The Mechanism of Action of Ursolic Acid as a Potential Anti-Toxoplasmosis Agent, and Its Immunomodulatory Effects
Abstract
1. Introduction
2. Results
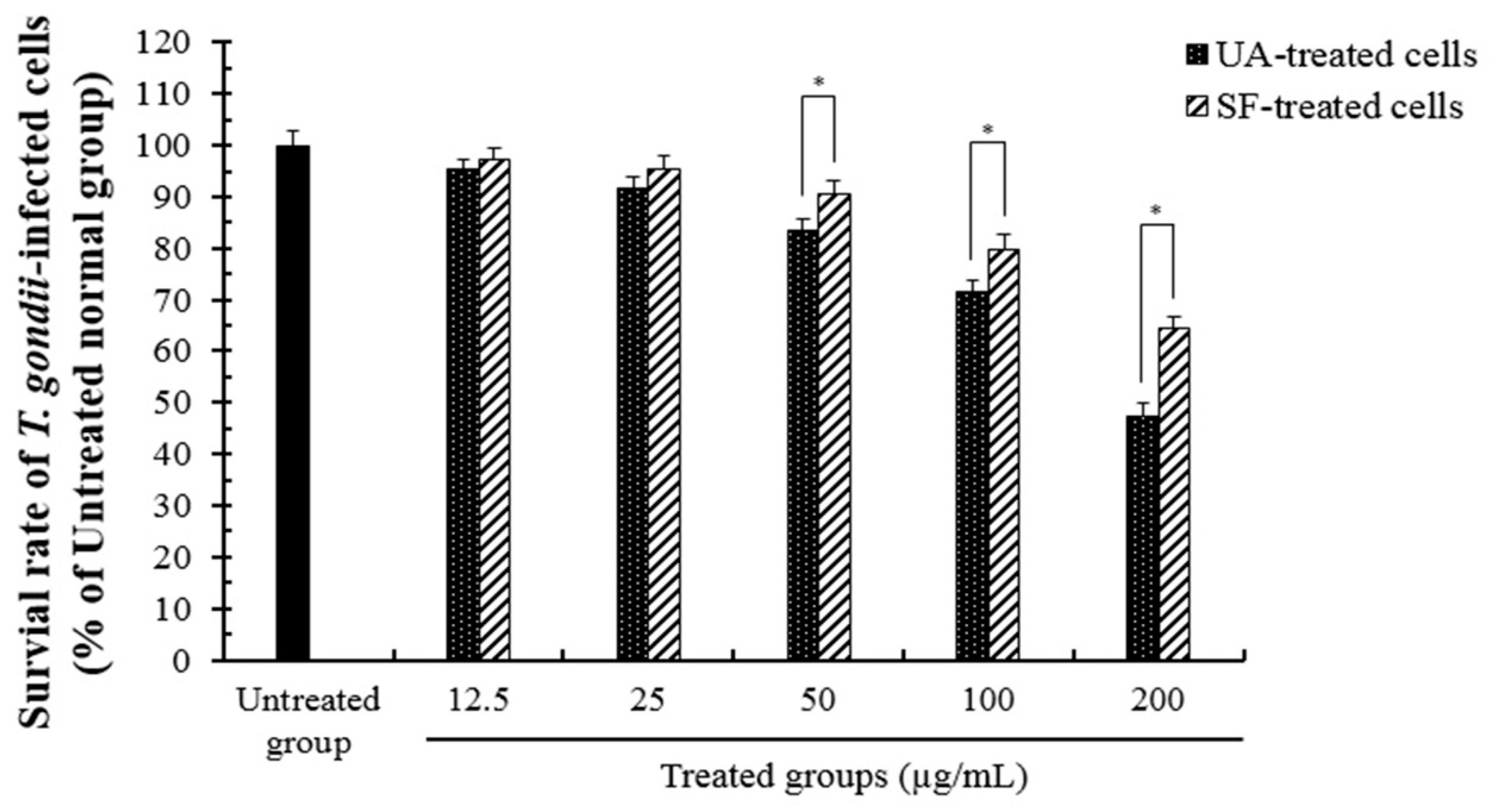
2.1. Anti-Parasitic Effect of Ursolic Acid Against the Viability of T. gondii
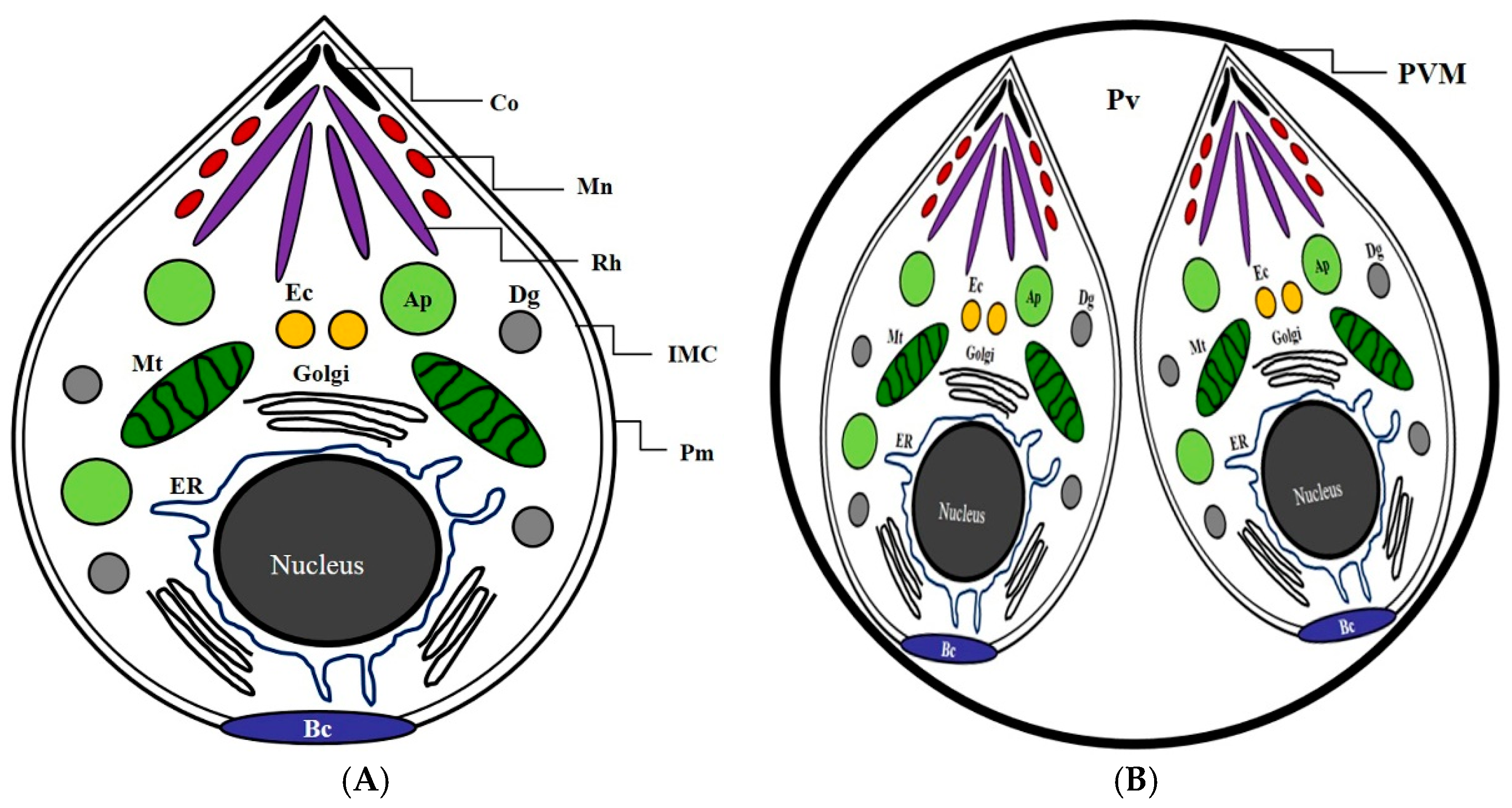
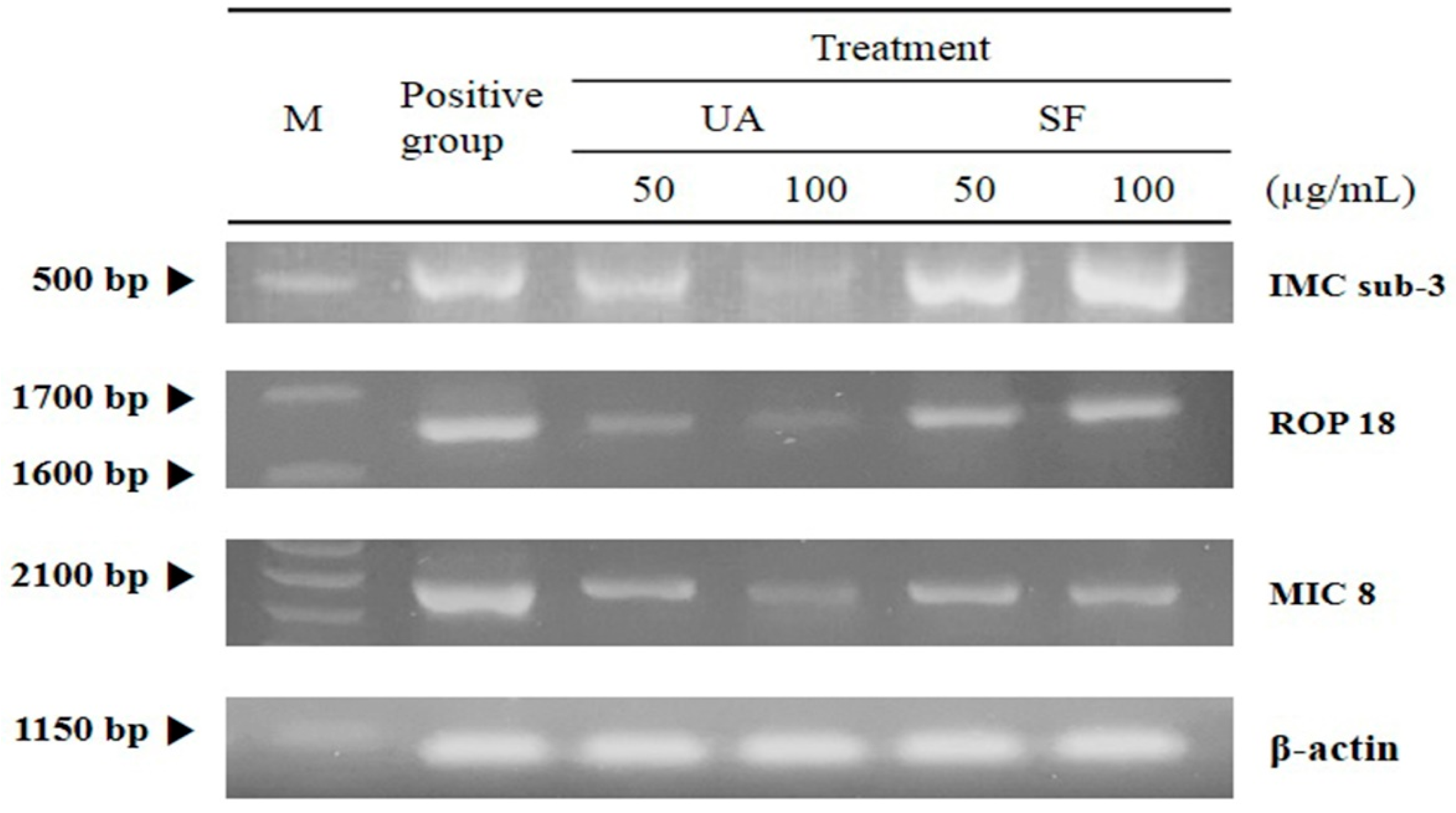
2.2. The Inhibitory Effects of Subcellular Organelles of T. gondii
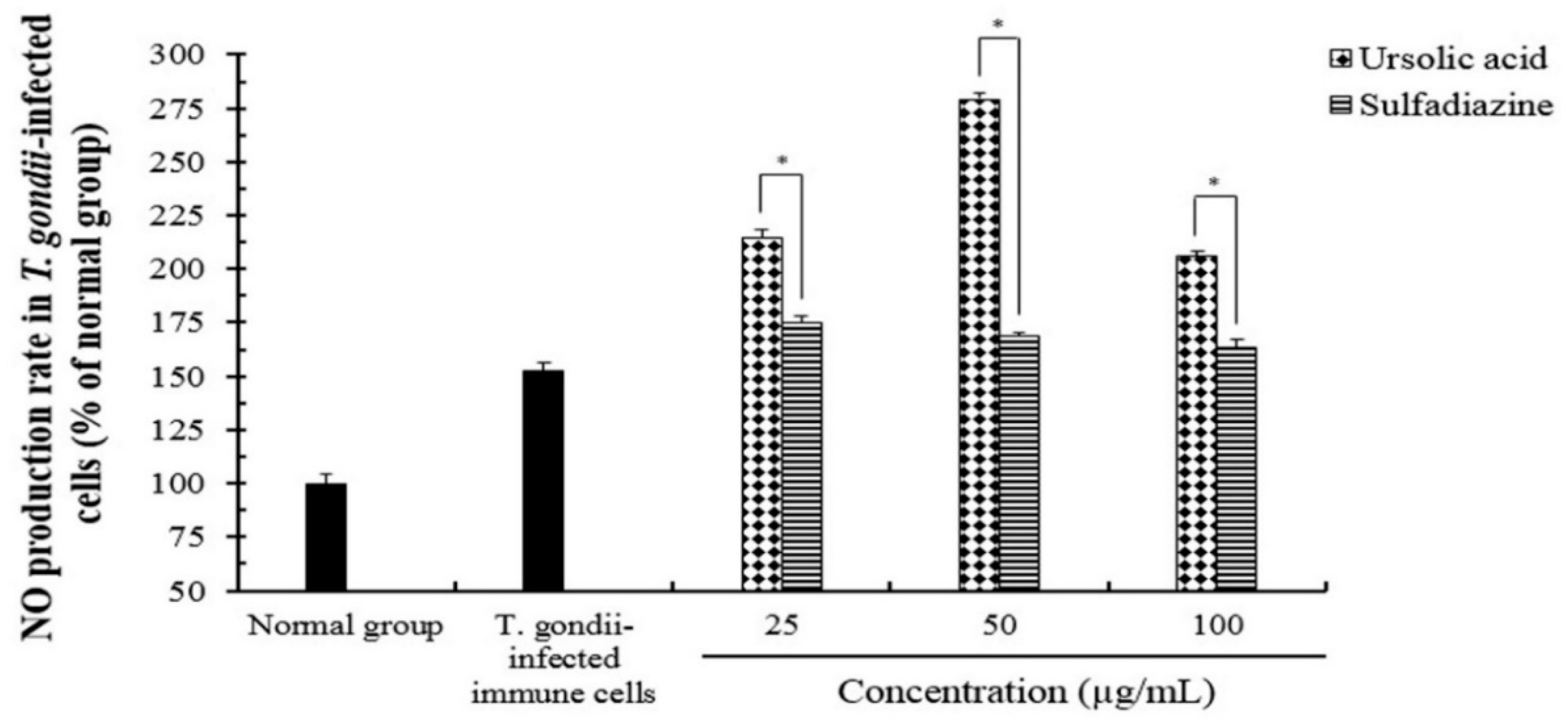
2.3. The Effect of Reactive Oxygen Species (ROS) and Nitric Oxide (NO) Production of Ursolic acid in T. gondii-Infected Cells
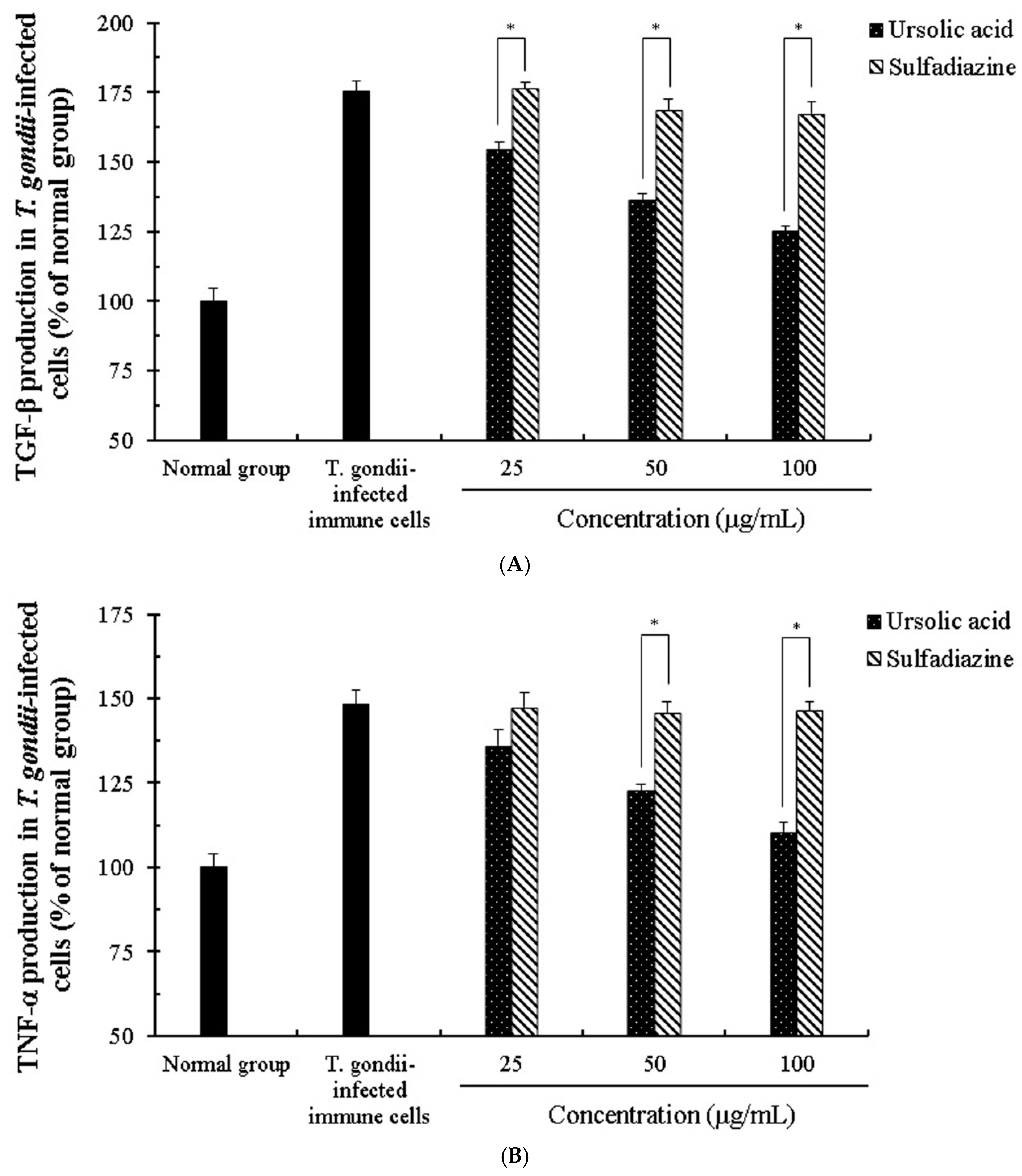
2.4. The Change of Cytokines by Interaction of Ursolic Acid and Immune cells
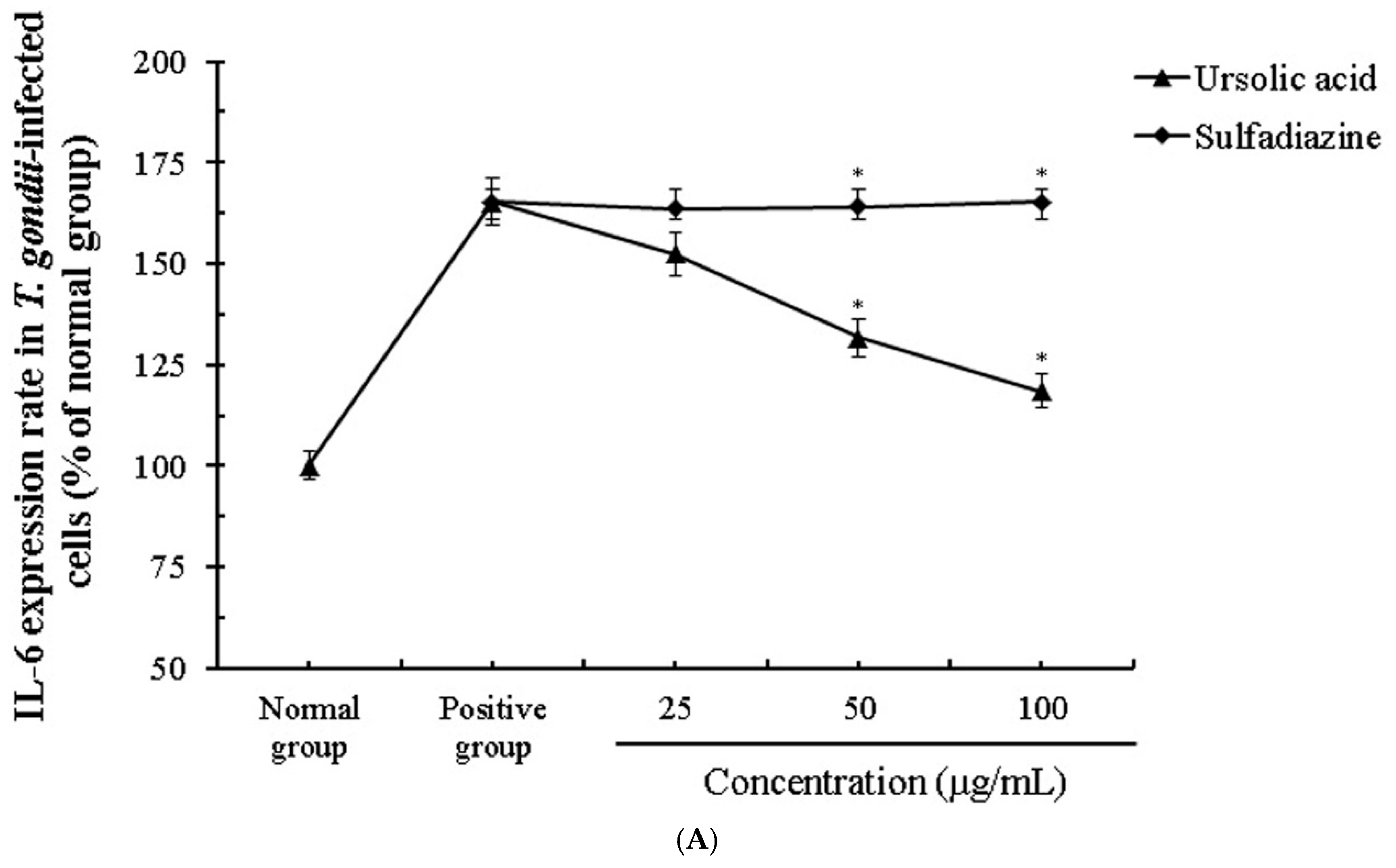
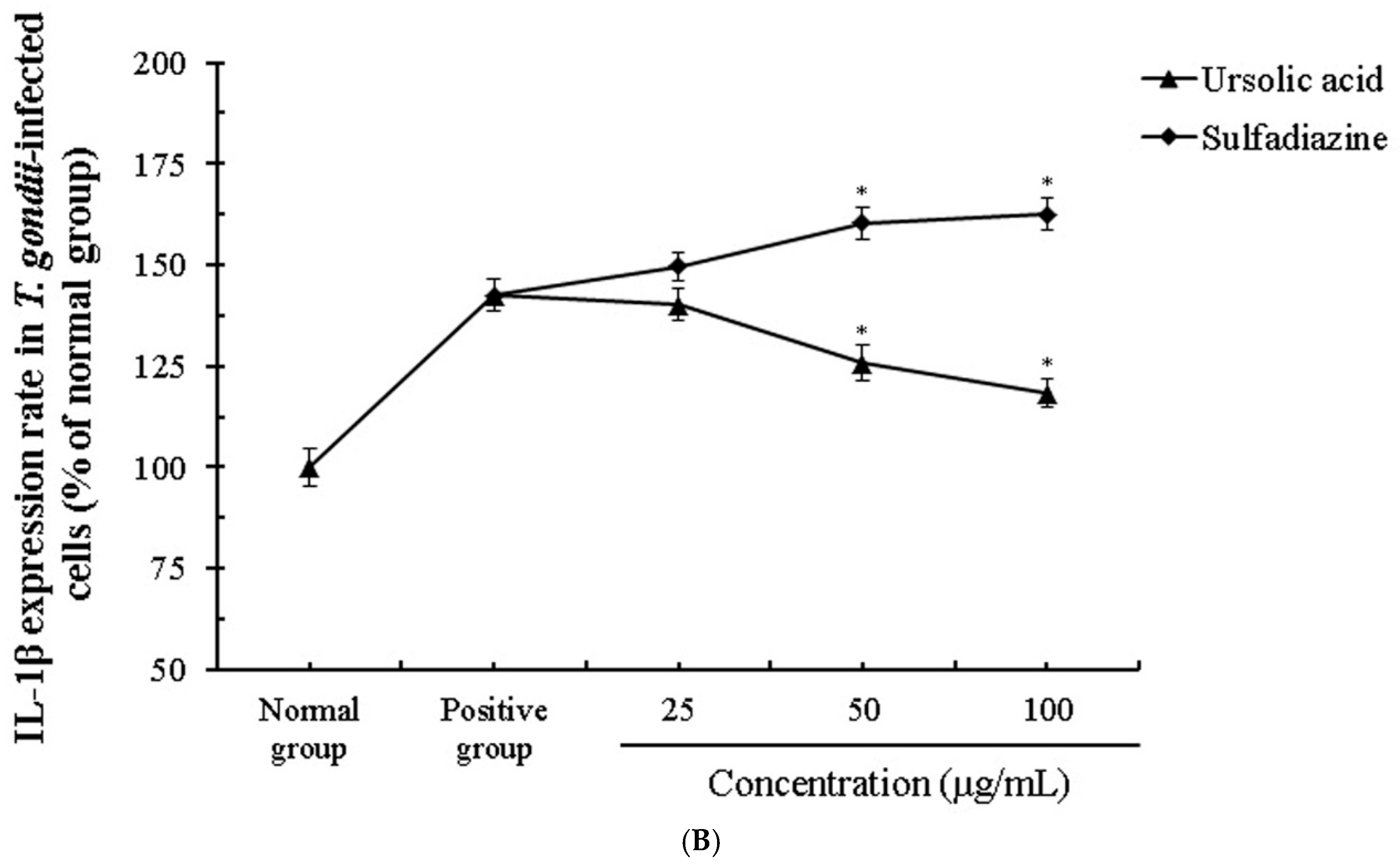
2.5. The Activity and Change of Cytokines by Stimulation of Ursolic acid
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Preparation of Drugs
4.3. Culture Condition of T. gondii and Cell Lines
4.4. The Viability of T. gondii and T. gondii-Infected Cells
4.5. RT-PCR Analysis of T. gondii
4.6. Determination of NO and ROS Production
4.7. The Cytokine of T. gondii-Infected Immune Cells
4.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| GM-CSF | Granulocyte macrophage colony stimulating factor |
| GRAs | Dense granule proteins |
| IL-1β | Interleukin 1 beta |
| IL-6 | Interleukin 6 |
| IL-10 | Interleukin 10 |
| IL-12 | Interleukin 12 |
| IMC sub-3 | Inner membrane complex sub-compartment protein 3 |
| MIC 8 | Microneme protein 8 |
| NK | Natural killer cell |
| NO | Nitric oxide |
| ROP 18 | Rhoptry protein 18 |
| ROS | Reactive oxygen species |
| SAGs | Surface antigens |
| SF | Sulfadiazine |
| T. gondii | Toxoplasma gondii |
| TGF-β1 | Transforming growth factor beta 1 |
| TNF-α | Tumor necrosis factor alpha |
| UA | Ursolic acid |
References
- Joiner, K.A.; Roos, D.S. Secretory traffic in the eukaryotic parasite Toxoplasma gondii: Less is more. J. Cell Biol. 2002, 157, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.R.; Rodriguez-Fernandez, I.A.; de Leon, J.C.; Huynh, M.H.; Carruthers, V.B.; Morrissette, N.S.; Bradley, P.J. A novel family of Toxoplasma IMC proteins displays a hierarchical organization and functions in coordinating parasite division. PLoS Pathog. 2010, 6, e1001094. [Google Scholar] [CrossRef]
- Harding, C.R.; Meissner, M. The inner membrane complex through development of Toxoplasma gondii and Plasmodium. Cell. Microbiol. 2014, 16, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.; Harrison, B.; Dangoudoubiyam, S.; Bandini, G.; Cheng, K.; Kosber, A.; Agop-Nersesian, C.; Howe, D.K.; Samuelson, J.; Ferguson, D.J.P.; et al. Differential Roles for Inner Membrane Complex Proteins across Toxoplasma gondii and Sarcocystis neurona Development. mSphere 2017, 2, e00409-17. [Google Scholar] [CrossRef] [PubMed]
- Varberg, J.M.; Coppens, I.; Arrizabalaga, G.; Gaji, R.Y. TgTKL1 Is a Unique Plant-Like Nuclear Kinase That Plays an Essential Role in Acute Toxoplasmosis. mBio 2018, 9, e00301-18. [Google Scholar] [CrossRef] [PubMed]
- Berry, L.; Chen, C.T.; Francia, M.E.; Guerin, A.; Graindorge, A.; Saliou, J.M.; Grandmougin, M.; Wein, S.; Bechara, C.; Morlon-Guyot, J.; et al. Toxoplasma gondii chromosomal passenger complex is essential for the organization of a functional mitotic spindle: A prerequisite for productive endodyogeny. Cell. Mol. Life Sci. 2018, 75, 4417–4443. [Google Scholar] [CrossRef]
- Bonhomme, A.; Bouchot, A.; Pezzella, N.; Gomez, J.; Le, M.H.; Pinon, J.M. Signaling during the invasion of host cells by Toxoplasma gondii. FEMS Microbiol. Rev. 1999, 23, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Rome, M.E.; Beck, J.R.; Turetzky, J.M.; Webster, P.; Bradley, P.J. Intervacuolar Transport and Unique Topology of GRA14, a Novel Dense Granule Protein in Toxoplasma gondii. Infect. Immun. 2008, 76, 4865–4875. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.H.; Lee, I.A. Evaluation of Anti-Toxoplasma gondii Effect of Ursolic Acid as a Novel Toxoplasmosis Inhibitor. Pharmaceuticals 2018, 11, 43. [Google Scholar] [CrossRef]
- Kavitha, N.; Noordin, R.; Chan, K.L.; Sasidharan, S. In vitro anti-Toxoplasma gondii activity of root extract/fractions of Eurycoma longifolia Jack. BMC Complement. Altern. Med. 2012, 12, 91. [Google Scholar] [CrossRef]
- Moine, E.; Moire, N.; Dimier-Poisson, I.; Brunet, K.; Couet, W.; Colas, C.; Van Langendonck, N.; Enguehard-Gueiffier, C.; Gueiffier, A.; Heraut, B.; et al. Imidazo[1,2-b]pyridazines targeting Toxoplasma gondii calcium-dependent protein kinase 1 decrease the parasite burden in mice with acute toxoplasmosis. Int. J. Parasitol. 2018, 48, 561–568. [Google Scholar] [CrossRef]
- Si, H.; Xu, C.; Zhang, J.; Zhang, X.; Li, B.; Zhou, X.; Zhang, J. Licochalcone A: An effective and low-toxicity compound against Toxoplasma gondii in vitro and in vivo. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 238–245. [Google Scholar] [CrossRef]
- Munera Lopez, J.; Ganuza, A.; Bogado, S.S.; Muñoz, D.; Ruiz, D.M.; Sullivan, W.J., Jr.; Vanagas, L.; Angel, S.O. Evaluation of ATM Kinase Inhibitor KU-55933 as Potential Anti-Toxoplasma gondii Agent. Front. Cell. Infect. Microbiol. 2019, 9, 26. [Google Scholar] [CrossRef]
- Silveira, G.R.; Campelo, K.A.; Lima, G.R.S.; Carvalho, L.P.; Samarao, S.S.; Vieira-da-Motta, O.; Mathias, L.; Matos, C.R.R.; Vieira, I.J.C.; Melo, E.J.T.; et al. In Vitro Anti-Toxoplasma gondii and Antimicrobial Activity of Amides Derived from Cinnamic Acid. Molecules 2018, 23, 774. [Google Scholar] [CrossRef] [PubMed]
- Alomar, M.L.; Rasse-Suriani, F.O.; Ganuza, A.; Coceres, V.M.; Cabrerizo, F.M.; Angel, S.O. In vitro evaluation of b-carboline alkaloids as potential anti-Toxoplasma agents. BMC Res. Notes 2013, 6, 193. [Google Scholar] [CrossRef]
- Xu, M.; Lee, E.M.; Wen, Z.; Cheng, Y.; Huang, W.K.; Qian, X.; Tcw, J.; Kouznetsova, J.; Ogden, S.C.; Hammack, C.; et al. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat. Med. 2016, 22, 1101–1107. [Google Scholar] [CrossRef]
- Choi, W.H. Novel pharmacological activity of artesunate and artemisinin: Their potential as anti-tubercular agents. J. Clin. Med. 2017, 6, 30. [Google Scholar] [CrossRef]
- Upton, A.M.; Cho, S.; Yang, T.J.; Kim, Y.; Wang, Y.; Lu, Y.; Wang, B.; Xu, J.; Mdluli, K.; Ma, Z.; et al. In vitro and in vivo activities of the nitroimidazole TBA-354 against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2015, 59, 136–144. [Google Scholar] [CrossRef]
- Choi, W.H. Evaluation of anti-tubercular activity of linolenic acid and conjugated-linoleic acid as effective inhibitors against Mycobacterium tuberculosis. Asian Pac. J. Trop. Med. 2016, 9, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Altamirano, R.; Monzote, L.; Pinon-Tapanes, A.; Vibrans, H.; Rivero-Cruz, J.F.; Ibarra-Alvarado, C.; Rojas-Molina, A. In vitro antileishmanial activity of Mexican medicinal plants. Heliyon 2017, 3, e00394. [Google Scholar] [CrossRef] [PubMed]
- Jackwood, M.W.; Rosenbloom, R.; Petteruti, M.; Hilt, D.A.; McCall, A.W.; Williams, S.M. Avian coronavirus infectious bronchitis virus susceptibility to botanical oleoresins and essential oils in vitro and in vivo. Virus Res. 2010, 149, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.M.R.J.; Gandi, M.O.; Mendonça, J.S.; Carvalho, A.S.; Coutinho, J.P.; Aguiar, A.C.C.; Krettli, A.U.; Boechat, N. New hybrid trifluoromethylquinolines as antiplasmo dium agents. Bioorg. Med. Chem. 2019. [Google Scholar] [CrossRef]
- De Oliveira, L.H.G.; de Sousa, P.A.P.S.; Hilario, F.F.; Nascimento, G.J.; Morais, J.P.S.; de Medeiros, E.P.; de Sousa, M.F.; da Cruz Nunes, F. Agave sisalana extract induces cell death in Aedes aegypti hemocytes increasing nitric oxide production. Asian Pac. J. Trop. Biomed. 2016, 6, 396–399. [Google Scholar] [CrossRef]
- Xu, H.L.; Wang, X.T.; Cheng, Y.; Zhao, J.G.; Zhou, Y.J.; Yang, J.J.; Qi, M.Y. Ursolic acid improves diabetic nephropathy via suppression of oxidative stress and inflammation in streptozotocin-induced rats. Biomed. Pharmacother. 2018, 105, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, N.; Yan, S.; Liu, M.; Sun, B.; Lu, Y.; Shao, Y. Ursolic acid alleviates inflammation and against diabetes-induced nephropathy through TLR4-mediated inflammatory pathway. Mol. Med. Rep. 2018, 18, 4675–4681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, H.; Chen, R.; Wang, Z. Oral supplementation with ursolic acid ameliorates sepsis-induced acute kidney injury in a mouse model by inhibiting oxidative stress and inflammatory responses. Mol. Med. Rep. 2018, 17, 7142–7148. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Li, W.; Wu, R.; Guo, Y.; Cheng, D.; Yang, Y.; Androulakis, I.P.; Kong, A.N. Pharmacokinetics and Pharmacodynamics of the Triterpenoid Ursolic Acid in Regulating the Antioxidant, Anti-inflammatory, and Epigenetic Gene Responses in Rat Leukocytes. Mol. Pharm. 2017, 14, 3709–3717. [Google Scholar] [CrossRef]
- Kim, K.; Shin, E.A.; Jung, J.H.; Park, J.E.; Kim, D.S.; Shim, B.S.; Kim, S.H. Ursolic Acid Induces Apoptosis in Colorectal Cancer Cells Partially via Upregulation of MicroRNA-4500 and Inhibition of JAK2/STAT3 Phosphorylation. Int. J. Mol. Sci. 2018, 20, 114. [Google Scholar] [CrossRef]
- Manouchehri, J.M.; Kalafatis, M. Ursolic Acid Promotes the Sensitization of rhTRAIL-resistant Triple-negative Breast Cancer. Anticancer Res. 2018, 38, 6789–6795. [Google Scholar] [CrossRef]
- Chen, C.F.; Yang, J.S.; Chen, W.K.; Lu, C.C.; Chiang, J.H.; Chiu, H.Y.; Tsai, S.C.; Juan, Y.N.; Huang, H.J.; Way, T.D. Ursolic acid elicits intrinsic apoptotic machinery by downregulating the phosphorylation of AKT/BAD signaling in human cisplatin-resistant oral cancer CAR cells. Oncol. Rep. 2018, 40, 1752–1760. [Google Scholar] [CrossRef]
- Mu, D.; Zhou, G.; Li, J.; Su, B.; Guo, H. Ursolic acid activates the apoptosis of prostate cancer via ROCK/PTEN mediated mitochondrial translocation of cofilin-1. Oncol. Lett. 2018, 15, 3202–3206. [Google Scholar] [CrossRef]
- Wang, C.M.; Jhan, Y.L.; Tsai, S.J.; Chou, C.H. The Pleiotropic Antibacterial Mechanisms of Ursolic Acid against Methicillin-Resistant Staphylococcus aureus (MRSA). Molecules 2016, 21, 884. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Arellanes, A.; Luna-Herrera, J.; Cornejo-Garrido, J.; Lopez-Garcia, S.; Castro-Mussot, M.E.; Meckes-Fischer, M.; Mata-Espinosa, D.; Marquina, B.; Torres, J.; Hernández-Pando, R. Ursolic and oleanolic acids as antimicrobial and immunomodulatory compounds for tuberculosis treatment. BMC Complement. Altern. Med. 2013, 13, 258. [Google Scholar] [CrossRef]
- Wang, C.M.; Chen, H.T.; Wu, Z.Y.; Jhan, Y.L.; Shyu, C.L.; Chou, C.H. Antibacterial and Synergistic Activity of Pentacyclic Triterpenoids Isolated from Alstonia scholaris. Molecules 2016, 21, 139. [Google Scholar] [CrossRef] [PubMed]
- Jesus, J.A.; Fragoso, T.N.; Yamamoto, E.S.; Laurenti, M.D.; Silva, M.S.; Ferreira, A.F.; Lago, J.H.; Santos-Gomes, G.; Passero, L.F. Therapeutic effect of ursolic acid in experimental visceral leishmaniasis. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, F.G.; de Brum Vieira, P.; Meirelles, L.C.; Rigo, G.V.; da Silva, E.F.; Gnoatto, S.C.B.; Tasca, T. Anti-Trichomonas vaginalis activity of ursolic acid derivative: A promising alternative. Parasitol. Res. 2018, 117, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.S.; Gomes, A.O.; Barbosa, B.F.; Angeloni, M.B.; Silva, N.M.; Teixeira-Carvalho, A.; Martins-Filho, O.A.; Silva, D.A.; Mineo, J.R.; Ferro, E.A. Azithromycin and spiramycin induce anti-inflammatory response in human trophoblastic (BeWo) cells infected by Toxoplasma gondii but are able to control infection. Placenta 2011, 32, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Borges, I.P.; Castanheira, L.E.; Barbosa, B.F.; de Souza, D.L.; da Silva, R.J.; Mineo, J.R.; Tudini, K.A.; Rodrigues, R.S.; Ferro, E.A.; de Melo, R.V. Anti-parasitic effect on Toxoplasma gondii induced by BnSP-7, a Lys49-phospholipase A2 homologue from Bothrops pauloensis venom. Toxicon 2016, 119, 84–91. [Google Scholar] [CrossRef]
- Hao, P.; Alaraj, I.Q.; Dulayymi, J.R.; Baird, M.S.; Liu, J.; Liu, Q. Sterculic Acid and Its Analogues Are Potent Inhibitors of Toxoplasma gondii. Korean J. Parasitol. 2016, 54, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, O.S.; Murata, Y.; Sugi, T.; Han, Y.; Kato, K. Modulation of host HIF-1α activity and the tryptophan pathway contributes to the anti-Toxoplasma gondii potential of nanoparticles. Biochem. Biophys. Rep. 2017, 11, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Sanfelice, R.A.; da Silva, S.S.; Bosqui, L.R.; Miranda-Sapla, M.M.; Barbosa, B.F.; Silva, R.J.; Ferro, E.A.V.; Panagio, L.A.; Navarro, I.T.; Bordignon, J.; et al. Pravastatin and simvastatin inhibit the adhesion, replication and proliferation of Toxoplasma gondii (RH strain) in HeLa cells. Acta Trop. 2017, 167, 208–215. [Google Scholar] [CrossRef]
- Degbe, M.; Debierre-Grockiego, F.; Tete-Benissan, A.; Debare, H.; Aklikokou, K.; Dimier-Poisson, I.; Gbeassor, M. Extracts of Tectona grandis and Vernonia amygdalina have anti-Toxoplasma and pro-inflammatory properties in vitro. Parasite 2018, 25, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.B.; Shen, Q.K.; Wang, H.; Jin, C.; Jin, C.M.; Quan, Z.S. Synthesis and evaluation of novel arctigenin derivatives as potential anti-Toxoplasma gondii agents. Eur. J. Med. Chem. 2018, 158, 414–427. [Google Scholar] [CrossRef] [PubMed]
- Dubremetz, J.F. Rhoptries are major players in Toxoplasma gondii invasion and host cell interaction. Cell. Microbiol. 2007, 9, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Besteiro, S.; Michelin, A.; Poncet, J.; Dubremetz, J.F.; Lebrun, M. Export of a Toxoplasma gondii rhoptry neck protein complex at the host cell membrane to form the moving junction during invasion. PLoS Pathog. 2009, 5, e1000309. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, K.; Weitzman, J.B. Host-parasite interactions: An intimate epigenetic relationship. Cell. Microbiol. 2015, 17, 1121–1132. [Google Scholar] [CrossRef]
- Guerin, A.; Corrales, R.M.; Parker, M.L.; Lamarque, M.H.; Jacot, D.; El Hajj, H.; Soldati-Favre, D.; Boulanger, M.J.; Lebrun, M. Efficient invasion by Toxoplasma depends on the subversion of host protein networks. Nat. Microbiol. 2017, 2, 1358–1366. [Google Scholar] [CrossRef]
- Takemae, H.; Kobayashi, K.; Sugi, T.; Han, Y.; Gong, H.; Ishiwa, A.; Recuenco, F.C.; Murakoshi, F.; Takano, R.; Murata, Y.; et al. Toxoplasma gondii RON4 binds to heparan sulfate on the host cell surface. Parasitol. Int. 2018, 67, 123–130. [Google Scholar] [CrossRef]
- Paquet, C.; Yudin, M.H. Society of Obstetricians and Gynaecologists of Canada. Toxoplasmosis in pregnancy: Prevention, screening, and treatment. J. Obstet. Gynaecol. Can. 2013, 35, 78–81. [Google Scholar] [CrossRef]
- Oz, H.S. Fetomaternal and Pediatric Toxoplasmosis. J. Pediatr. Infect. Dis. 2017, 12, 202–208. [Google Scholar] [CrossRef]
- An, R.; Tang, Y.; Chen, L.; Cai, H.; Lai, D.H.; Liu, K.; Wan, L.; Gong, L.; Yu, L.; Luo, Q.; et al. Encephalitis is mediated by ROP18 of Toxoplasma gondii, a severe pathogen in AIDS patients. Proc. Natl. Acad. Sci. USA 2018, 115, E5344–E5352. [Google Scholar] [CrossRef] [PubMed]
- Şenoglu, S.; Yeşilbag, Z.; Altuntaş Aydın, O.; Kumbasar Karaosmanoglu, H.; Kart Yaşar, K. Toxoplasma gondii IgG Seroprevalence in Patients with HIV/AIDS. Turkiye Parazitolojii Dergisi 2018, 42, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Muniz-Feliciano, L.; Van Grol, J.; Portillo, J.A.; Liew, L.; Liu, B.; Carlin, C.R.; Carruthers, V.B.; Matthews, S.; Subauste, C.S. Toxoplasma gondii-induced activation of EGFR prevents autophagy protein-mediated killing of the parasite. PLoS Pathog. 2013, 9, e1003809. [Google Scholar] [CrossRef] [PubMed]
- Hippe, D.; Weber, A.; Zhou, L.; Chang, D.C.; Hacker, G.; Luder, C.G. Toxoplasma gondii infection confers resistance against BimS-induced apoptosis by preventing the activation and mitochondrial targeting of pro-apoptotic Bax. J. Cell Sci. 2009, 122, 3511–3521. [Google Scholar] [CrossRef][Green Version]
- Blader, I.J.; Coleman, B.I.; Chen, C.T.; Gubbels, M.J. Lytic Cycle of Toxoplasma gondii: 15 Years Later. Annu. Rev. Microbiol. 2015, 69, 463–485. [Google Scholar] [CrossRef]
- Naumov, A.; Kratzer, S.; Ting, L.M.; Kim, K.; Suvorova, E.S.; White, M.W. The Toxoplasma Centrocone Houses Cell Cycle Regulatory Factors. MBio 2017, 8, e00579-17. [Google Scholar] [CrossRef]
- Letscher-Bru, V.; Pfaff, A.W.; Abou-Bacar, A.; Filisetti, D.; Antoni, E.; Villard, O.; Klein, J.P.; Candolfi, E. Vaccination with Toxoplasma gondii SAG-1 protein isprotective against congenital toxoplasmosis in BALB/c mice but not in CBA/J mice. Infect. Immun. 2003, 71, 6615–6619. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dlugonska, H. Toxoplasma rhoptries: Unique secretory organelles andsource of promising vaccine proteins for immunoprevention of toxoplasmosis. J. Biomed. Biotechnol. 2008, 2008, 632424. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yin, H. Research progress on surface antigen 1 (SAG1) of Toxoplasma gondii. Parasit. Vectors 2014, 7, 180. [Google Scholar] [CrossRef]
- Lakhrif, Z.; Moreau, A.; Herault, B.; Di-Tommaso, A.; Juste, M.; Moire, N.; Dimier-Poisson, I.; Mevelec, M.N.; Aubrey, N. Targeted Delivery of Toxoplasma gondii Antigens to Dendritic Cells Promote Immunogenicity and Protective Efficiency against Toxoplasmosis. Front. Immunol. 2018, 9, 317. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, W.H.; Lee, I.A. The Mechanism of Action of Ursolic Acid as a Potential Anti-Toxoplasmosis Agent, and Its Immunomodulatory Effects. Pathogens 2019, 8, 61. https://doi.org/10.3390/pathogens8020061
Choi WH, Lee IA. The Mechanism of Action of Ursolic Acid as a Potential Anti-Toxoplasmosis Agent, and Its Immunomodulatory Effects. Pathogens. 2019; 8(2):61. https://doi.org/10.3390/pathogens8020061
Chicago/Turabian StyleChoi, Won Hyung, and In Ah Lee. 2019. "The Mechanism of Action of Ursolic Acid as a Potential Anti-Toxoplasmosis Agent, and Its Immunomodulatory Effects" Pathogens 8, no. 2: 61. https://doi.org/10.3390/pathogens8020061
APA StyleChoi, W. H., & Lee, I. A. (2019). The Mechanism of Action of Ursolic Acid as a Potential Anti-Toxoplasmosis Agent, and Its Immunomodulatory Effects. Pathogens, 8(2), 61. https://doi.org/10.3390/pathogens8020061



