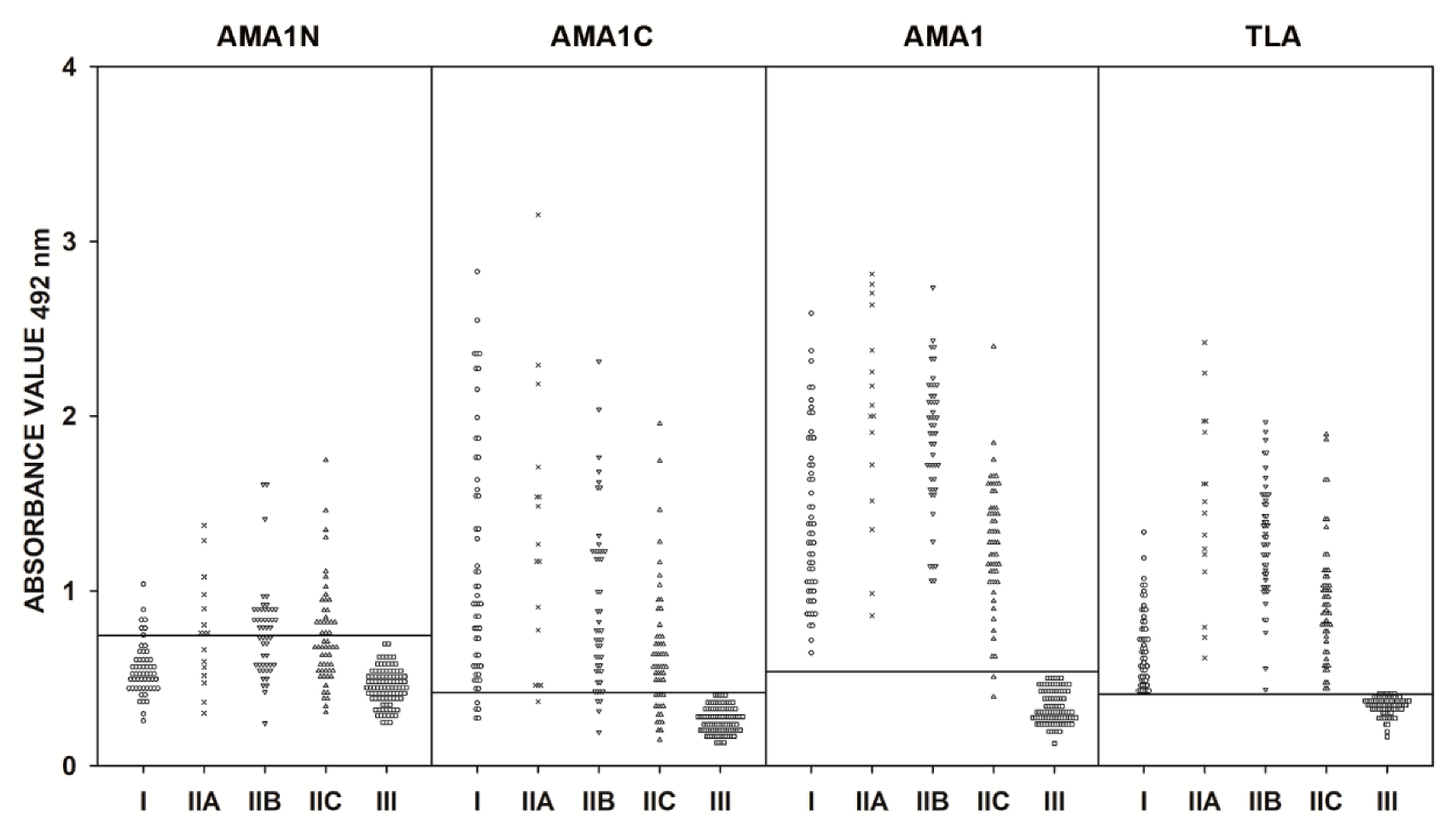
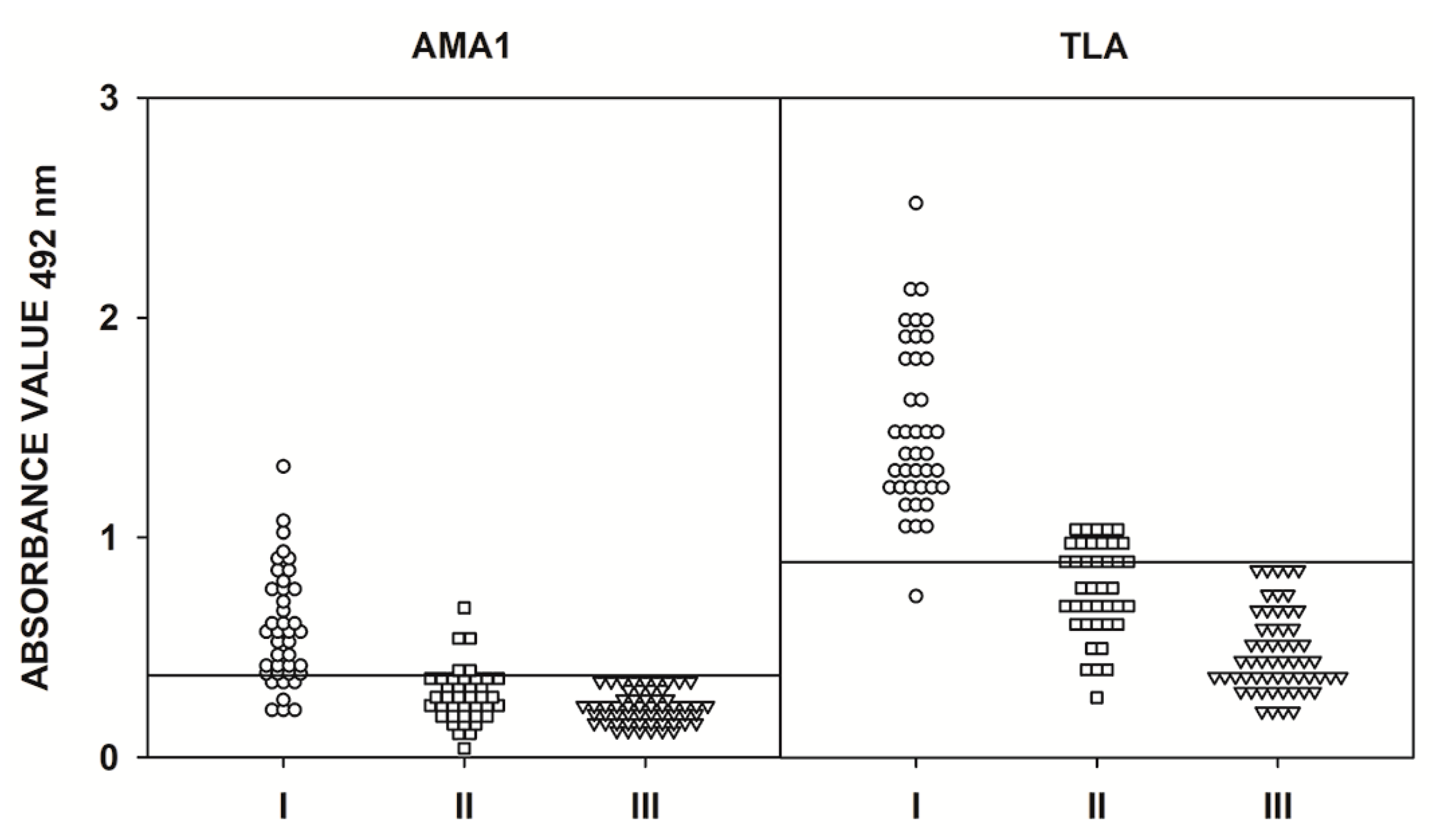
| Antigen | Time after Challenge (Weeks) | IgM | IgG | ||
|---|---|---|---|---|---|
| Median | p-Value | Median | p-Value | ||
| AMA1N | 0 | 0.165 | - | 0.118 | - |
| 2 | 0.411 | 0.021 | 0.270 | 0.002 | |
| 3 | 0.328 | 0.041 | 0.594 | 0.002 | |
| 6 | 0.184 | 0.485 | 1.512 | 0.002 | |
| 12 | 0.223 | 0.065 | 1.235 | 0.002 | |
| AMA1C | 0 | 0.110 | - | 0.091 | - |
| 2 | 0.284 | <0.001 | 0.188 | 0.002 | |
| 3 | 0.217 | <0.001 | 0.403 | 0.004 | |
| 6 | 0.201 | <0.001 | 1.404 | <0.001 | |
| 12 | 0.181 | <0.001 | 1.167 | 0.002 | |
| AMA1 | 0 | 0.162 | - | 0.125 | - |
| 2 | 1.118 | <0.001 | 1.390 | <0.001 | |
| 3 | 0.560 | 0.002 | 1.619 | 0.002 | |
| 6 | 0.264 | 0.024 | 1.910 | <0.001 | |
| 12 | 0.231 | 0.065 | 1.809 | <0.001 | |
| TLA | 0 | 0.318 | - | 0.159 | - |
| 2 | 2.784 | <0.001 | 2.392 | <0.001 | |
| 3 | 2.018 | 0.002 | 2.539 | 0.002 | |
| 6 | 1.343 | 0.002 | 2.732 | <0.001 | |
| 12 | 0.862 | <0.001 | 2.846 | <0.001 | |
| Antigen | Calculated Cut-off | ROC Cut-off | AUC | Sensitivity, % | Specificity, % |
|---|---|---|---|---|---|
| AMA1N | 0.7454 | 0.7453 | 0.811 | 36.6 | 100 |
| AMA1C | 0.4185 | 0.4208 | 0.953 | 84.6 | 100 |
| AMA1 | 0.5371 | 0.5043 | 0.998 | 99.4 | 100 |
| TLA | 0.4092 | 0.4130 | 1.000 | 100 | 100 |
| ELISA Test | Group | AMA1N | AMA1C | AMA1 | |||
|---|---|---|---|---|---|---|---|
| r | p Value | r | p Value | r | p Value | ||
| IgM | I | - | - | - | - | 0.449 | <0.001 |
| II | - | - | - | - | 0.539 | <0.001 | |
| III | - | - | - | - | 0.536 | <0.001 | |
| All | - | - | - | - | 0.781 | <0.001 | |
| IgG | I | 0.033 | 0.814 | 0.529 | <0.001 | 0.382 | 0.004 |
| IIA | 0.208 | 0.440 | 0.075 | 0.782 | 0.530 | 0.035 | |
| IIB | 0.514 | <0.001 | 0.154 | 0.291 | 0.376 | 0.008 | |
| IIC | 0.339 | 0.011 | 0.449 | 0.001 | 0.311 | 0.021 | |
| IIA-C | 0.344 | <0.001 | 0.378 | <0.001 | 0.574 | <0.001 | |
| III | 0.104 | 0.274 | 0.195 | 0.039 | 0.176 | 0.063 | |
| All | 0.587 | <0.001 | 0.523 | <0.001 | 0.793 | <0.001 | |
| Antigen | IgM | IgG | ||||
|---|---|---|---|---|---|---|
| Group | Median | p-Value | Group | Median | p-Value | |
| AMA1N | - | - | - | I | 0.524 | <0.001 |
| IIA | 0.755 | <0.001 | ||||
| IIB | 0.779 | <0.001 | ||||
| IIC | 0.685 | <0.001 | ||||
| III | 0.459 | - | ||||
| AMA1C | - | - | - | I | 0.928 | <0.001 |
| IIA | 1.226 | <0.001 | ||||
| IIB | 0.774 | <0.001 | ||||
| IIC | 0.562 | <0.001 | ||||
| III | 0.245 | - | ||||
| AMA1 | I II III | 0.558 0.271 0.216 | <0.001 0.014 - | I | 1.324 | <0.001 |
| IIA | 2.035 | <0.001 | ||||
| IIB | 1.919 | <0.001 | ||||
| IIC | 1.279 | <0.001 | ||||
| III | 0.264 | - | ||||
| TLA | I II III | 1.392 0.770 0.431 | <0.001 <0.001 - | I | 0.650 | <0.001 |
| IIA | 1.478 | <0.001 | ||||
| IIB | 1.301 | <0.001 | ||||
| IIC | 0.913 | <0.001 | ||||
| III | 0.345 | - | ||||
| Gene Fragment | Primer Sequence | Corresponding Protein Residues/Plasmid Size |
|---|---|---|
| ama1N | 5’-TGGACAGCCCAGATCGCACGTCGGGGAATCCC-3’ 5’-GAATTCGGATCCGATATCTTTGGGCATTTACTGATGAA CGCATCTG-3’ | 67–287 (221 aa)/6057 bp |
| ama1C | 5’-TGGACAGCCCAGATCCAAATCAAGCTCTTCGCGG GTACAG-3’ 5’-GGTGGTGGTGCTCGAAGTAATCCCCCTCGACCATAACA-3’ | 287–569 (283 aa)/6192 bp |
| ama1 | 5’-TGGACAGCCCAGATCGCACGTCGGGGAATCCC-3’ 5’-GGTGGTGGTGCTCGAAGTAATCCCCCTCGACCATAACA-3’ | 67–569 (503 aa)/6855 bp |
| ELISA | Group of Serum Samples | Number of Serum Samples | Total |
|---|---|---|---|
| IgM | I (IgG+; IgM+; low avidity–suspected acute phase) | 40 | 156 |
| II (IgG+; IgM-; high avidity–chronic phase) | 40 | ||
| III (IgG-; IgM-–not infected individuals) | 76 | ||
| IgG | I (IgG+; IgM+; low avidity–suspected acute phase) | 55 | 287 |
| II (IgG+; IgM-; high avidity–chronic phase) | 120 | ||
| IIA (IgG > 300 IU/mL) | 16 | ||
| IIB (IgG 101–300 IU/mL) | 49 | ||
| IIC (IgG ≤ 100 IU/mL) | 55 | ||
| III (IgG-; IgM-–not infected individuals) | 112 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferra, B.; Holec-Gąsior, L.; Gatkowska, J.; Dziadek, B.; Dzitko, K. Toxoplasma gondii Recombinant antigen AMA1: Diagnostic Utility of Protein Fragments for the Detection of IgG and IgM Antibodies. Pathogens 2020, 9, 43. https://doi.org/10.3390/pathogens9010043
Ferra B, Holec-Gąsior L, Gatkowska J, Dziadek B, Dzitko K. Toxoplasma gondii Recombinant antigen AMA1: Diagnostic Utility of Protein Fragments for the Detection of IgG and IgM Antibodies. Pathogens. 2020; 9(1):43. https://doi.org/10.3390/pathogens9010043
Chicago/Turabian StyleFerra, Bartłomiej, Lucyna Holec-Gąsior, Justyna Gatkowska, Bożena Dziadek, and Katarzyna Dzitko. 2020. "Toxoplasma gondii Recombinant antigen AMA1: Diagnostic Utility of Protein Fragments for the Detection of IgG and IgM Antibodies" Pathogens 9, no. 1: 43. https://doi.org/10.3390/pathogens9010043
APA StyleFerra, B., Holec-Gąsior, L., Gatkowska, J., Dziadek, B., & Dzitko, K. (2020). Toxoplasma gondii Recombinant antigen AMA1: Diagnostic Utility of Protein Fragments for the Detection of IgG and IgM Antibodies. Pathogens, 9(1), 43. https://doi.org/10.3390/pathogens9010043







