Genome Mining and Comparative Analysis of Streptococcus intermedius Causing Brain Abscess in a Child
Abstract
1. Introduction
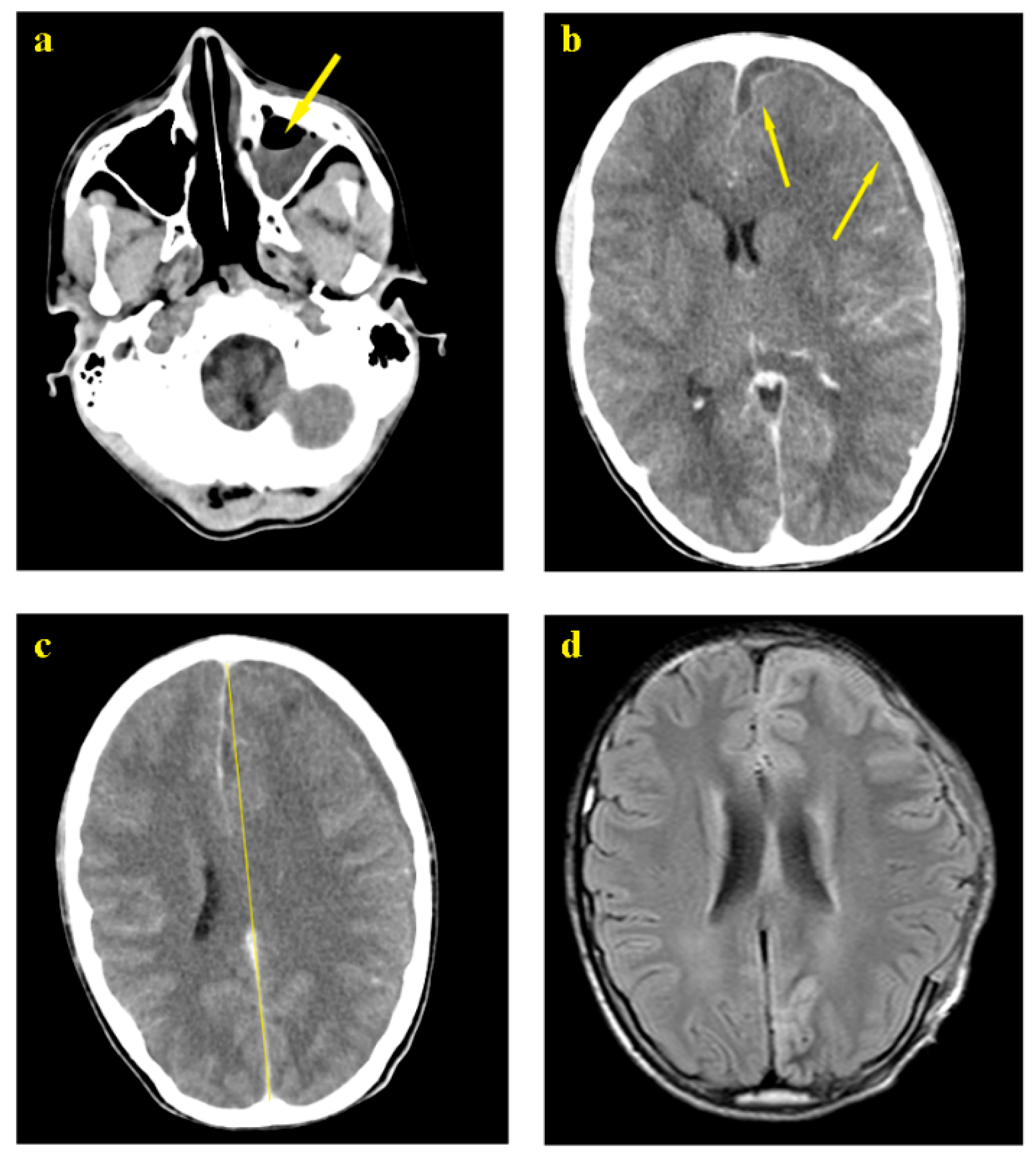
Case Presentation
2. Results
2.1. Genomic Features
2.2. Resistance Profile
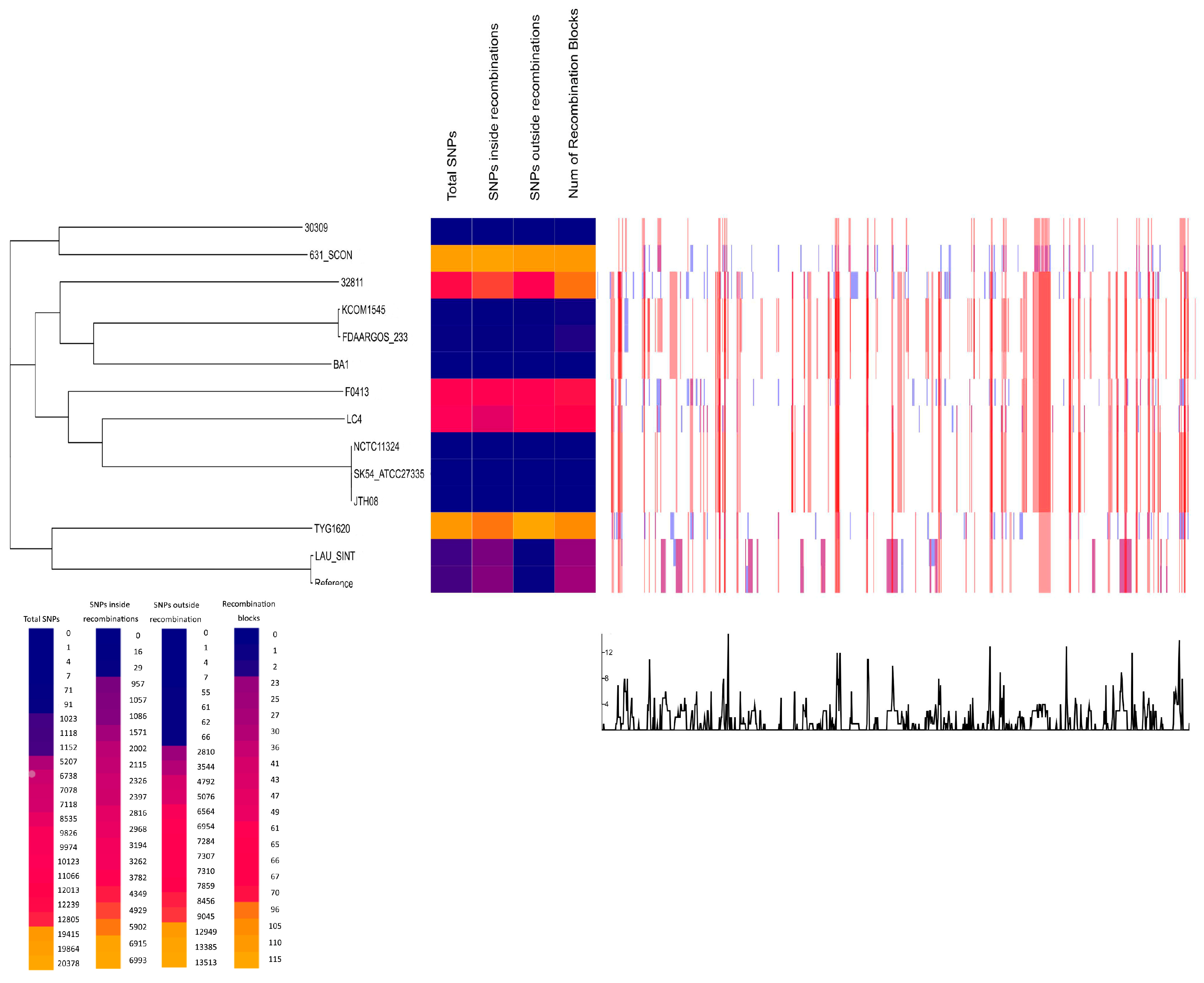
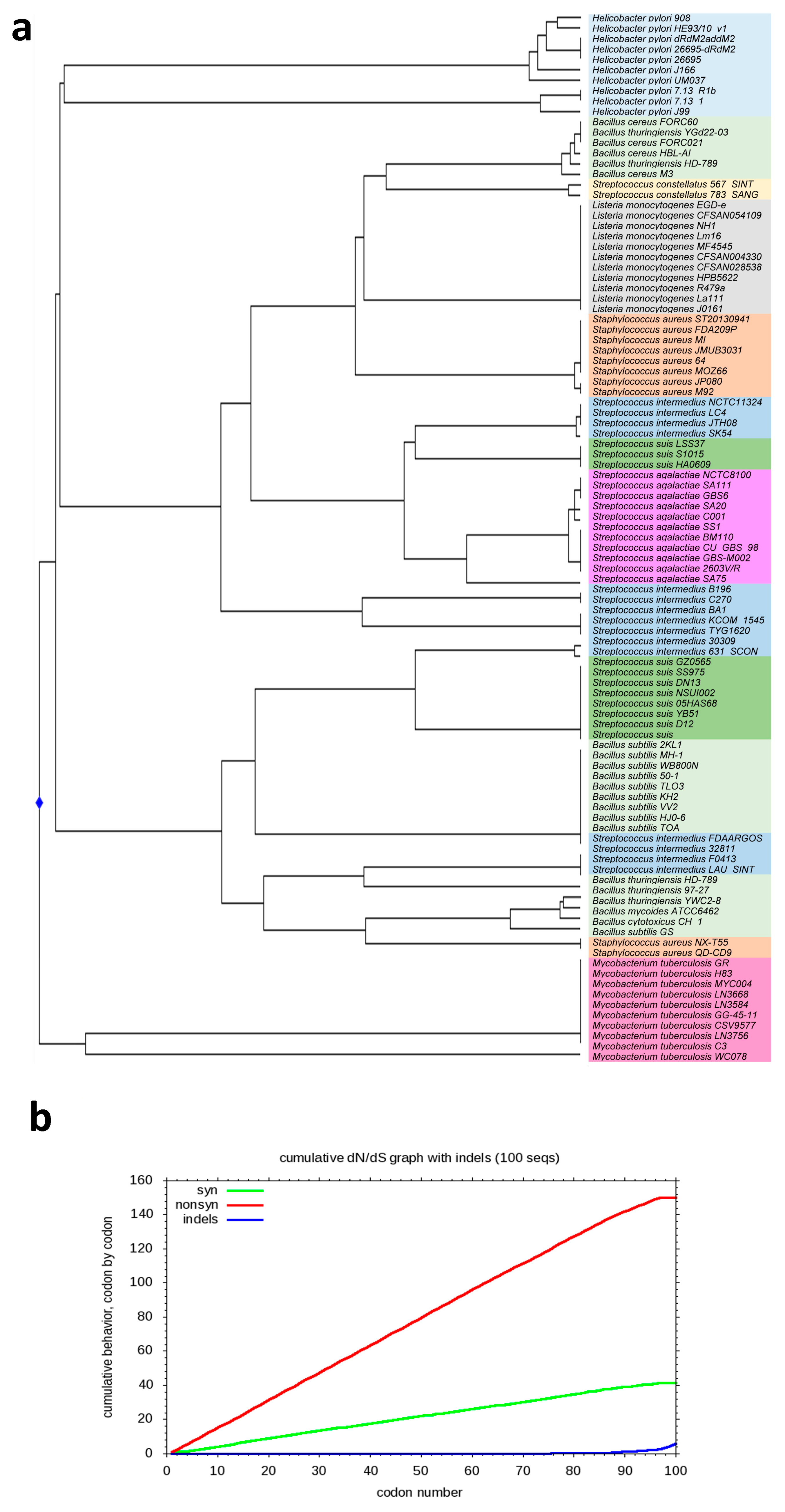
2.3. cg-SNPs Phylogeny and Recombination
2.4. dN/dS Ratio
2.5. Phage Content
2.6. Virulence Factors
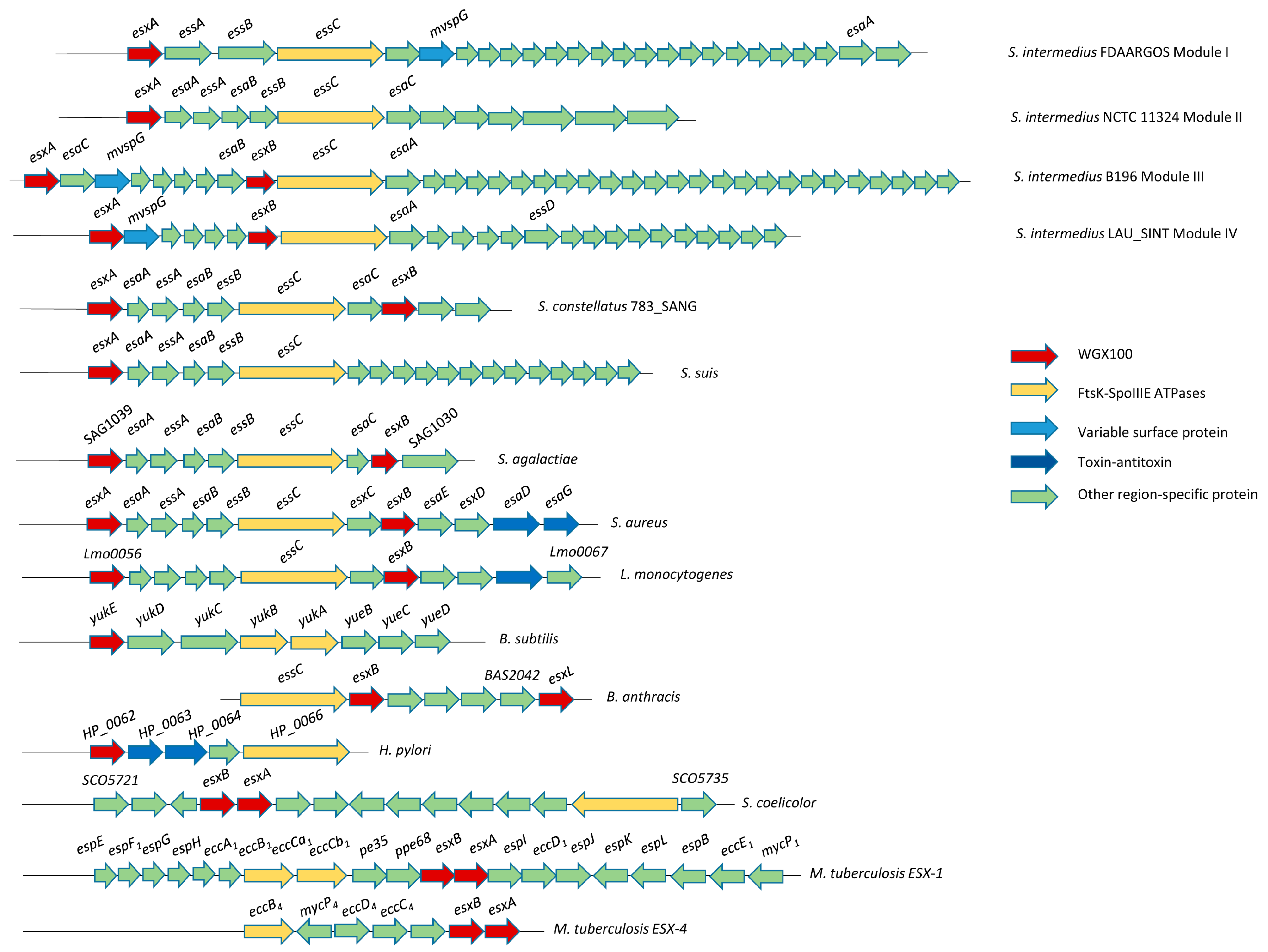
2.7. Type VII Secretion System
2.8. Comparative Genome Analysis
2.9. Pan-Genome Analysis
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Antimicrobial Susceptibility Testing
4.3. DNA Extraction
4.4. 16 S rRNA Sequencing
4.5. Genome Sequencing
4.6. Genome Assembly and Annotation
4.7. cgSNPs Analysis
4.8. 16S rRNA Based Phylogeny
4.9. Comparative Genome Analysis
4.10. Pan-Genome Analysis
4.11. Data Availability
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jensen, A.; Hoshino, T.; Kilian, M. Taxonomy of the Anginosus group of the genus Streptococcus and description of Streptococcus anginosus subsp. whileyi subsp. nov. and Streptococcus constellatus subsp. viborgensis subsp. nov. Int. J. Syst. Evol. Microbiol. 2013, 63, 2506–2519. [Google Scholar] [CrossRef] [PubMed]
- Facklam, R. What happened to the Streptococci: Overview of taxonomic and nomenclature changes. Clin. Microbiol. Rev. 2002, 15, 613–630. [Google Scholar] [CrossRef] [PubMed]
- El Kamouni, Y.; Arsalane, L.; Allali, A.; Beddou, G.; Zouhair, S. Rare case of pyogenic brain abscess in a immunocompetent children caused by Streptococcus intermedius. Int. J. Med. Sci. Clin. Invent. 2017, 4, 2980–2983. [Google Scholar] [CrossRef]
- Vajramani, G.V.; Nagmoti, M.B.; Patil, C.S. Neurobrucellosis presenting as an intra-medullary spinal cord abscess. Ann. Clin. Microbiol. Antimicrob. 2005, 4, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Junckerstorff, R.K.; Robinson, J.O.; Murray, R.J. Invasive Streptococcus anginosus group infection—Does the species predict the outcome? Int. J. Infect. Dis. 2014, 18, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Nagamune, H.; Whiley, R.A.; Goto, T.; Inai, Y.; Maeda, T.; Hardie, J.M.; Kourai, H. Distribution of the intermedilysin gene among the anginosus group streptococci and correlation between intermedilysin production and deep-seated infection with Streptococcus intermedius. J. Clin. Microbiol. 2000, 38, 220–226. [Google Scholar] [PubMed]
- Faden, H.S. Infections associated with Streptococcus intermedius in children. Pediatr. Infect. Dis. J. 2016, 35, 1047–1048. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, J.G.; Pauli, S.U. Epidural abscess after dental extraction. Age Ageing 2015, 44, 901. [Google Scholar] [CrossRef]
- Hasegawa, N.; Sekizuka, T.; Sugi, Y.; Kawakami, N.; Ogasawara, Y.; Kato, K.; Yamashita, A.; Takeuchi, F.; Kuroda, M. Characterization of the pathogenicity of Streptococcus intermedius TYG1620 isolated from a human brain abscess based on the complete genome sequence with transcriptome analysis and transposon mutagenesis in a murine subcutaneous abscess model. Infect. Immun. 2017, 85, e00886-16. [Google Scholar] [CrossRef]
- Petti, C.A.; Simmon, K.E.; Bender, J.; Blaschke, A.; Webster, K.A.; Conneely, M.F.; Schreckenberger, P.C.; Origitano, T.C.; Challapalli, M. Culture-negative intracerebral abscesses in children and adolescents from Streptococcus anginosus group infection: A case series. Clin. Infect. Dis. 2008, 46, 1578–1580. [Google Scholar] [CrossRef]
- Giddings, K.S.; Zhao, J.; Sims, P.J.; Tweten, R.K. Human CD59 is a receptor for the cholesterol-dependent cytolysin intermedilysin. Nat. Struct. Mol. Biol. 2004, 11, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Tomoyasu, T.; Tabata, A.; Hiroshima, R.; Imaki, H.; Masuda, S.; Whiley, R.A.; Aduse-Opoku, J.; Kikuchi, K.; Hiramatsu, K.; Nagamune, H. Role of catabolite control protein A in the regulation of intermedilysin production by Streptococcus intermedius. Infect. Immun. 2010, 78, 4012–4021. [Google Scholar] [CrossRef] [PubMed]
- Pecharki, D.; Petersen, F.C.; Scheie, A.A. Role of hyaluronidase in Streptococcus intermedius biofilm. Microbiology 2008, 154, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Proft, T.; Fraser, J. Superantigens: Just like peptides only different. J. Exp. Med. 1998, 187, 819–821. [Google Scholar] [CrossRef] [PubMed]
- Moses, A.E.; Wessels, M.R.; Zalcman, K.; Albertí, S.; Natanson-Yaron, S.; Menes, T.; Hanski, E. Relative contributions of hyaluronic acid capsule and M protein to virulence in a mucoid strain of the group A Streptococcus. Infect. Immun. 1997, 65, 64–71. [Google Scholar] [PubMed]
- Petersen, F.C.; Pasco, S.; Ogier, J.; Klein, J.P.; Assev, S.; Scheie, A.A. Expression and functional properties of the Streptococcus intermedius surface protein antigen I/II. Infect. Immun. 2001, 69, 4647–4653. [Google Scholar] [CrossRef] [PubMed]
- Kielian, T.; Barry, B.; Hickey, W.F. CXC chemokine receptor-2 ligands are required for neutrophil-mediated host defense in experimental brain abscesses. J. Immunol. 2001, 166, 4634–4643. [Google Scholar] [CrossRef]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef]
- Tajima, F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 1989, 123, 585–595. [Google Scholar]
- Sawyer, R.T.; Drevets, D.A.; Campbell, P.A.; Potter, T.A. Internalin A can mediate phagocytosis of Listeria monocytogenes by mouse macrophage cell lines. J. Leukoc. Biol. 1996, 60, 603–610. [Google Scholar] [CrossRef]
- Lai, L.; Dai, J.; Tang, H.; Zhang, S.; Wu, C.; Qiu, W.; Lu, C.; Yao, H.; Fan, H.; Wu, Z. Streptococcus suis serotype 9 strain GZ0565 contains a type VII secretion system putative substrate EsxA that contributes to bacterial virulence and a vanZ-like gene that confers resistance to teicoplanin and dalbavancin in Streptococcus agalactiae. Vet. Microbiol. 2017, 205, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Attwood, N.; Whitehead, R.; Shukla, A.; Parikh, R.; Pallen, M.; Anthony, M. The WXG100 protein secretion system of Streptococcus agalactiae. In Proceedings of the Neonatal Society 2007 Summer Meeting, Portsmouth, UK, 28–29 June 2007. [Google Scholar]
- Olson, A.B.; Kent, H.; Sibley, C.D.; Grinwis, M.E.; Mabon, P.; Ouellette, C.; Tyson, S.; Graham, M.; Tyler, S.D.; Van Domselaar, G.; et al. Phylogenetic relationship and virulence inference of Streptococcus Anginosus Group: Curated annotation and whole-genome comparative analysis support distinct species designation. BMC Genom. 2013, 14, 895. [Google Scholar] [CrossRef]
- Page, A.J.; Cummins, C.A.; Hunt, M.; Wong, V.K.; Reuter, S.; Holden, M.T.; Fookes, M.; Falush, D.; Keane, J.A.; Parkhill, J. Roary: Rapid large-scale prokaryote pan genome analysis. Bioinformatics 2015, 31, 691–3693. [Google Scholar] [CrossRef] [PubMed]
- Green, E.R.; Mecsas, J. Bacterial secretion systems—An overview. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Sitkiewicz, I.; Green, N.M.; Guo, N.; Mereghetti, L.; Musser, J.M. Lateral gene transfer of streptococcal ICE element RD2 (region of difference 2) encoding secreted proteins. BMC Microbiol. 2011, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ma, Q.; Su, D.; Li, Q.; Yao, W.; Wang, C. Identification of horizontal gene transfer and recombination of PsaA gene in streptococcus mitis group. Microbiol. Immunol. 2010, 54, 313–319. [Google Scholar] [CrossRef]
- Hytönen, J.; Haataja, S.; Finne, J. Streptococcus pyogenes glycoprotein-binding strepadhesin activity is mediated by a surface-associated carbohydrate-degrading enzyme, pullulanase. Infect. Immun. 2003, 71, 784–793. [Google Scholar] [CrossRef]
- Salim, K.Y.; De Azavedo, J.C.; Bast, D.J.; Cvitkovitch, D.G. Regulation of sagA, siaA and scpC by SilCR, a putative signaling peptide of Streptococcus pyogenes. FEMS Microbial. Lett. 2008, 289, 119–125. [Google Scholar] [CrossRef]
- Mendonca, M.L.; Szamosi, J.C.; Lacroix, A.M.; Fontes, M.E.; Bowdish, D.M.; Surette, M.G. The sil locus in Streptococcus anginosus group: Interspecies competition and a hotspot of genetic diversity. Front. Microbiol. 2017, 7, 2156. [Google Scholar] [CrossRef]
- Morona, J.K.; Morona, R.; Paton, J.C. Comparative genetics of capsular polysaccharide biosynthesis in Streptococcus pneumoniae types belonging to serogroup 19. J. Bacteriol. 1999, 181, 5355–5364. [Google Scholar]
- Smith, H.E.; de Vries, R.; Smits, M.A. The cps locus of Streptococcus suis serotype 2: Genetic determinant for the synthesis of sialic acid. Microb. Pathog. 2000, 29, 27–134. [Google Scholar] [CrossRef] [PubMed]
- Jordan, I.K.; Rogozin, I.B.; Wolf, Y.I.; Koonin, E.V. Essential genes are more evolutionarily conserved than are nonessential genes in bacteria. Genome Res. 2002, 12, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Pecharki, D.; Petersen, F.C.; Scheie, A.A. LuxS and expression of virulence factors in Streptococcus intermedius. Oral Microbiol. Immunol. 2008, 23, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Polekhina, G.; Giddings, K.S.; Tweten, R.K.; Parker, M.W. Insights into the action of the superfamily of cholesterol-dependent cytolysins from studies of intermedilysin. Proc. Natl. Acad. Sci. USA 2005, 102, 600–605. [Google Scholar] [CrossRef] [PubMed]
- LaRocca, T.J.; Stivison, E.A.; Hod, E.A.; Spitalnik, S.L.; Cowan, P.J.; Randis, T.M.; Ratner, A.J. Human-specific bacterial pore-forming toxins induce programmed necrosis in erythrocytes. mBio 2014, 5, e01251-14. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Kelly, S.J.; Lamani, E.; Ferraroni, M.; Jedrzejas, M.J. Structural basis of hyaluronan degradation by Streptococcus pneumoniae hyaluronate lyase. EMBO J. 2000, 19, 1228–1240. [Google Scholar] [CrossRef] [PubMed]
- Takao, A.; Nagashima, H.; Usui, H.; Sasaki, F.; Maeda, N.; Ishibashi, K.; Fujita, H. Hyaluronidase activity in human pus from which Streptococcus intermedius was isolated. Microbiol. Immunol. 1997, 41, 795–798. [Google Scholar] [CrossRef]
- Hynes, W.L.; Walton, S.L. Hyaluronidases of Gram-positive bacteria. FEMS Microbiol. Lett. 2000, 183, 201–207. [Google Scholar] [CrossRef]
- Tong, Z.; Zhou, L.; Li, J.; Jiang, W.; Ma, L.; Ni, L. In vitro evaluation of the antibacterial activities of MTAD in combination with nisin against Enterococcus Faecalis. J. Endod. 2011, 37, 1116–1120. [Google Scholar] [CrossRef]
- Willner, D.; Furlan, M.; Schmieder, R.; Grasis, J.A.; Pride, D.T.; Relman, D.A.; Angly, F.E.; McDole, T.; Mariella, R.P.; Rohwer, F.; et al. Metagenomic detection of phage-encoded platelet-binding factors in the human oral cavity. Proc. Natl. Acad. Sci. USA 2011, 108, 4547–4553. [Google Scholar] [CrossRef]
- Das, C.; Ghosh, T.S.; Mande, S.S. In silico dissection of Type VII Secretion System components across bacteria: New directions towards functional characterization. J. Biosci. 2016, 41, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Ates, L.S.; Houben, E.N.; Bitter, W. Type VII secretion: A highly versatile secretion system. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Abdallah, A.M.; Van Pittius, N.C.G.; Champion, P.A.D.; Cox, J.; Luirink, J.; Vandenbroucke-Grauls, C.M.; Appelmelk, B.J.; Bitter, W. Type VII secretion—Mycobacteria show the way. Nature Rev. Microbiol. 2007, 5, 883. [Google Scholar] [CrossRef] [PubMed]
- Hammer, N.D.; Skaar, E.P. The impact of metal sequestration on Staphylococcus aureus metabolism. Curr. Opin. Microbiol. 2012, 15, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Honsa, E.S.; Johnson, M.D.; Rosch, J.W. The roles of transition metals in the physiology and pathogenesis of Streptococcus pneumoniae. Front. Cell Infect. Microbiol. 2013, 3, 92. [Google Scholar] [CrossRef]
- Whitney, J.C.; Peterson, S.B.; Kim, J.; Pazos, M.; Verster, A.J.; Radey, M.C.; Goo, Y.A. A broadly distributed toxin family mediates contact-dependent antagonism between gram-positive bacteria. eLife 2017, 6, e26938. [Google Scholar] [CrossRef]
- Unnikrishnan, M.; Constantinidou, C.; Palmer, T.; Pallen, M.J. The enigmatic Esx proteins: Looking beyond mycobacteria. Trends Microbiol. 2017, 25, 192–204. [Google Scholar] [CrossRef]
- Korea, C.; Balsamo, G.; Pezzicoli, A.; Merakou, C.; Tavarini, S.; Bagnoli, F.; Serruto, D.; Unnikrishnan, M. Staphylococcal Esx proteins modulate apoptosis and release of intracellular Staphylococcus aureus during infection in epithelial cells. Infect. Immun. 2017, 82, 4144–4153. [Google Scholar] [CrossRef]
- Sutcliffe, I.C. New insights into the distribution of WXG100 protein secretion systems. Antonie Van Leeuwenhoek 2011, 99, 127–131. [Google Scholar] [CrossRef]
- Uchiyama, I.; Albritton, J.; Fukuyo, M.; Kojima, K.K.; Yahara, K.; Kobayashi, I. A novel approach to Helicobacter pylori pan-genome analysis for identification of genomic islands. PLoS ONE 2016, 11, e0159419. [Google Scholar] [CrossRef]
- Pallen, M.J. The ESAT-6/WXG100 superfamily–and a new Gram-positive secretion system? Trends Microbiol. 2002, 10, 209–212. [Google Scholar] [CrossRef]
- Burts, M.L.; DeDent, A.C.; Missiakas, D.M. EsaC substrate for the ESAT-6 secretion pathway and its role in persistent infections of Staphylococcus aureus. Mol. Microbiol. 2008, 69, 736–746. [Google Scholar] [CrossRef] [PubMed]
- European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters; Version 5.0; European Committee on Antimicrobial Susceptibility Testing (EUCAST): Basel, Switzerland, 2015; Available online: http://www.eucast.org/clinical_breakpoints/ (accessed on 20 January 2018).
- Schmidt, T.M.; DeLong, E.F.; Pace, N.R. Analysis of a marine picoplankton community by 16S rRNA gene cloning and sequencing. J. Bacteriol. 1991, 173, 4371–4378. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data; Babraham Institute: Cambridge, UK, 2010. [Google Scholar]
- Krueger, F. Trim Galore!: A Wrapper Tool Around Cutadapt and FastQC to Consistently Apply Quality and Adapter Trimming to FastQ Files; Babraham Institute: Cambridge, UK, 2015. [Google Scholar]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016, 44, W16–W21. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid annotations using subsystems technology. BMC Gen. 2008, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, C.; Laird, M.R.; Williams, K.P.; Simon Fraser University Research Computing Group; Lau, B.Y.; Hoad, G.; Winsor, G.L.; Brinkman, F.S. IslandViewer 4: Expanded prediction of genomic islands for larger-scale datasets. Nucleic Acids Res. 2017, 45, W30–W35. [Google Scholar] [CrossRef]
- Omasits, U.; Ahrens, C.H.; Müller, S.; Wollscheid, B. Protter: Interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics 2013, 30, 884–886. [Google Scholar] [CrossRef] [PubMed]
- Korber, B. HIV Signature and Sequence Variation Analysis. In Computational Analysis of HIV Molecular Sequences; Springer: New York, NY, USA, 2000; pp. 55–72. [Google Scholar]
- Seemann, T. Snippy: Fast Bacterial Variant Calling from NGS Reads. Available online: https://github.com/tseemann/snippy (accessed on 25 December 2018).
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Croucher, N.J.; Page, A.J.; Connor, T.R.; Delaney, A.J.; Keane, J.A.; Bentley, S.D.; Parkhill, J.; Harris, S.R. Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using Gubbins. Nucleic Acids Res. 2014, 43, e15. [Google Scholar] [CrossRef] [PubMed]
- Hadfield, J.; Croucher, N.J.; Goater, R.J.; Abudahab, K.; Aanensen, D.M.; Harris, S.R. Phandango: An interactive viewer for bacterial population genomics. Bioinformatics 2017, 34, 292–293. [Google Scholar] [CrossRef] [PubMed]
- Bikandi, J.; Millán, R.S.; Rementeria, A.; Garaizar, J. In silico analysis of complete bacterial genomes: PCR, AFLP–PCR and endonuclease restriction. Bioinformatics 2004, 20, 798–799. [Google Scholar] [CrossRef] [PubMed]
- Alikhan, N.F.; Petty, N.K.; Zakour, N.L.B.; Beatson, S.A. BLAST Ring Image Generator (BRIG): Simple prokaryote genome comparisons. BMC Genom. 2011, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Goris, J.; Konstantinidis, K.T.; Klappenbach, J.A.; Coenye, T.; Vandamme, P.; Tiedje, J.M. DNA–DNA hybridization values and their relationship to whole-genome sequence similarities. Int. J. Syst. Evol. Microbiol. 2007, 57, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [PubMed]
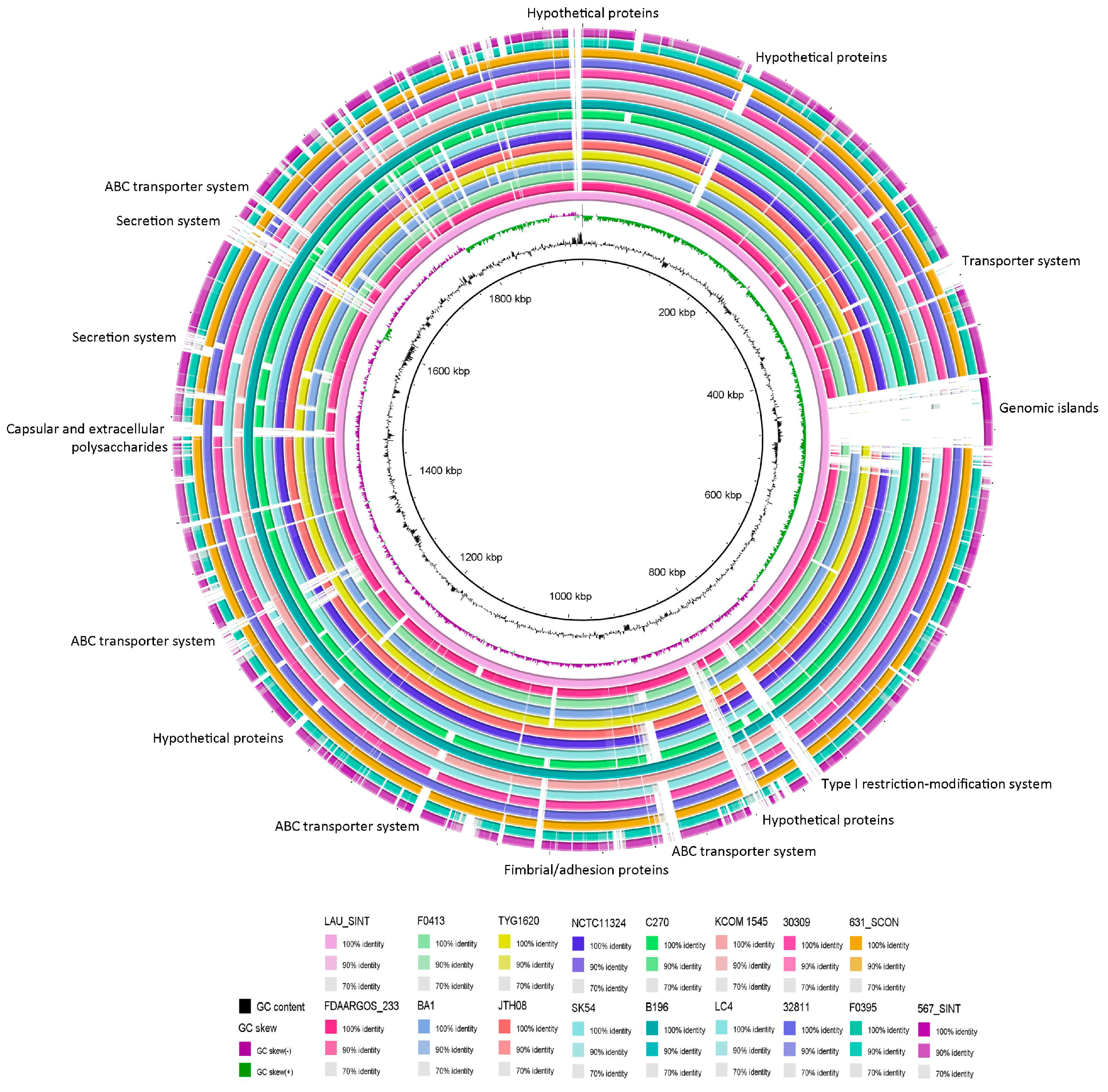
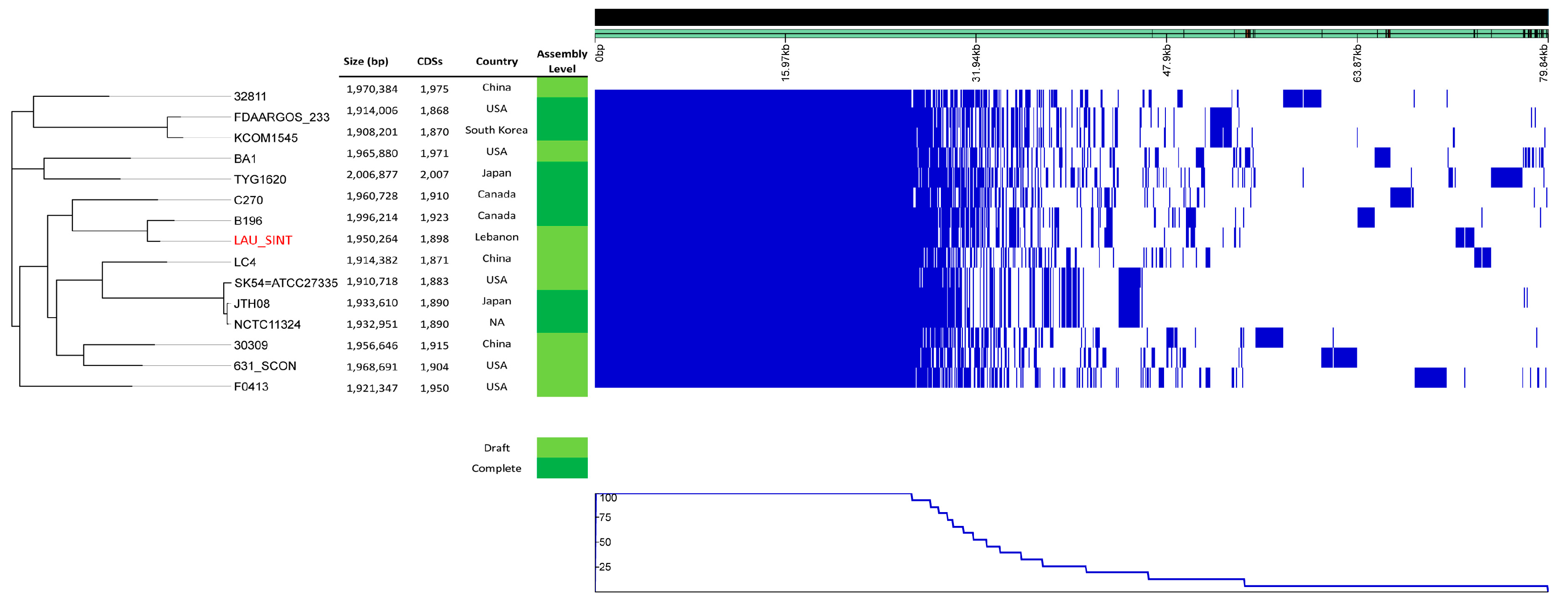
| # | Isolate | Size (bp) | GC% | RNA | CDS | Sample Information | Year | Country | Ref. | Accession # | Assembly Level |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F0395 | 1,927,278 | 37.9 | 69 | 1958 | NA | 2011 | USA | NA | GCA_000234015.1 | Draft |
| 2 | F0413 | 1,921,347 | 37.6 | 69 | 1950 | NA | 2011 | USA | NA | GCA_000234035.1 | Draft |
| 3 | BA1 | 1,965,880 | 37.7 | 74 | 1971 | Epidural abscess | 2012 | USA | Planet et al., 2013 | GCA_000313655.1 | Draft |
| 4 | SK54 = ATCC 27335 | 1,910,718 | 37.6 | 31 | 1883 | NA | 2012 | USA | NA | GCA_000258445.1 | Draft |
| 5 | JTH08 | 1,933,610 | 37.6 | 79 | 1890 | NA | 2012 | Japan | NA | GCA_000306805.1 | Complete |
| 6 | C270 | 1,960,728 | 37.6 | 72 | 1910 | Human bronchopulmonary abscess | 2013 | Canada | Olson et al., 2013 | GCA_000463385.1 | Complete |
| 7 | B196 | 1,996,214 | 37.6 | 72 | 1923 | Broncho-pulmonary abscess, septic arthritis, osteomyelitis, pyomyositis | 2013 | Canada | Olson et al., 2013 | GCA_000463355.1 | Complete |
| 8 | 567_SINT | 2,069,671 | 38.0 | 56 | 2073 | NA | 2015 | USA | NA | GCA_001073405.1 | Draft |
| 9 | 631_SCON | 1,968,691 | 37.8 | 57 | 1904 | NA | 2015 | USA | NA | GCA_001073635.1 | Draft |
| 10 | KCOM 1545 | 1,908,201 | 37.6 | 37 | 1870 | Oral Cavity, enonotic infection; collected in 2005 | 2015 | South Korea | NA | GCA_001296205.1 | Complete |
| 11 | TYG1620 | 2,006,877 | 37.6 | 72 | 2007 | Brain abscess in an infant | 2016 | Japan | Hasegawa et al., 2017 | GCA_002356055.1 | Complete |
| 12 | 30309 | 1,956,646 | 37.5 | 46 | 1915 | Abscess, pyogenic fluids of percutaneous drainage; collected in 2015 | 2018 | China | NA | GCA_002879585.1 | Draft |
| 13 | 32811 | 1,970,384 | 37.7 | 48 | 1975 | Abscess, pyogenic fluids of percutaneous drainage; collected in 2015 | 2018 | China | NA | GCA_002879575.1 | Draft |
| 14 | FDAARGOS_233 | 1,914,006 | 37.7 | 72 | 1868 | Abscess in an 15-months female; collected in 2014 | 2018 | USA | NA | GCA_002073355.2 | Complete |
| 15 | LAU_SINT | 1,950,264 | 37.7 | 54 | 1898 | Meningitis and brain abscess in an 13-years old male | 2018 | Lebanon | This study | GCA_003284685.1 | Draft |
| 16 | NCTC11324 | 1,932,951 | 37.7 | 72 | 1890 | NA | 2018 | NA | NA | GCA_900475975.1 | Complete |
| 17 | LC4 | 1,914,382 | 37.8 | 43 | 1871 | Abscess, pyogenic fluids of percutaneous drainage; collected in 2015 | 2018 | China | NA | GCA_002879755.1 | Draft |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Issa, E.; Salloum, T.; Panossian, B.; Ayoub, D.; Abboud, E.; Tokajian, S. Genome Mining and Comparative Analysis of Streptococcus intermedius Causing Brain Abscess in a Child. Pathogens 2019, 8, 22. https://doi.org/10.3390/pathogens8010022
Issa E, Salloum T, Panossian B, Ayoub D, Abboud E, Tokajian S. Genome Mining and Comparative Analysis of Streptococcus intermedius Causing Brain Abscess in a Child. Pathogens. 2019; 8(1):22. https://doi.org/10.3390/pathogens8010022
Chicago/Turabian StyleIssa, Elio, Tamara Salloum, Balig Panossian, David Ayoub, Edmond Abboud, and Sima Tokajian. 2019. "Genome Mining and Comparative Analysis of Streptococcus intermedius Causing Brain Abscess in a Child" Pathogens 8, no. 1: 22. https://doi.org/10.3390/pathogens8010022
APA StyleIssa, E., Salloum, T., Panossian, B., Ayoub, D., Abboud, E., & Tokajian, S. (2019). Genome Mining and Comparative Analysis of Streptococcus intermedius Causing Brain Abscess in a Child. Pathogens, 8(1), 22. https://doi.org/10.3390/pathogens8010022




