Mechanisms Involved in the Persistence of Babesia canis Infection in Dogs
Abstract
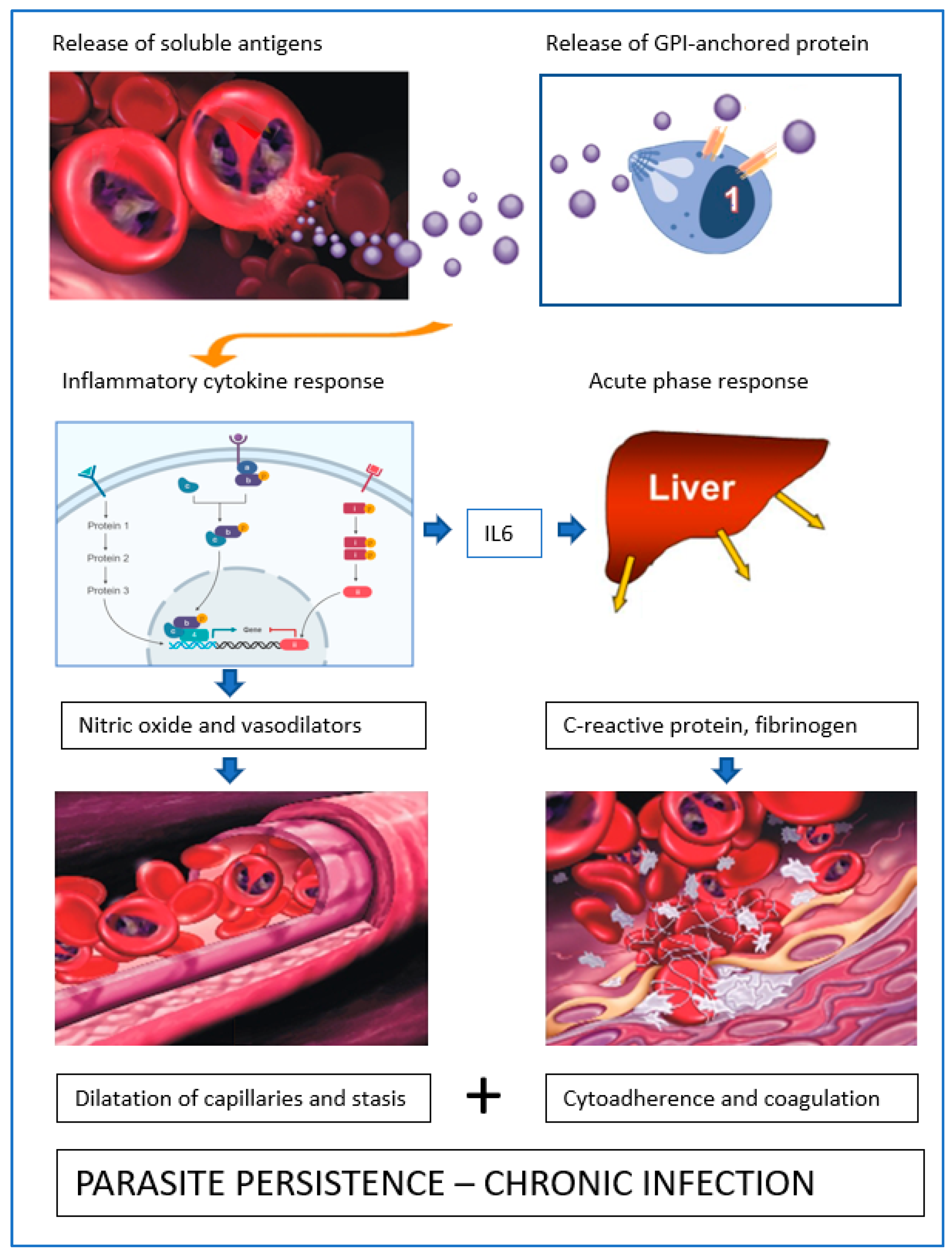
1. Introduction
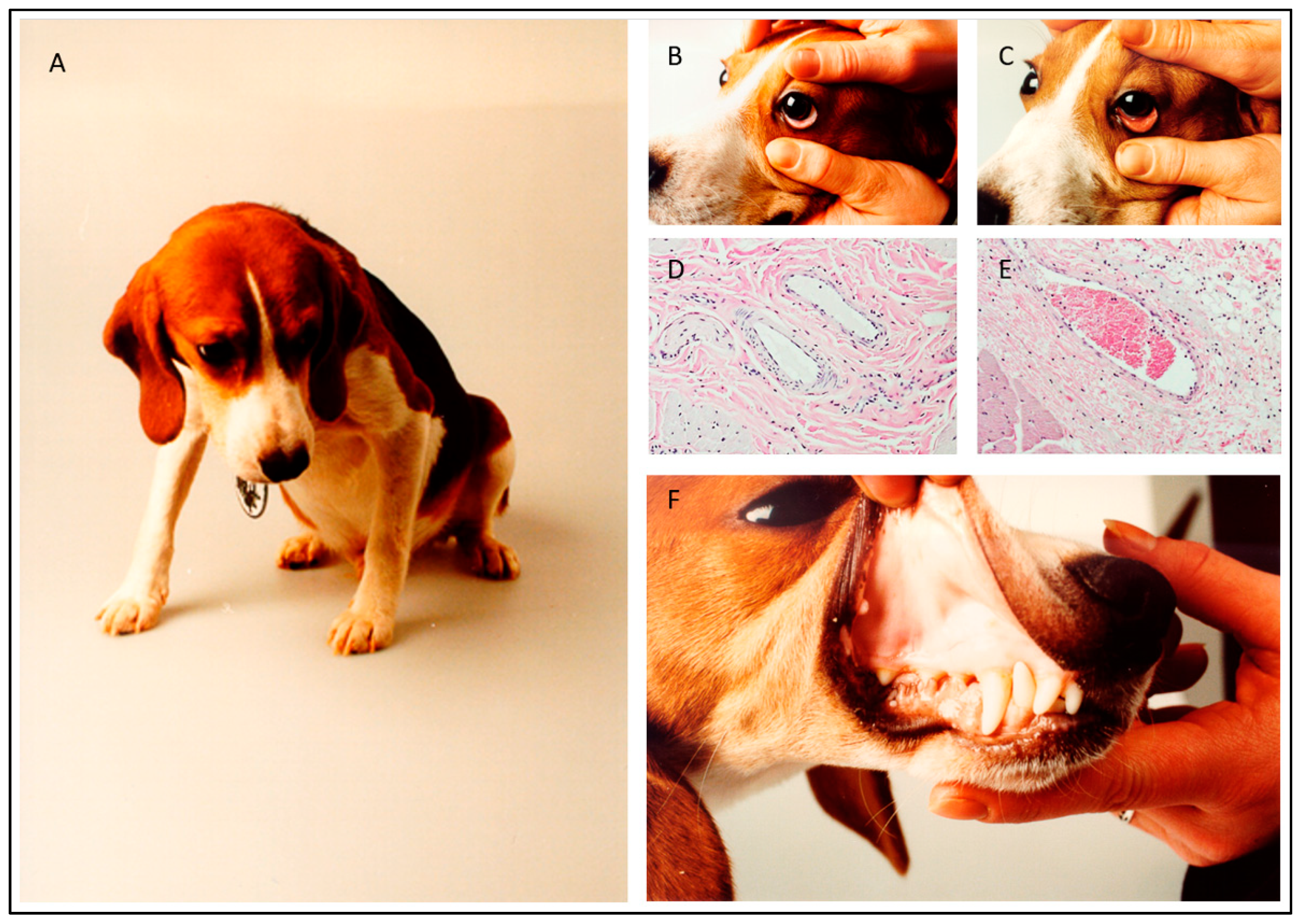
2. Experimental Infection Models
3. Blood Circulation Problems
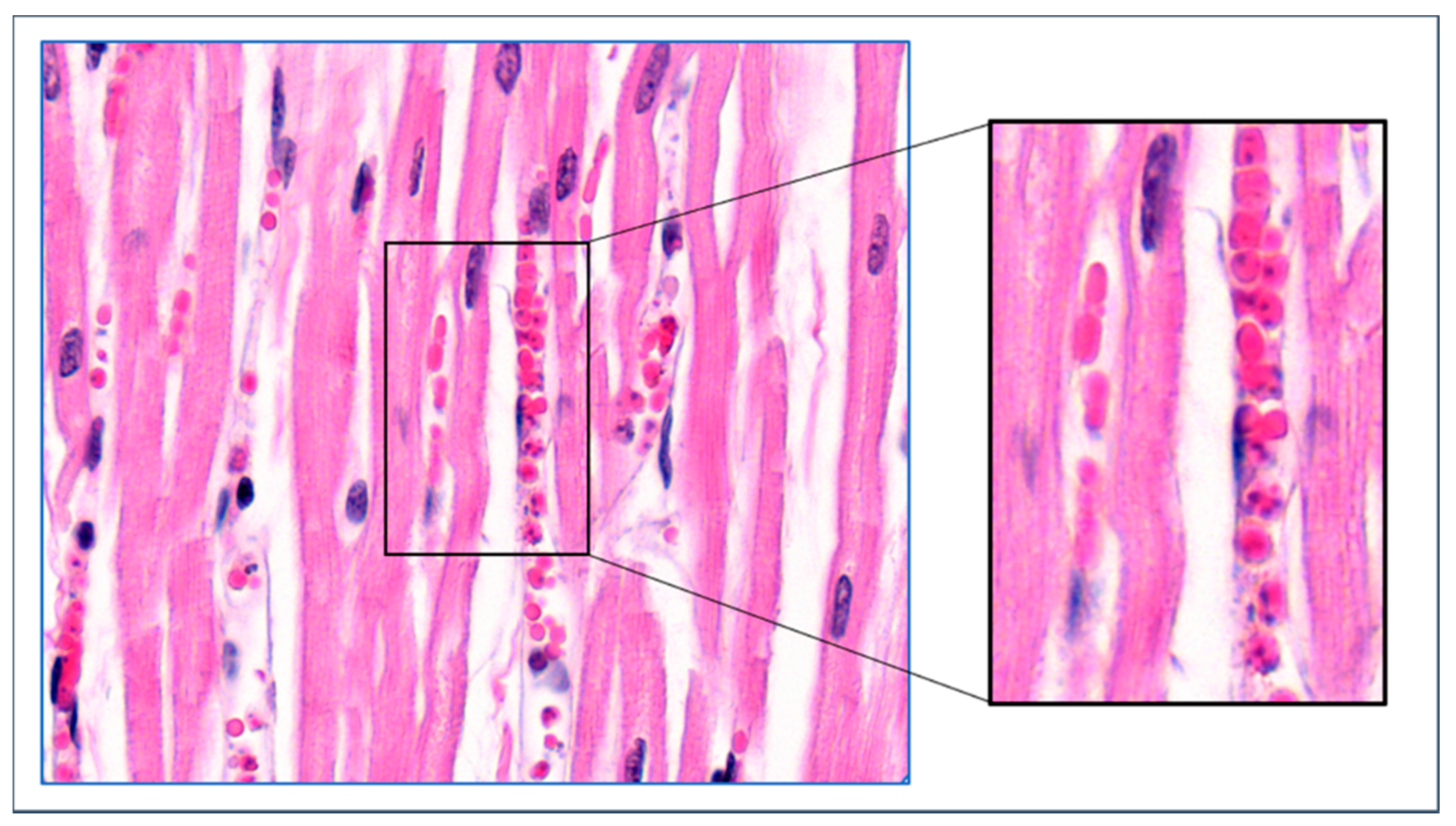
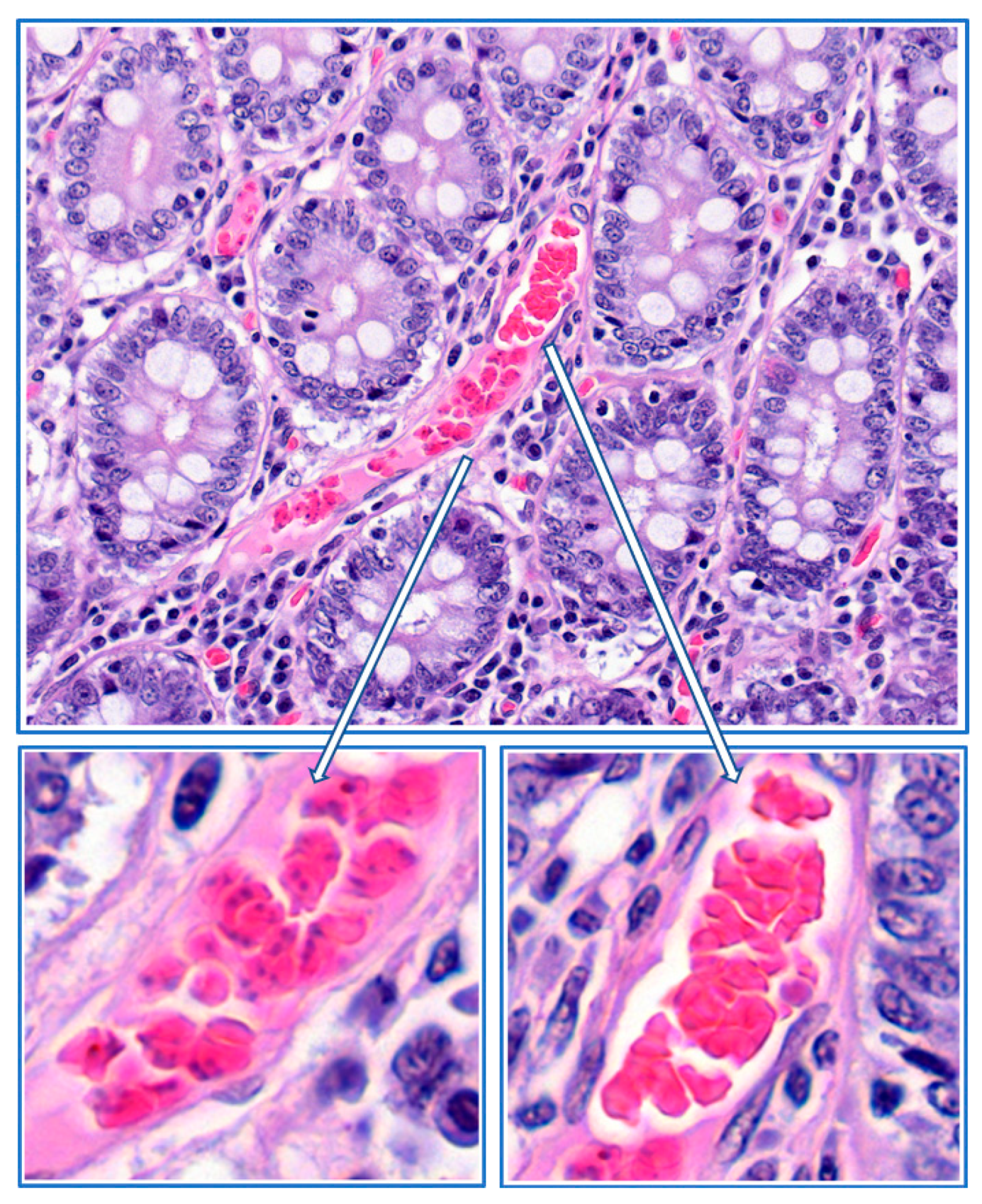
4. Mechanisms of Obstruction of Capillaries
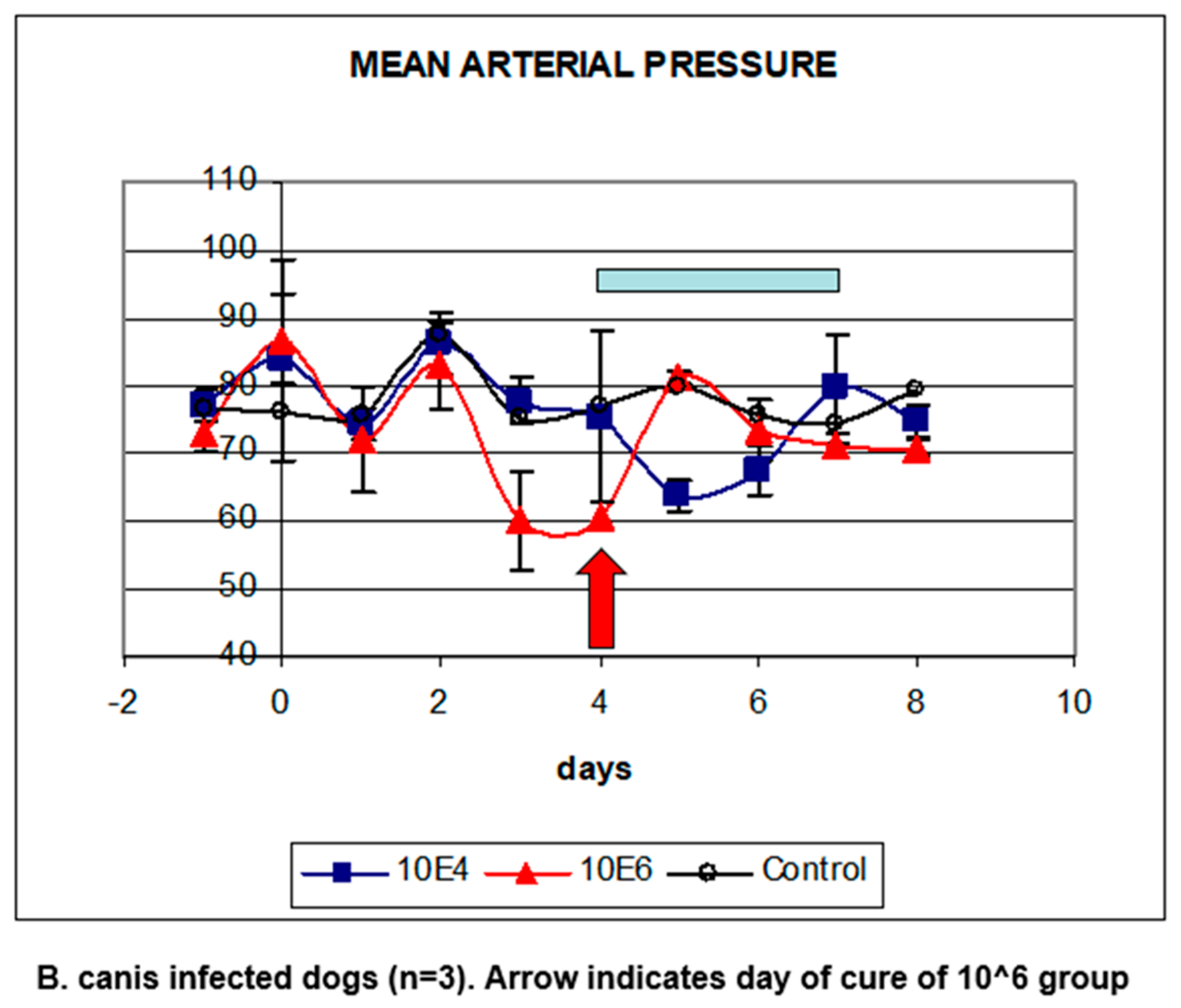
5. Babesia Infection Induces Hypotension
6. Compensated Hypotension
7. Acute Phase Response-Coagulation
8. Parasite Sequestration
9. Local Proliferation
10. Role of Soluble Proteins from B. canis-Infected Erythrocytes
Funding
Acknowledgments
Conflicts of Interest
References
- Reyers, F.; Leisewitz, A.L.; Lobetti, R.G.; Milner, R.J.; Jacobson, L.S.; Van Zyl, M. Canine babesiosis in South-Africa—More than one disease? Does this serve as a model for falciparum malaria? Ann. Trop. Med. Parasitol. 1998, 92, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, L.S. The South African form of severe and complicated canine babesiosis: Clinical advances 1994–2004. Vet. Parasitol. 2006, 138, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Matijatko, V.; Torti, M.; Schetters, T.H.P.M. Canine babesiosis in Europe: How many diseases? Trends Parasitol. 2012, 28, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Schetters, T.H.; Kleuskens, J.; Scholtes, N.; Moubri, K.; Gorenflot, A. Different Babesia canis isolates, different diseases. Parasitology 1997, 115, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Bourdoiseau, G. Canine babesiosis in France. Vet. Parasitol. 2006, 138, 118–125. [Google Scholar] [CrossRef]
- Matjila, T.P.; Carcy, B.; Leisewitz, A.L.; Schetters, T.H.; Jongejan, F.; Penzhorn, B.L. Preliminary evaluation of the BrEMA1 gene as a tool for associating Babesia rossi genotypes and clinical manifestation of canine babesiosis. J. Clin. Microbiol. 2009, 47, 3586–3592. [Google Scholar] [CrossRef] [PubMed]
- Shortt, H.E. Babesia canis: The life cycle and laboratory maintenance in its arthropod and mammalian hosts. Int. J. Parasitol. 1973, 3, 119–148. [Google Scholar] [CrossRef]
- Schetters, T.H.P.M.; Kleuskens, J.; Van De Crommert, J.; De Leeuw, P.; Finizio, A.-L.; Gorenflot, A. Systemic inflammatory responses in dogs experimentally infected with Babesia canis: A haematological study. Vet. Parasitol. 2009, 162, 7–15. [Google Scholar] [CrossRef]
- Beugnet, F.; Halos, L.; Larsen, D.; Labuschagne, M.; Erasmus, H.; Fourie, J. The ability of an oral formulation of afoxolaner to block the transmission of Babesia canis by Dermacentor reticulatus ticks to dogs. Parasit. Vectors 2014, 7, 283. [Google Scholar] [CrossRef]
- Nuttall, G.H.F. Canine piroplasmosis. I. J. Hyg. 1904, 4, 219–252. [Google Scholar] [CrossRef]
- Schetters, T.H.P.M. Vaccination against canine babesiosis. Trends Parasitol. 2005, 21, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Schetters, T.H.P.M.; Kleuskens, J.A.G.M.; Scholtes, N.C.; Bos, H.J. Vaccination of dogs against Babesia canis infection using parasite antigens derived from in vitro culture. Parasite Immunol. 1992, 14, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Nocard, E.; Motas, C.S. Contribution à l’étude de la piroplasmose canine. Ann. Inst. Pasteur 1902, 16, 257–290. [Google Scholar]
- Schetters, T.H.; Montenegro-James, S. Vaccines against babesiosis using soluble parasite antigens. Parasitol. Today 1995, 11, 456–462. [Google Scholar] [CrossRef]
- Schetters, T.H.P.M.; Kleuskens, J.A.G.M.; Scholtes, N.C.; Pasman, J.W.; Bos, H.J. Vaccination of dogs against Babesia canis infection using antigen from culture supernatant with an emphasis on clinical babesiosis. Vet. Parasitol. 1994, 52, 219–233. [Google Scholar] [CrossRef]
- Graham-Smith, G.S. Canine piroplasmosis. III. Morbid Anatomy. J. Hyg. 1905, 5, 250–267. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wright, I.G.; Kerr, J.D. Hypotension in acute Babesia bovis (=B. argentina) infections of splenectomised calves. J. Comp. Pathol. 1977, 87, 531–537. [Google Scholar] [CrossRef]
- Jacobson, L.S.; Lobetti, R.G.; Vaughan-Scott, T. Blood pressure changes in dogs with babesiosis. J. S. Afr. Vet. Assoc. 2000, 71, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Feldman, H.A.; Murphy, M.D. The effect of alterations in blood volume in the anemia and hypoproteinemia of human malaria. J. Clin. Investig. 1945, 24, 780–792. [Google Scholar] [CrossRef]
- Brooks, M.H.; Malloy, J.P.; Bartelloni, P.J.; Tigertt, W.D.; Sheehy, T.W.; Barry, K.G. Pathophysiology of acute falciparum malaria. I. Correlation of clinical and biochemical abnormalities. Am. J. Med. 1967, 43, 735–744. [Google Scholar] [CrossRef]
- Sitprija, V. Altered fluid, electrolyte and mineral status in tropical disease, with an emphasis on malaria and leptospirosis. Nat. Clin. Pract. Nephrol. 2008, 4, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Maegraith, B.; Gilles, H.M.; Devakul, K. Pathological processes in Babesia canis infections. Z. Tropenmed. Parasit. 1957, 8, 485–514. [Google Scholar]
- Schetters, T.H.P.M.; Kleuskens, J.A.G.M.; Scholtes, N.C.; Goovaerts, D. Parasite localisation and dissemination in the Babesia-infected host. Ann. Trop. Med. Parasitol. 1998, 92, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Konto, M.; Biu, A.A.; Ahmed, M.I.; Mbaya, A.W.; Luka, J. Clinico-biochemical responses of dogs to experimental infection with Babesia canis. Vet. World 2014, 7, 113–118. [Google Scholar] [CrossRef][Green Version]
- Wright, I.G.; Goodger, B.V. Pathogenesis of babesiosis. In Babesiosis of Domestic Animals and Man; Ristic, M., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 1988; pp. 99–118. [Google Scholar]
- Goddard, A.; Wiinberg, B.; Schoeman, J.P.; Kristensen, A.T.; Kjelgaard-Hansen, M. Mortality in virulent canine babesiosis is associated with consumptive coagulopathy. Vet. J. 2013, 196, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Seydel, K.B.; Milner, D.A.; Kamiza, S.B.; Molyneux, M.E.; Taylor, T.E. The distribution and intensity of parasite sequestration in comatose Malawian children. J. Inf. Dis. 2006, 194, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Allred, D.R. Immune evasion by Babesia bovis and Plasmodium falciparum: Cliff-dwellers of the parasite world. Parasitol. Today 1995, 11, 100–105. [Google Scholar] [CrossRef]
- Eichenberger, R.; Ramakrishnan, C.; Russo, G.; Deplazes, P.; Hehl, A. Genome-wide analysis of gene expression and protein secretion of Babesia canis during virulent infection identifies potential pathogenicity factors. Sci. Rep. 2017, 7, 3357. [Google Scholar] [CrossRef]
- Schetters, T.H.P.M.; Moubri, K.; Cooke, B. Comparison of Babesia rossi and Babesia canis isolates with emphasis on effects of vaccination with soluble parasite antigens. J. S. Afr. Vet. Assoc. 2009, 80, 75–78. [Google Scholar] [CrossRef]
- Schetters, T.H.; Goovaerts, D. Sequestration or proliferation? Parasitol. Today 1996, 12, 84. [Google Scholar] [CrossRef]
- Silamut, K.; Phu, N.H.; Whitty, C.; Turner, G.D.H.; Louwrier, K.; Mai, N.T.H.; Simpson, J.A.; Hien, T.T.; White, N.J. A quantitative analysis of the microvascular sequestration of malaria parasites in the human brain. Am. J. Pathol. 1999, 155, 395–410. [Google Scholar] [CrossRef]
- Schetters, T.H.P.M.; Scholtes, N.C.; Kleuskens, J.A.G.M.; Bos, H.J. Not peripheral parasitaemia but the level of soluble parasite antigen in plasma correlates with vaccine efficacy against Babesia canis. Parasite Immunol. 1996, 18, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Finizio, A.-L.; Kleuskens, J.A.G.M.; Van de Crommert, J.; Gorenflot, A.; Carcy, B.; Schetters, T.H.P.M. Soluble parasite antigens from Babesia canis do not directly activate the kallikrein system in dogs infected with Babesia canis. Vet. Parasitol. 2011, 176, 132–138. [Google Scholar] [CrossRef] [PubMed]









| Parasite Distribution in Dogs That Succumbed To Babesiosis (Adapted From Graham-Smith 1905) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peripheral Blood | Brain Capillaries | Spinal cord Vessels | Lungs Capillaries | Heart Capillaries | Liver Capillaries | Spleen Pulp | Capillaries | Kidney Capillaries | Adrenals Capillaries | Pancreas Capillaries | Lymph nodes Capillaries | Marrow femur | |
| Dilatation | + | + | ++ | ++ | +++ | yes | ++ | + | +++ | + | na | ||
| PE-high | 6% | 88% | neg | 52% | 72% | 53% | 12% | 48% | 95% | 70% | Numerous | 53.5% | |
| PE-low | 0.10% | nd | neg | 4 | nd | 23% | 3.7% | nd | nd | nd | nd | 2.0% | |
| # Free Par to PE | 0.06 | 0.50 | na | 1.50 | nd | 0.40 | 0.11 | 0.45 | 0.21 | 0.11 | 0.13 | ||
| #Par/RBC | % | % | % | % | % | % | % | % | % | % | |||
| 1 | 67.76 | 38.33 | 38.97 | 40.84 | 46.27 | 48.37 | 34.33 | 50.93 | 40.03 | 29.59 | |||
| 2 | 28.66 | 39.97 | 52.37 | 50.08 | 42.06 | 43.52 | 52.00 | 44.14 | 46.04 | 52.06 | |||
| 3 | 0.71 | 2.02 | 1.06 | 1.25 | 1.14 | 1.89 | 1.71 | 0.53 | 0.32 | 1.94 | |||
| 4 | 2.54 | 14.75 | 6.70 | 6.81 | 8.79 | 5.23 | 10.01 | 4.12 | 8.86 | 12.39 | |||
| 5 | 0.04 | 0.25 | 0.07 | 0.19 | 0.26 | 0.15 | 0.21 | 0.16 | 0.40 | ||||
| 6 | 0.12 | 1.38 | 0.31 | 0.22 | 0.48 | 0.39 | 0.78 | 0.16 | 0.94 | 1.19 | |||
| 7 | 0.03 | 0.05 | 0.02 | 0.03 | 0.16 | 0.18 | |||||||
| 8 | 0.12 | 2.52 | 0.31 | 0.47 | 0.84 | 0.36 | 0.85 | 0.10 | 2.69 | 1.80 | |||
| 9 | 0.01 | 0.03 | |||||||||||
| 10 | 0.01 | 0.10 | 0.04 | 0.09 | 0.07 | 0.31 | 0.11 | ||||||
| 11 | |||||||||||||
| 12 | 0.01 | 0.25 | 0.02 | 0.16 | 0.02 | ||||||||
| 13 | 0.01 | ||||||||||||
| 14 | 0.13 | 0.07 | 0.02 | 0.01 | |||||||||
| 15 | 0.13 | ||||||||||||
| 16 | 0.01 | 0.25 | 0.02 | 0.02 | 0.32 | 0.15 | |||||||
| Total COUNTED | 22,589 | 793 | 2910 | 5345 | 5347 | 5792 | 1398 | 1869 | 632 | 8660 | |||
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schetters, T. Mechanisms Involved in the Persistence of Babesia canis Infection in Dogs. Pathogens 2019, 8, 94. https://doi.org/10.3390/pathogens8030094
Schetters T. Mechanisms Involved in the Persistence of Babesia canis Infection in Dogs. Pathogens. 2019; 8(3):94. https://doi.org/10.3390/pathogens8030094
Chicago/Turabian StyleSchetters, Theo. 2019. "Mechanisms Involved in the Persistence of Babesia canis Infection in Dogs" Pathogens 8, no. 3: 94. https://doi.org/10.3390/pathogens8030094
APA StyleSchetters, T. (2019). Mechanisms Involved in the Persistence of Babesia canis Infection in Dogs. Pathogens, 8(3), 94. https://doi.org/10.3390/pathogens8030094





