Hepatitis C Virus among Female Sex Workers: A Cross-Sectional Study Conducted along Rivers and Highways in the Amazon Region
Abstract
:1. Introduction
2. Results
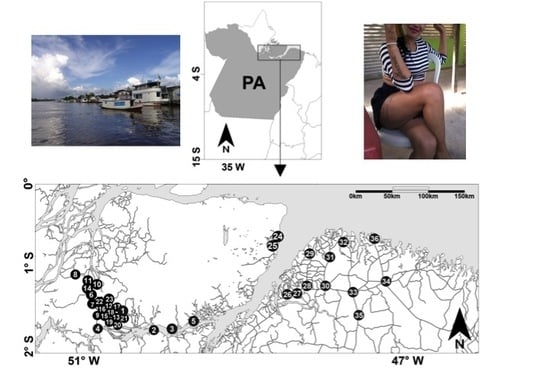
2.1. Study Sample
2.2. Characteristics of FSWs
2.3. Prevalence of Infections and Frequency of HCV Genotypes
2.4. Factors Associated with HCV Exposure
2.5. Substitutions Associated with Resistance
3. Discussion
4. Materials and Methods
4.1. Study Design and Participants
4.2. Laboratory Tests
4.3. Statistical Analysis
4.4. HCV Phylogenetic Analysis and Genotyping
4.5. Analysis of Mutations Associated with Resistance to Protease Inhibitors
4.6. Availability of Data
4.7. Ethical Review
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lanini, S.; Easterbrook, P.J.; Zumla, A.; Ippolito, G. Hepatitis C: Global epidemiology and strategies for control. Clin. Microbiol. Infect. 2016, 22, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Te, H.S.; Jensen, D.M. Epidemiology of hepatitis B and C viruses: A global overview. Clin. Liver Dis. 2010, 14, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, R.H.; Dusheiko, G. Natural history of hepatitis C. J. Hepatol. 2014, 61, S58–S68. [Google Scholar] [CrossRef]
- Prati, D. Transmission of hepatitis C virus by blood transfusions and other medical procedures: A global review. J. Hepatol. 2006, 45, 607–616. [Google Scholar] [CrossRef]
- Oliveira-Filho, A.B.; Santos, F.J.A.; Silva, F.Q.; Raiol, N.C.; Costa, C.C.S.; Piauiense, J.N.F. Hepatitis C virus infection status and associated factors among a multi-site sample of people who used illicit drugs in the Amazon region. BMC Infect. Dis. 2019, 19, 634. [Google Scholar] [CrossRef]
- Ferreira-Júnior, O.D.C.; Guimarães, M.D.C.; Damacena, G.N.; de Almeida, W.D.S.; de Souza-Júnior, P.R.B.; Szwarcwald, C.L. Prevalence estimates of HIV, syphilis, hepatitis B and C among female sex workers (FSW) in Brazil, 2016. Medicine 2018, 97, S3–S8. [Google Scholar] [CrossRef]
- Nijmeijer, B.M.; Koopsen, J.; Schinkel, J.; Prins, M.; Geijtenbeek, T.B. Sexually transmitted hepatitis C virus infections: Current trends, and recent advances in understanding the spread in men who have sex with men. J. Int. AIDS Soc. 2019, 22, e25348. [Google Scholar] [CrossRef]
- Petruzziello, A.; Marigliano, S.; Loquercio, G.; Cozzolino, A.; Cacciapuoti, C. Global epidemiology of hepatitis C virus infection: An up-date of the distribution and circulation of hepatitis C virus genotypes. World J. Gastroenterol. 2016, 22, 7824–7840. [Google Scholar] [CrossRef]
- Carter, W.; Connelly, S.; Struble, K. Reinventing HCV treatment: Past and future perspectives. J. Clin. Pharmacol. 2017, 57, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Jensen, D.M. A new era of hepatitis C therapy begins. N. Engl. J. Med. 2011, 364, 1272–1274. [Google Scholar] [CrossRef] [PubMed]
- Asselah, T.; Boyer, N.; Saadoun, D.; Martinot-Peignoux, M.; Marcellin, P. Direct-acting antivirals for the treatment of hepatitis C virus infection: Optimizing current IFN-free treatment and future perspectives. Liver Int. 2016, 36, S47–S57. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, I.M.; McHutchison, J.G.; Dusheiko, G.; Di Bisceglie, A.M.; Reddy, K.R.; Bzowej, N.H. Telaprevir for previously untreated chronic hepatitis C virus infection. N. Engl. J. Med. 2011, 364, 2405–2416. [Google Scholar] [CrossRef] [PubMed]
- Poordad, F.; McCone, J., Jr.; Bacon, B.R.; Bruno, S.; Manns, M.P.; Sulkowski, M.S. Boceprevir for untreated chronic HCV genotype 1 infection. N. Engl. J. Med. 2011, 364, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Lobato, C.M.O.; Codes, L.; Silva, G.F.; Souza, A.F.M.; Coelho, H.S.M.; Pedroso, M.L.A. Direct antiviral therapy for treatment of hepatitis C: A real-world study from Brazil. Ann. Hepatol. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health, Brazil. Boletim Epidemiológico de Hepatites Virais. Brasília: MS; 2018. Available online: http://www.aids.gov.br/pt-br/pub/2018/boletim-epidemiologico-de-hepatites-virais-2018 (accessed on 25 September 2019).
- Lampe, E.; Lewis-Ximenez, L.; Espírito-Santo, M.P.; Delvaux, N.M.; Pereira, S.A.; Peres-da-Silva, A. Genetic diversity of HCV in Brazil. Antivir. Ther. 2013, 18, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Lampe, E.; Espirito-Santo, M.P.; Martins, R.M.; Bello, G. Epidemic history of Hepatitis C virus in Brazil. Infect. Genet. Evol. 2010, 10, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Sawada, L.; Pinheiro, A.C.; Locks, D.; Pimenta, A.S.; Rezende, P.R.; Crespo, D.M. Distribution of hepatitis C virus genotypes among different exposure categories in the State of Pará, Brazilian Amazon. Rev. Soc. Bras. Med. Trop. 2011, 44, 8–12. [Google Scholar] [CrossRef]
- Nishiya, A.S.; de Almeida-Neto, C.; Ferreira, S.C.; Alencar, C.S.; Di-Lorenzo-Oliveira, C.; Levi, J.E. HCV genotypes, characterization of mutations conferring drug resistance to protease inhibitors, and risk factors among blood donors in São Paulo, Brazil. PLoS ONE 2014, 9, e86413. [Google Scholar] [CrossRef]
- Oliveira-Filho, A.B.; Pimenta, A.S.; Rojas, M.F.; Chagas, M.C.; Crescente, J.A.; Crespo, D.M. Prevalence and genotyping of hepatitis C virus in blood donors in the state of Pará, Northern Brazil. Mem. Inst. Oswaldo Cruz. 2010, 105, 103–106. [Google Scholar] [CrossRef]
- De Almeida, M.K.; Dos Santos, K.N.; Fecury, A.A.; de Oliveira, C.S.; Freitas, A.S.; Quaresma, J.A. Prevalence of viral hepatitis B and C in riverside communities of the Tucuruí Dam, Pará, Brazil. J. Med. Virol. 2012, 84, 1907–1912. [Google Scholar] [CrossRef]
- De Freitas, M.J.R.; Fecury, A.A.; de Almeida, M.K.; Freitas, A.S.; de Souza Guimarães, V.; da Silva, A.M. Prevalence of hepatitis C virus infection and genotypes in patient with chronic kidney disease undergoing hemodialysis. J. Med. Virol. 2013, 85, 1741–5174. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.S.; Silva, A.V.; Dos Santos, K.N.; Fecury, A.A.; Almeida, M.K.; Fernandes, A.P. Hepatitis B and C virus infection among Brazilian Amazon riparians. Rev. Soc. Bras. Med. Trop. 2011, 44, 546–550. [Google Scholar] [CrossRef]
- Succi, R.C.; Bensabath, G.; Soares, M.C.; Saraiva, A.S.; Peres, L.V. Hepatitis C virus (HCV) in children and adolescent hemophiliacs. J. Pediatr. 1998, 74, 325–332. [Google Scholar] [CrossRef]
- Soares, M.C.; Menezes, R.C.; Martins, S.J.; Bensabath, G. Epidemiology of hepatitis B, C and D viruses among indigenous Parakanã tribe in the Eastern Brazilian Amazon Region. Bol. Oficina Sanit. Panam. 1994, 117, 124–135. [Google Scholar] [PubMed]
- Oliveira-Filho, A.B.; Pimenta, A.S.; Rojas, M.F.; Chagas, M.C.; Crespo, D.M.; Crescente, J.A. Likely transmission of hepatitis C virus through sharing of cutting and perforating instruments in blood donors in the State of Pará, Northern Brazil. Cad. Saude Publica 2010, 26, 837–844. [Google Scholar] [CrossRef]
- Valois, R.C.; Maradei-Pereira, L.M.; Crescente, J.Â.; Oliveira-Filho, A.B.; Lemos, J.A. HCV infection through perforating and cutting material among candidates for blood donation in Belém, Brazilian Amazon. Rev. Inst. Med. Trop. Sao Paulo 2014, 56, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Filho, A.B.; Sawada, L.; Pinto, L.C.; Locks, D.; Bahia, S.L.; Castro, J.A. Epidemiological aspects of HCV infection in non-injecting drug users in the Brazilian state of Pará, eastern Amazon. Virol. J. 2014, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, S.D.; Silva-Oliveira, G.C.; Maradei-Pereira, L.M.; Crescente, J.Â.; Lemos, J.A.; Oliveira-Filho, A.B. Prevalence of HCV infection and associated factors among illicit drug users in Breves, State of Pará, northern Brazil. Rev. Soc. Bras. Med. Trop. 2014, 47, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Frade, P.C.; Raiol, N.C.; da Costa, L.M.; Pinheiro, L.M.; Silva-Oliveira, G.C.; Pinho, J.R. Prevalence and genotyping of hepatitis B virus: A cross-sectional study conducted with female sex workers in the Marajó Archipelago, Brazil. Int. J. STD AIDS 2019, 30, 902–910. [Google Scholar] [CrossRef]
- Puga, M.A.M.; Bandeira, L.M.; Weis, S.M.D.S.; Fernandes, F.R.P.; Castro, L.S.; Tanaka, T.S.O. High-risk behaviors for hepatitis B and C infections among female sex workers. Rev. Soc. Bras. Med. Trop. 2018, 51, 198–202. [Google Scholar] [CrossRef]
- Marx, M.A.; Murugavel, K.G.; Tarwater, P.M.; SriKrishnan, A.K.; Thomas, D.L.; Solomon, S. Association of hepatitis C virus infection with sexual exposure in southern India. Clin. Infect. Dis. 2003, 37, 514–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tohme, R.A.; Holmberg, S.D. Is sexual contact a major mode of hepatitis C virus transmission? Hepatology 2010, 52, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Barua, P.; Mahanta, J.; Medhi, G.K.; Dale, J.; Paranjape, R.S.; Thongamba, G. Sexual activity as risk factor for hepatitis C virus (HCV) transmission among the female sex workers in Nagaland. Indian J. Med. Res. 2012, 136, 30–35. [Google Scholar]
- Cavalcante, N.D.S.; Lima, H.R.R.; Tabosa, D.F.; Barbosa, E.D.S.S.; Costa, N.P.D.S.; Costa, L.M.D. Syphilis in female sex workers: An epidemiological study of the highway system of the state of Pará, northern Brazil. Rev. Soc. Bras. Med. Trop. 2019, 52, e20180064. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.Q.; Santos, F.J.A.; Andrade, A.P.; Pacheco, S.D.B.; Fischer, B.; Pinho, J.R.R. Hepatitis C virus infection among illicit drug users in an archipelago of the Amazon. Arch. Virol. 2018, 163, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, L.M.; Vieira, M.R.M.D.S.; Oliveira, J.F.G.; Trindade, J.Q.; Brasiliense, D.M.; Ferrari, S.F. High prevalence of sexual Chlamydia trachomatis infection in young women from Marajó Island, in the Brazilian Amazon. PLoS ONE 2018, 13, e0207853. [Google Scholar] [CrossRef]
- De Aguiar, S.A.; de Souza França, S.A.; Santana, B.B.; Santos, M.B.; Freitas, F.B.; Ferreira, G. Human T-lymphotropic virus 1aA circulation and risk factors for sexually transmitted infections in an Amazon geographic area with lowest human development index (Marajó Island, Northern Brazil). BMC Infect. Dis. 2017, 17, 758. [Google Scholar] [CrossRef] [Green Version]
- Anteneh, Z.A.; Agumas, Y.A.; Tarekegn, M. Sexually transmitted diseases among female commercial sex workers in Finote Selam town, northwest Ethiopia: A community-based cross-sectional study. HIV AIDS 2017, 9, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Aquino, P.S.; Nicolau, A.I.O.; Moura, E.R.F.; Pinheiro, A.K.B. Socio-demographic and sexual behavior profile of prostitutes in Fortaleza-CE. Texto Contexto Enferm. 2008, 17, 427–434. [Google Scholar] [CrossRef] [Green Version]
- Caetano, K.A.; França, D.D.; Carneiro, M.A.; Martins, R.M.; Stefani, M.M.; Kerr, L.R. Prevalence and virologic profile of HIV infections among female sex workers in Goiânia City, central Brazil. AIDS Patient Care STDs 2013, 27, 1–4. [Google Scholar] [CrossRef]
- Ishizaki, A.; Tran, V.T.; Nguyen, C.H.; Tanimoto, T.; Hoang, H.T.T.; Pham, H.V. Discrepancies in prevalence trends for HIV, hepatitis B virus, and hepatitis C virus in Haiphong, Vietnam from 2007 to 2012. PLoS ONE 2017, 12, e0179616. [Google Scholar] [CrossRef] [PubMed]
- Kazerooni, P.A.; Motazedian, N.; Motamedifar, M.; Sayadi, M.; Sabet, M.; Lari, M.A. The prevalence of human immunodeficiency virus and sexually transmitted infections among female sex workers in Shiraz, South of Iran: By respondent-driven sampling. Int. J. STD AIDS 2014, 25, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Penha, J.C.; Aquino, C.B.Q.; Neri, E.A.R.; Reis, T.G.O.; Aquino, P.S.; Pinheiro, A.K.B. Risk factors for sexually transmitted diseases among sex workers in the interior of Piaui, Brazil. Rev. Gaucha Enferm. 2015, 36, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.C.; Yim, Y.L.; Lynn, H. Sexually transmitted infections among female sex workers in Hong Kong: The role of migration status. J. Travel Med. 2011, 18, 1–7. [Google Scholar] [CrossRef]
- Souza, J.C.; Crispim, M.A.E.; Abrahim, C.; Fraiji, N.A.; Kiesslich, D.; Stefani, M.M.A. High rate of seromarkers for HIV, HBV and syphilis among blood donors using confidential unit exclusion, before and after HIV-NAT implementation at a major public blood bank in the Brazilian Amazon. Transfusion 2019, 59, 629–638. [Google Scholar] [CrossRef]
- Mason, L.M.; Duffell, E.; Veldhuijzen, I.K.; Petriti, U.; Bunge, E.M.; Tavoschi, L. Hepatitis B and C prevalence and incidence in key population groups with multiple risk factors in the EU/EEA: A systematic review. Eurosurveillance 2019, 24. [Google Scholar] [CrossRef]
- Trickey, A.; Fraser, H.; Lim, A.G.; Peacock, A.; Colledge, S.; Walker, J.G. The contribution of injection drug use to hepatitis C virus transmission globally, regionally, and at country level: A modelling study. Lancet Gastroenterol. Hepatol. 2019, 4, 435–444. [Google Scholar] [CrossRef] [Green Version]
- Boettiger, D.C.; Salazar-Vizcaya, L.; Dore, G.J.; Gray, R.T.; Law, M.G.; Callander, D. Can Australia reach the World Health Organization Hepatitis C elimination goal by 2025 among HIV-positive gay and bisexual men? Clin. Infect. Dis. 2019, 28, ciz164. [Google Scholar] [CrossRef]
- Lockart, I.; Matthews, G.V.; Danta, M. Sexually transmitted hepatitis C infection: The evolving epidemic in HIV-positive and HIV-negative MSM. Curr. Opin. Infect. Dis. 2019, 32, 31–37. [Google Scholar] [CrossRef]
- Leruez-Ville, M.; Kunstmann, J.M.; De Almeida, M.; Rouzioux, C.; Chaix, M.L. Detection of hepatitis C virus in the semen of infected men. Lancet 2000, 356, 42–43. [Google Scholar] [CrossRef]
- Manavi, M.; Watkins-Riedel, T.; Kucera, E.; Czerwenka, K.; Hofmann, H. Evidence of hepatitis C virus in cervical smears. J. Infect. 1999, 38, 60–61. [Google Scholar] [CrossRef]
- Nyamathi, A.; Robbins, W.A.; Fahey, J.L.; Wiley, D.; Pekler, V.A.; Longshore, D. Presence and predictors of hepatitis C virus RNA in the semen of homeless men. Biol. Res. Nurs. 2002, 4, 22–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halfon, P.; Riflet, H.; Renou, C.; Quentin, Y.; Cacoub, P. Molecular evidence of male-to-female sexual transmission of hepatitis C virus after vaginal and anal intercourse. J. Clin. Microbiol. 2001, 39, 1204–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosn, J.; Thibault, V.; Delaugerre, C.; Fontaine, H.; Lortholary, O.; Rouzioux, C. Sexually transmitted hepatitis C virus superinfection in HIV/hepatitis C virus co-infected men who have sex with men. AIDS 2008, 22, 658–661. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho-Mello, I.M.; Filho, J.E.; Gomes-Gouvêa, M.S.; de Mello Malta, F.; de Queiróz, A.T.; Pinho, J.R. Molecular evidence of horizontal transmission of hepatitis C virus within couples. J. Gen. Virol. 2010, 91, 691–696. [Google Scholar] [CrossRef]
- Zeminian, L.B.; Padovani, J.L.; Corvino, S.M.; Silva, G.F.; Pardini, M.I.; Grotto, R.M. Variability and resistance mutations in the hepatitis C virus NS3 protease in patients not treated with protease inhibitors. Mem. Inst. Oswaldo Cruz. 2013, 108, 13–17. [Google Scholar] [CrossRef]
- Hoffmann, L.; Ramos, J.A.; Souza, E.V.; Araújo Ramos, A.L.; Villela-Nogueira, C.A.; Urményi, T.P. Dynamics of resistance mutations to NS3 protease inhibitors in a cohort of Brazilian patients chronically infected with hepatitis C virus (genotype 1) treated with pegylated interferon and ribavirin: A prospective longitudinal study. Virol. J. 2013, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Peres-da-Silva, A.; de Almeida, A.J.; Lampe, E. Mutations in hepatitis C virus NS3 protease domain associated with resistance to specific protease inhibitors in antiviral therapy naïve patients. Arch. Virol. 2010, 155, 807–811. [Google Scholar] [CrossRef]
- Frade, P.C.R.; da Costa, L.M.; Lisboa, B.L.A.; Pinheiro, L.M.L.; Martins, L.C.; Silva-Oliveira, G.C. Strategies and actions to access and assist in the health promotion of female sex workers. In Public Health and Collective Health: Dialogue on Thematic Interfaces, 1st ed.; Silva Neto, B.R., Ed.; Atena Editora: Ponta Grossa, Brazil, 2019; Volume 4, pp. 214–224. [Google Scholar] [CrossRef]
- Wang, G.P.; Terrault, N.; Reeves, J.D.; Liu, L.; Li, E.; Zhao, L. Prevalence and impact of baseline resistance-associated substitutions on the efficacy of ledipasvir/sofosbuvir or simeprevir/sofosbuvir against GT1 HCV infection. Sci. Rep. 2018, 8, 3199. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Z.W.; Li, H.; Ren, H.; Hu, P. Prevalence of hepatitis C virus-resistant association substitutions to direct-acting antiviral agents in treatment-naïve hepatitis C genotype 1b-infected patients in western China. Infect. Drug Resist. 2017, 10, 377–392. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Wang, M.; Gong, Q.; Yu, D.; Chen, P.; Lin, J. Comparison of naturally occurring resistance-associated substitutions between 2008 and 2016 in Chinese patients with chronic hepatitis C virus Infection. Microb. Drug Resist. 2019, 25, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Grgic, I.; Planinic, A.; Santak, M.; Gorenec, L.; Lepej, S.Z.; Vince, A. High prevalence of Q80K among NS3 resistance-associated substitutions in subtype 1a patients with chronic hepatitis C prior to treatment with direct acting antivirals: The Croatian Data. Hepat. Mon. 2017, 17, e45543. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T. Female Sex Workers and STI/HIV in Ba Ria—Vung Tau Province, Vietnam: Transmission, Knowledge, Attitudes and Sexual Behaviors. Ph.D Thesis, Griffith University, Queensland, Australia, 2016. Available online: https://research-repository.griffith.edu.au (accessed on 25 September 2018).
- Enomoto, N.; Takada, A.; Nakao, T.; Date, T. There are two major types of hepatitis C virus in Japan. Biochem. Biophys. Res. Commun. 1990, 170, 1021–1025. [Google Scholar] [CrossRef]
- Larsson, A. AliView: A fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 2014, 30, 3276–3278. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefort, V.; Longueville, J.E.; Gascuel, O. SMS: Smart Model Selection in PhyML. Mol. Biol. Evol. 2017, 34, 2422–2424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anisimova, M.; Gascuel, O. Approximate likelihood-ratio test for branches: A fast, accurate, and powerful alternative. Syst. Biol. 2006, 55, 539–552. [Google Scholar] [CrossRef]
- Sarrazin, C.; Kieffer, T.L.; Bartels, D.; Hanzelka, B.; Müh, U.; Welker, M. Dynamic hepatitis C virus genotypic and phenotypic changes in patients treated with the protease inhibitor telaprevir. Gastroenterology 2007, 132, 1767–1777. [Google Scholar] [CrossRef]
- Sarrazin, C.; Rouzier, R.; Wagner, F.; Forestier, N.; Larrey, D.; Gupta, S.K. SCH 503034, a novel hepatitis C virus protease inhibitor, plus pegylated interferon alpha-2b for genotype 1 nonresponders. Gastroenterology 2007, 132, 1270–1278. [Google Scholar] [CrossRef]
- Curry, S.; Qiu, P.; Tong, X. Analysis of HCV resistance mutations during combination therapy with protease inhibitor boceprevir and PEG-IFN alpha-2b using TaqMan mismatch amplification mutation assay. J. Virol. Methods 2008, 153, 156–162. [Google Scholar] [CrossRef]
- Susser, S.; Welsch, C.; Wang, Y.; Zettler, M.; Domingues, F.S.; Karey, U. Characterization of resistance to the protease inhibitor boceprevir in hepatitis C virus-infected patients. Hepatology 2009, 50, 1709–1718. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | N Total | Anti-HCV+ (%) | Anti-HCV- (%) | p *** |
|---|---|---|---|---|
| Total | 412 | 44 (10.7) | 368 (89.3) | - |
| Age (years) | ||||
| 18–24 | 172 | 22 (12.8) | 150 (87.2) | 0.49 |
| 25–30 | 167 | 15 (9.0) | 152 (91.0) | |
| >30 | 73 | 7 (9.6) | 66 (90.4) | |
| Color/Race (self-declaration) | ||||
| White | 105 | 12 (11.4) | 93 (88.6) | 0.61 |
| Brown (mixed race) | 192 | 21 (10.9) | 171 (89.1) | |
| Black | 115 | 9 (7.8) | 106 (92.2) | |
| Origin | ||||
| Born in the state of Pará | 318 | 36 (11.3) | 282 (88.7) | 0.44 |
| Not born in the state of Pará | 94 | 8 (8.5) | 86 (91.5) | |
| Sexual orientation | ||||
| Heterosexual | 381 | 40 (10.5) | 341 (89.5) | 0.68 |
| Same sex (including bisexual) | 31 | 4 (12.9) | 27 (87.1) | |
| Education Level | ||||
| Illiterate | 72 | 11 (15.3) | 61 (84.7) | 0.17 |
| Elementary school (incomplete/complete) | 221 | 26 (11.8) | 195 (88.2) | |
| High school (incomplete/complete) | 110 | 6 (5.5) | 104 (94.5) | |
| University (incomplete) | 9 | 1 (11.1) | 8 (88.9) | |
| Marital status * | ||||
| Married or co-habitating | 33 | 5 (15.2) | 28 (84.8) | 0.39 |
| Single, separated or widowed | 379 | 39 (10.3) | 340 (89.7) | |
| Monthly income (minimum wage) * | ||||
| ≤1 ** | 307 | 38 (12.4) | 269 (87.6) | 0.15 |
| 2–3 | 79 | 5 (6.3) | 74 (93.7) | |
| >3 | 26 | 1 (3.8) | 25 (96.2) |
| Marker (Laboratory Test) | Prevalence | 95% CI | |
|---|---|---|---|
| Positive/Total | % | ||
| HCV infection | |||
| All (EIA+) | 44/412 | 10.7 | 5.8–15.1 |
| Active (EIA+ and PCR+) | 32/412 | 7.8 | 2.5–12.5 |
| Non-active (EIA+ Immuno Blot+ and PCR-) | 12/412 | 2.9 | 0.0–8.2 |
| Exposed (EIA+ and Immuno Blot+ or PCR+) | 44/412 | 10.7 | 5.8–15.1 |
| HCV genotypes | |||
| Genotype 1 | 26/32 | 81.3 | 76.9–86.5 |
| Genotype 3 | 6/32 | 18.7 | 14.7–23.4 |
| Risk Factors | N Total | N Anti-HCV+ | Bivariate OR (95% CI) | Multivariate aOR (95% CI) |
|---|---|---|---|---|
| Up to elementary school vs. high school or more | 293 | 38 | 2.8 (1.2–6.8) | 2.3 (1.3–6.3) |
| Up to one minimum wage vs. more than one minimum wage * | 307 | 41 | 5.3 (1.6–17.4) | 5.5 (1.7–16.8) |
| Illicit drug use (injectable or inhaled) vs. did not use illicit drugs * | 130 | 34 | 9.7 (4.3–20.1) | 9.4 (3.9–19.5) |
| Unprotected sex vs. protected sex ** | 156 | 42 | 35.8 (11.1–86.3) | 32.1 (10.8–74.3) |
| More than five sexual partners vs. up to five sexual partners ** | 191 | 28 | 2.3 (1.2–4.5) | 2.5 (1.3–4.2) |
| Condom exemption for clients paying extra vs. condom use for clients paying extra ** | 131 | 38 | 13.4 (5.3–31.4) | 14.2 (4.9–28.4) |
| More than seven years working in the sex trade vs. up to seven years working in the sex trade | 188 | 33 | 4.1 (2.1–8.3) | 4.6 (1.8–7.6) |
| Changes in genitalia (wart, wound, and/or itching) vs. no changes in genitalia * | 249 | 35 | 2.9 (1.3–5.9) | 3.2 (1.2–6.0) |
| Did not perform medical/gynecological examination vs. performed medical/gynecological examination * | 225 | 36 | 4.3 (1.9–9.5) | 4.5 (1.7–8.2) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira-Filho, A.B.; Aires, D.W.F.; Cavalcante, N.S.; Raiol, N.C.; Lisboa, B.L.A.; Frade, P.C.R.; da Costa, L.M.; Pinheiro, L.M.L.; Machado, L.F.A.; Martins, L.C.; et al. Hepatitis C Virus among Female Sex Workers: A Cross-Sectional Study Conducted along Rivers and Highways in the Amazon Region. Pathogens 2019, 8, 236. https://doi.org/10.3390/pathogens8040236
Oliveira-Filho AB, Aires DWF, Cavalcante NS, Raiol NC, Lisboa BLA, Frade PCR, da Costa LM, Pinheiro LML, Machado LFA, Martins LC, et al. Hepatitis C Virus among Female Sex Workers: A Cross-Sectional Study Conducted along Rivers and Highways in the Amazon Region. Pathogens. 2019; 8(4):236. https://doi.org/10.3390/pathogens8040236
Chicago/Turabian StyleOliveira-Filho, Aldemir B., Diego Wendel F. Aires, Natalia S. Cavalcante, Nairis Costa Raiol, Brenda Luena A. Lisboa, Paula Cristina R. Frade, Luana M. da Costa, Luiz Marcelo L. Pinheiro, Luiz Fernando A. Machado, Luisa C. Martins, and et al. 2019. "Hepatitis C Virus among Female Sex Workers: A Cross-Sectional Study Conducted along Rivers and Highways in the Amazon Region" Pathogens 8, no. 4: 236. https://doi.org/10.3390/pathogens8040236
APA StyleOliveira-Filho, A. B., Aires, D. W. F., Cavalcante, N. S., Raiol, N. C., Lisboa, B. L. A., Frade, P. C. R., da Costa, L. M., Pinheiro, L. M. L., Machado, L. F. A., Martins, L. C., Silva-Oliveira, G. C., Pinho, J. R. R., Kupek, E., & Lemos, J. A. R. (2019). Hepatitis C Virus among Female Sex Workers: A Cross-Sectional Study Conducted along Rivers and Highways in the Amazon Region. Pathogens, 8(4), 236. https://doi.org/10.3390/pathogens8040236