Lessons Learned from Protective Immune Responses to Optimize Vaccines against Cryptosporidiosis
Abstract
1. Introduction
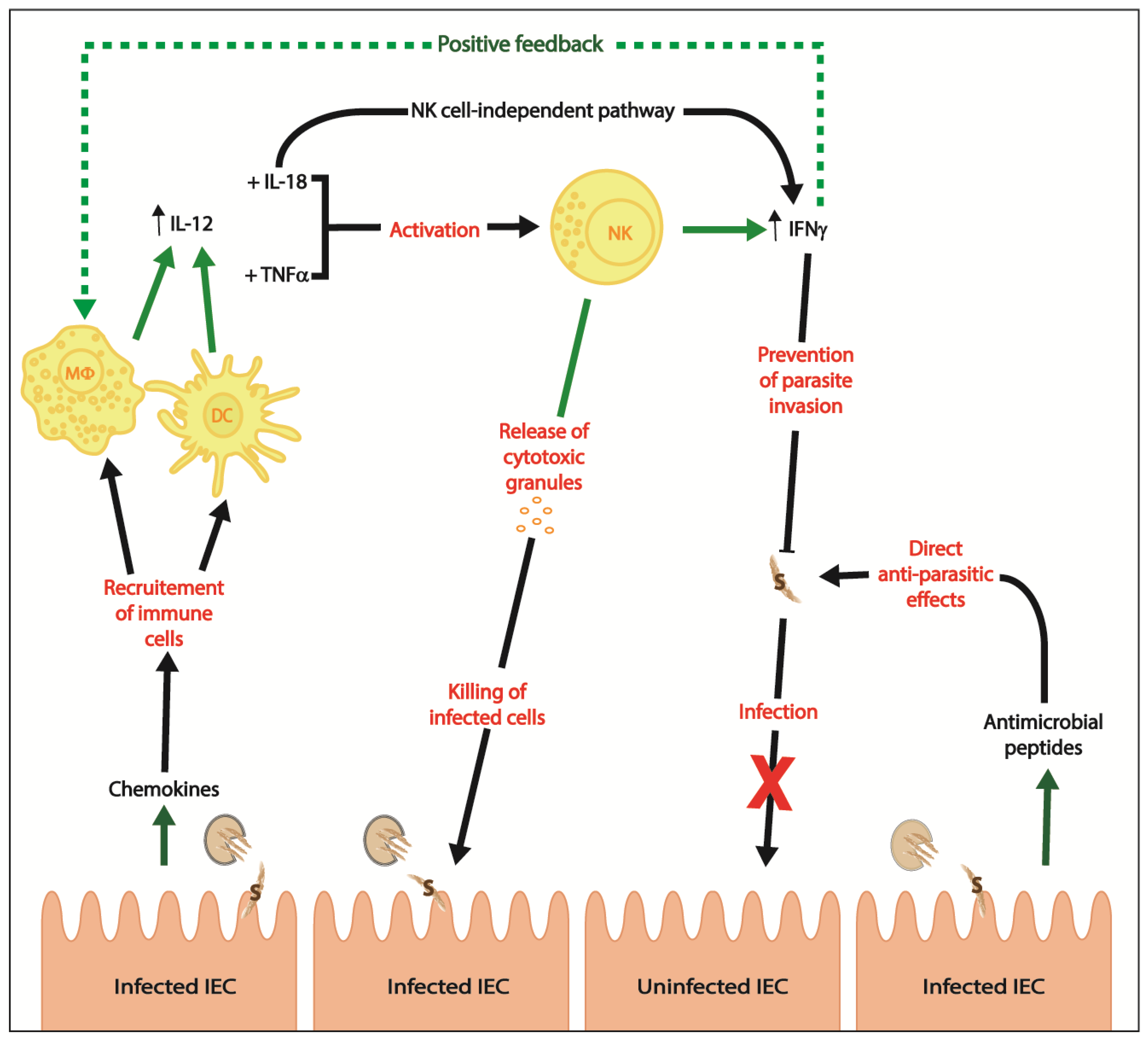
2. Innate Immunity
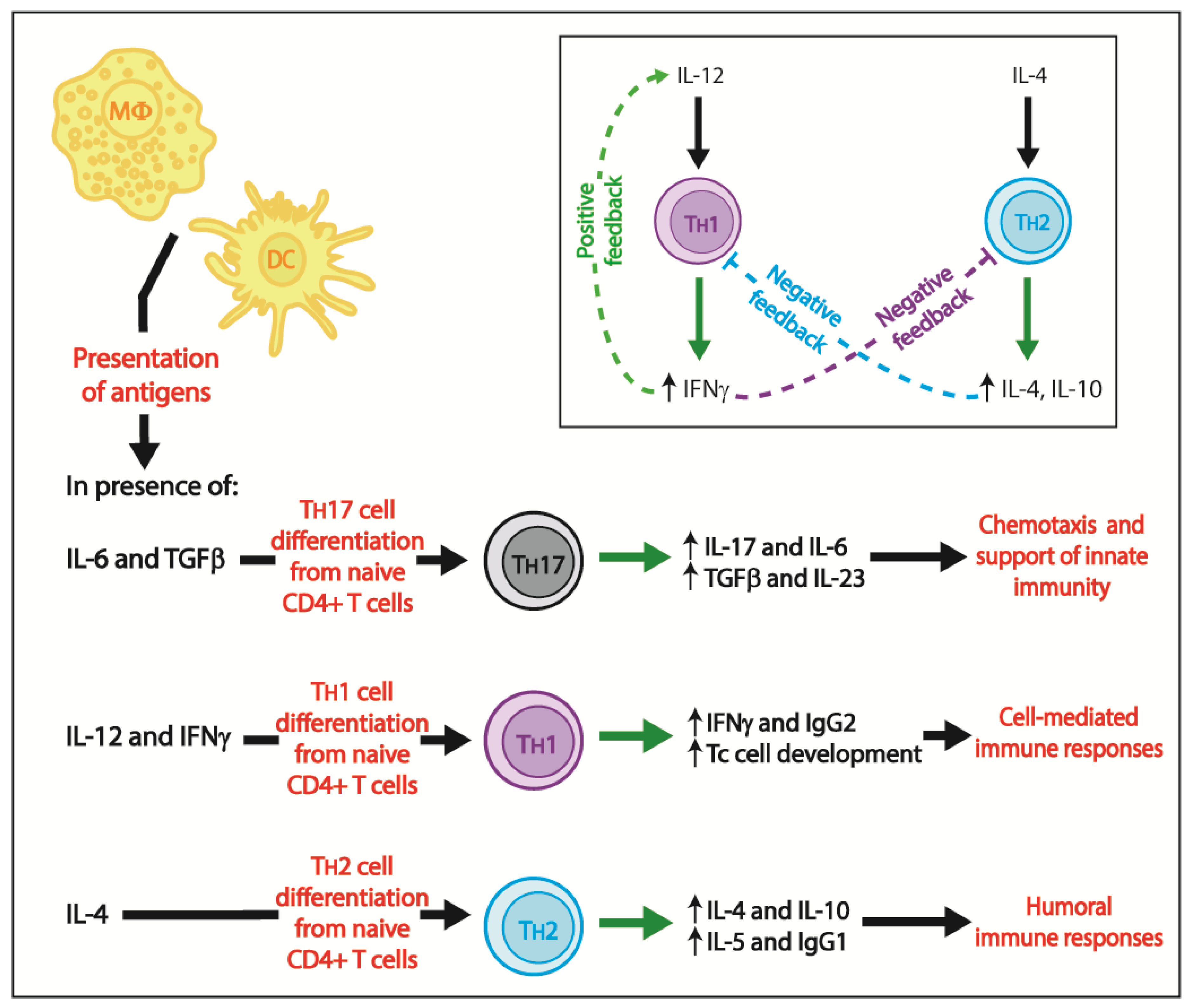
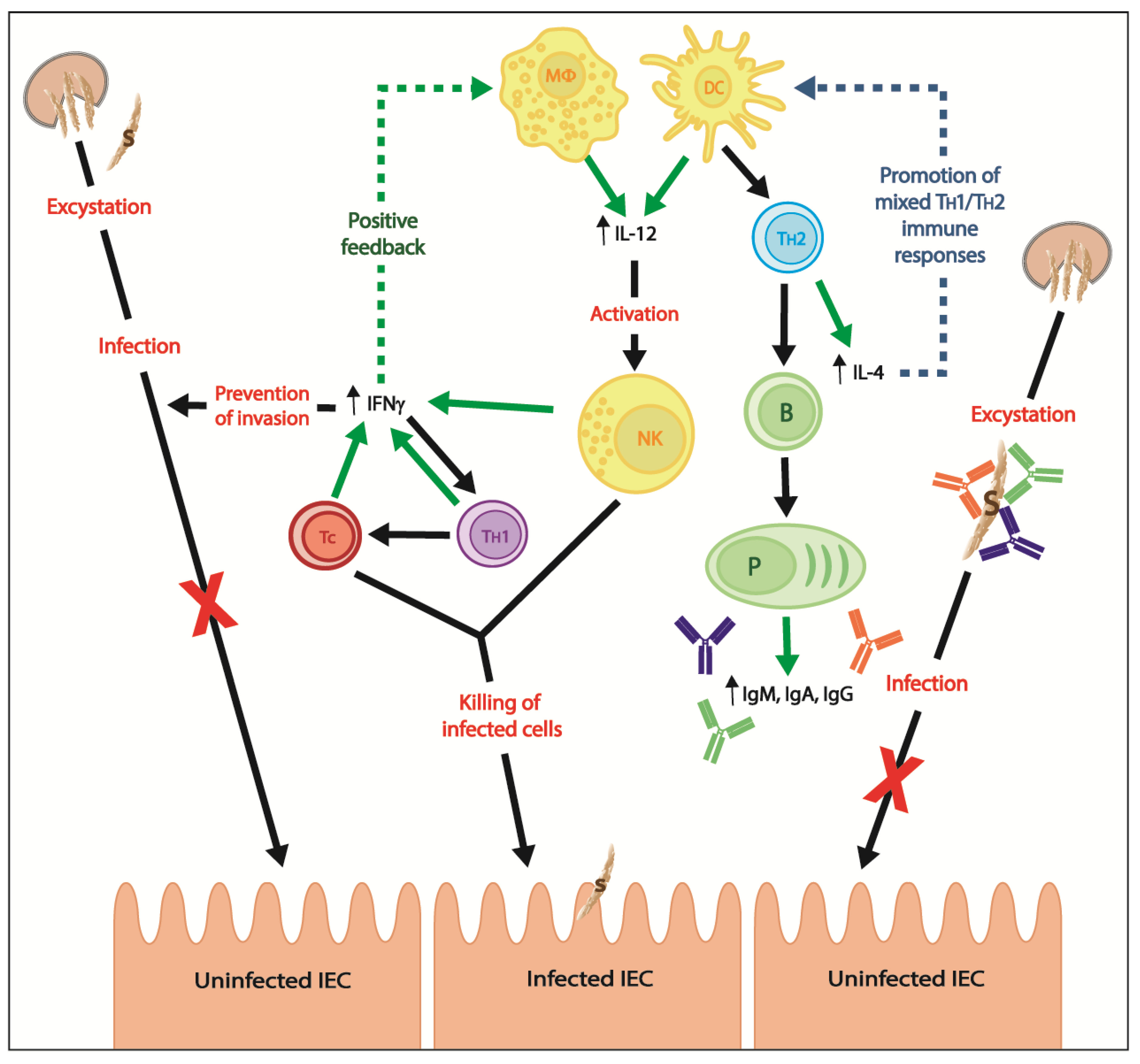
3. Adaptive Immunity
3.1. Cell-Mediated Immune Responses
3.2. Humoral Immune Responses
3.2.1. Bovine Cryptosporidiosis and Colostrum-Treatment of Calves
3.2.2. Antibody Treatment of Immunocompromised Mice
3.2.3. Treatment of Immunocompromised Cryptosporidium spp.-Infected Patients with Hyperimmune Bovine Colostrum
4. Vaccines against Cryptosporidium spp. Infection
4.1. DNA Vaccines and Subunit Vaccines
4.2. Live-Attenuated Vaccine
4.3. Vaccine Vectors
4.4. Prime-Pull Vaccine Approach
5. Conclusions and Future Directions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Troeger, C.; Forouzanfar, M.; Rao, P.C.; Khalil, I.; Brown, A.; Reiner, R.C.; Fullman, N.; Thompson, R.L.; Abajobir, A.; Ahmed, M.; et al. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017, 17, 909–948. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- Korpe, P.S.; Haque, R.; Gilchrist, C.; Valencia, C.; Niu, F.; Lu, M.; Ma, J.Z.; Petri, S.E.; Reichman, D.; Kabir, M.; et al. Natural history of cryptosporidiosis in a longitudinal study of slum-dwelling Bangladeshi children: Association with severe malnutrition. PLoS Negl. Trop. Dis. 2016, 10, e0004564. [Google Scholar] [CrossRef] [PubMed]
- Checkley, W.; Epstein, L.D.; Gilman, R.H.; Black, R.E.; Cabrera, L.; Sterling, C.R. Effects of Cryptosporidium parvum infection in peruvian children: Growth faltering and subsequent catch-up growth. Am. J. Epidemiol. 1998, 148, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Guerrant, D.I.; Moore, S.R.; Lima, A.A.; Patrick, P.D.; Schorling, J.B.; Guerrant, R.L. Association of early childhood diarrhea and cryptosporidiosis with impaired physical fitness and cognitive function four-seven years later in a poor urban community in northeast Brazil. Am. J. Trop. Med. Hyg. 1999, 61, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Sow, S.O.; Muhsen, K.; Nasrin, D.; Blackwelder, W.C.; Wu, Y.; Farag, T.H.; Panchalingam, S.; Sur, D.; Zaidi, A.K.; Faruque, A.S.; et al. The burden of Cryptosporidium diarrheal disease among children <24 months of age in moderate/high mortality regions of sub-Saharan Africa and south Asia, utilizing data from the Global Enteric Multicenter Study (GEMS). PLoS Negl. Trop. Dis. 2016, 10, e0004729. [Google Scholar] [CrossRef] [PubMed]
- Ryan, U.; Zahedi, A.; Paparini, A. Cryptosporidium in humans and animals—A one health approach to prophylaxis. Parasite Immunol. 2016, 38, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.F.; Levy, M.G.; Walker, R.L.; Crittenden, S. Cryptosporidiosis in veterinary students. J. Am. Vet. Med. Assoc. 1988, 193, 1413–1414. [Google Scholar] [PubMed]
- Preiser, G.; Preiser, L.; Madeo, L. An outbreak of cryptosporidiosis among veterinary science students who work with calves. J. Am. Coll. Health 2003, 51, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Gait, R.; Soutar, R.H.; Hanson, M.; Fraser, C.; Chalmers, R. Outbreak of cryptosporidiosis among veterinary students. Vet. Rec. 2008, 162, 843–845. [Google Scholar] [CrossRef] [PubMed]
- Ruecker, N.J.; Braithwaite, S.L.; Topp, E.; Edge, T.; Lapen, D.R.; Wilkes, G.; Robertson, W.; Medeiros, D.; Sensen, C.W.; Neumann, N.F. Tracking host sources of Cryptosporidium spp. in raw water for improved health risk assessment. Appl. Environ. Microbiol. 2007, 73, 3945–3957. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Villeneuve, A. Les Zoonoses Parasitaires: L’infection Chez les Animaux et Chez L’hommes; Presses de l’Université de Montréal: Montréal, QC, Canada, 2003; 499p, ISBN 2760618633. [Google Scholar]
- Dixon, B.; Parrington, L.; Cook, A.; Pollari, F.; Farber, J. Detection of Cyclospora, Cryptosporidium, and Giardia in ready-to-eat packaged leafy greens in Ontario, Canada. J. Food Prot. 2013, 76, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, R.M. Waterborne outbreaks of cryptosporidiosis. Annali Dell’istituto Superiore di Sanita 2012, 48, 429–446. [Google Scholar] [CrossRef] [PubMed]
- Mac Kenzie, W.R.; Hoxie, N.J.; Proctor, M.E.; Gradus, M.S.; Blair, K.A.; Peterson, D.E.; Kazmierczak, J.J.; Addiss, D.G.; Fox, K.R.; Rose, J.B.; et al. A massive outbreak in Milwaukee of cryptosporidium infection transmitted through the public water supply. N. Engl. J. Med. 1994, 331, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Hoxie, N.J.; Davis, J.P.; Vergeront, J.M.; Nashold, R.D.; Blair, K.A. Cryptosporidiosis-associated mortality following a massive waterborne outbreak in Milwaukee, Wisconsin. Am. J. Public Health 1997, 87, 2032–2035. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Singh, A.; Jiang, J.; Xiao, L. Molecular surveillance of Cryptosporidium spp. in raw wastewater in Milwaukee: Implications for understanding outbreak occurrence and transmission dynamics. J. Clin. Microbiol. 2003, 41, 5254–5257. [Google Scholar] [CrossRef] [PubMed]
- Corso, P.S.; Kramer, M.H.; Blair, K.A.; Addiss, D.G.; Davis, J.P.; Haddix, A.C. Cost of illness in the 1993 waterborne Cryptosporidium outbreak, Milwaukee, Wisconsin. Emerg. Infect. Dis. 2003, 9, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Fayer, R.; Xiao, L. Cryptosporidium and Cryptosporidiosis, 2nd ed.; CRC Press: Boca Raton, FL, USA; IWA Publishing: Boca Raton, FL, USA, 2008; 560p, ISBN 1420052268. [Google Scholar]
- Armson, A.; Thompson, R.C.; Reynoldson, J.A. A review of chemotherapeutic approaches to the treatment of cryptosporidiosis. Expert Rev. Anti-Infect. Ther. 2003, 1, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Tzipori, S.; Widmer, G. A hundred-year retrospective on cryptosporidiosis. Trends Parasitol. 2008, 24, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.D.; Neumann, A.U.; Perelson, A.S.; Chen, W.; Leonard, J.M.; Markowitz, M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 1995, 373, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Lok, J.J.; Bosch, R.J.; Benson, C.A.; Collier, A.C.; Robbins, G.K.; Shafer, R.W.; Hughes, M.D.; Team, A. Long-term increase in CD4+ T-cell counts during combination antiretroviral therapy for HIV-1 infection. AIDS 2010, 24, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Zardi, E.M.; Picardi, A.; Afeltra, A. Treatment of cryptosporidiosis in immunocompromised hosts. Chemotherapy 2005, 51, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Grube, H.; Ramratnam, B.; Ley, C.; Flanigan, T.P. Resolution of AIDS associated cryptosporidiosis after treatment with indinavir. Am. J. Gastroenterol. 1997, 92, 726. [Google Scholar] [PubMed]
- Mele, R.; Morales, M.A.G.; Tosini, F.; Pozio, E. Indinavir reduces Cryptosporidium parvum infection in both in vitro and in vivo models. Int. J. Parasitol. 2003, 33, 757–764. [Google Scholar] [CrossRef]
- Bowman, D.D.; Georgi, J.R. Georgis’ Parasitology for Veterinarians, 10th ed.; Elsevier: St. Louis, MO, USA, 2014; 477p, ISBN 9781455740062. [Google Scholar]
- Ruest, N.; Faubert, G.M.; Couture, Y. Prevalence and geographical distribution of Giardia spp. and Cryptosporidium spp. in dairy farms in Quebec. Can. Vet. J. 1998, 39, 697–700. [Google Scholar] [PubMed]
- Santin, M.; Trout, J.M.; Xiao, L.; Zhou, L.; Greiner, E.; Fayer, R. Prevalence and age-related variation of Cryptosporidium species and genotypes in dairy calves. Vet. Parasitol. 2004, 122, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Zhou, L.; Santin, M.; Yang, W.; Fayer, R. Distribution of Cryptosporidium parvum subtypes in calves in eastern United States. Parasitol. Res. 2007, 100, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Institut de l’élevage. Maladies Des Bovins, 3rd ed.; Éditions France Agricole: Paris, France, 2000; 540p, ISBN 2855570484. [Google Scholar]
- Tzipori, S. Cryptosporidiosis in animals and humans. Microbiol. Rev. 1983, 47, 84–96. [Google Scholar] [PubMed]
- DuPont, H.L.; Chappell, C.L.; Sterling, C.R.; Okhuysen, P.C.; Rose, J.B.; Jakubowski, W. The infectivity of Cryptosporidium parvum in healthy volunteers. N. Engl. J. Med. 1995, 332, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Striepen, B. Parasitic infections: Time to tackle cryptosporidiosis. Nature 2013, 503, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Ludington, J.G.; Ward, H.D. Systemic and mucosal immune responses to Cryptosporidium—Vaccine development. Curr. Trop. Med. Rep. 2015, 2, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Laurent, F.; Lacroix-Lamande, S. Innate immune responses play a key role in controlling infection of the intestinal epithelium by Cryptosporidium. Int. J. Parasitol. 2017, 47, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Kothavade, R.J. Challenges in understanding the immunopathogenesis of Cryptosporidium infections in humans. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
- Menzies, F.M.; Macphail, D.; Henriquez, F.L. The role of chemokines and their receptors during protist parasite infections. Parasitology 2016, 143, 1890–1901. [Google Scholar] [CrossRef] [PubMed]
- Lantier, L.; Lacroix-Lamande, S.; Potiron, L.; Metton, C.; Drouet, F.; Guesdon, W.; Gnahoui-David, A.; Le Vern, Y.; Deriaud, E.; Fenis, A.; et al. Intestinal CD103+ dendritic cells are key players in the innate immune control of Cryptosporidium parvum infection in neonatal mice. PLoS Pathog. 2013, 9, e1003801. [Google Scholar] [CrossRef] [PubMed]
- Auray, G.; Lacroix-Lamande, S.; Mancassola, R.; Dimier-Poisson, I.; Laurent, F. Involvement of intestinal epithelial cells in dendritic cell recruitment during C. parvum infection. Microbes Infect. Inst. Pasteur 2007, 9, 574–582. [Google Scholar] [CrossRef] [PubMed]
- De Sablet, T.; Potiron, L.; Marquis, M.; Bussiere, F.I.; Lacroix-Lamande, S.; Laurent, F. Cryptosporidium parvum increases intestinal permeability through interaction with epithelial cells and IL-1beta and TNFalpha released by inflammatory monocytes. Cell. Microbiol. 2016, 18, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Leitch, G.J.; He, Q. Arginine-derived nitric oxide reduces fecal oocyst shedding in nude mice infected with Cryptosporidium parvum. Infect. Immun. 1994, 62, 5173–5176. [Google Scholar] [PubMed]
- Nordone, S.K.; Gookin, J.L. Lymphocytes and not IFN-gamma mediate expression of iNOS by intestinal epithelium in murine cryptosporidiosis. Parasitol. Res. 2010, 106, 1507–1511. [Google Scholar] [CrossRef] [PubMed]
- Gookin, J.L.; Chiang, S.; Allen, J.; Armstrong, M.U.; Stauffer, S.H.; Finnegan, C.; Murtaugh, M.P. NF-kappaB-mediated expression of iNOS promotes epithelial defense against infection by Cryptosporidium parvum in neonatal piglets. Am. J. Physiol. Gastrointest. Liv. Physiol. 2006, 290, G164–G174. [Google Scholar] [CrossRef] [PubMed]
- Zaalouk, T.K.; Bajaj-Elliott, M.; George, J.T.; McDonald, V. Differential regulation of beta-defensin gene expression during Cryptosporidium parvum infection. Infect. Immun. 2004, 72, 2772–2779. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-M.; O’Hara, S.P.; Nelson, J.B.; Splinter, P.L.; Small, A.J.; Tietz, P.S.; Limper, A.H.; LaRusso, N.F. Multiple TLRs are expressed in human cholangiocytes and mediate host epithelial defense responses to Cryptosporidium parvum via activation of NF-kappaB. J. Immunol. 2005, 175, 7447–7456. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, M.; Meadows, S.K.; Basu, S.; Mselle, T.F.; Wira, C.R.; Sentman, C.L. TLRs mediate IFN-gamma production by human uterine NK cells in endometrium. J. Immunol. 2006, 176, 6219–6224. [Google Scholar] [CrossRef] [PubMed]
- Gelman, A.E.; Zhang, J.; Choi, Y.; Turka, L.A. Toll-like receptor ligands directly promote activated CD4+ T-cell survival. J. Immunol. 2004, 172, 6065–6073. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.H.; Taylor, D.K.; Turka, L.A. The contribution of direct TLR signaling to T-cell responses. Immunol. Res. 2009, 45, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Borad, A.; Ward, H. Human immune responses in cryptosporidiosis. Future Microbiol. 2010, 5, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cordon, G.; Yang, G.; Zhou, B.; Nie, W.; Li, S.; Shi, L.; Tzipori, S.; Feng, H. Interaction of Cryptosporidium parvum with mouse dendritic cells leads to their activation and parasite transportation to mesenteric lymph nodes. Pathog. Dis. 2014, 70, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Bedi, B.; McNair, N.N.; Mead, J.R. Dendritic cells play a role in host susceptibility to Cryptosporidium parvum infection. Immunol. Lett. 2014, 158, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Leav, B.A.; Yoshida, M.; Rogers, K.; Cohen, S.; Godiwala, N.; Blumberg, R.S.; Ward, H. An early intestinal mucosal source of gamma interferon is associated with resistance to and control of Cryptosporidium parvum infection in mice. Infect. Immun. 2005, 73, 8425–8428. [Google Scholar] [CrossRef] [PubMed]
- Pollok, R.C.; Farthing, M.J.; Bajaj-Elliott, M.; Sanderson, I.R.; McDonald, V. Interferon gamma induces enterocyte resistance against infection by the intracellular pathogen Cryptosporidium parvum. Gastroenterology 2001, 120, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Lodoen, M.B.; Lanier, L.L. Natural killer cells as an initial defense against pathogens. Curr. Opin. Immunol. 2006, 18, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Barakat, F.M.; McDonald, V.; Di Santo, J.P.; Korbel, D.S. Roles for NK cells and an NK cell-independent source of intestinal gamma interferon for innate immunity to Cryptosporidium parvum infection. Infect. Immun. 2009, 77, 5044–5049. [Google Scholar] [CrossRef] [PubMed]
- Choudhry, N.; Petry, F.; van Rooijen, N.; McDonald, V. A protective role for interleukin 18 in interferon gamma-mediated innate immunity to Cryptosporidium parvum that is independent of natural killer cells. J. Infect. Dis. 2012, 206, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Dann, S.M.; Wang, H.C.; Gambarin, K.J.; Actor, J.K.; Robinson, P.; Lewis, D.E.; Caillat-Zucman, S.; White, A.C., Jr. Interleukin-15 activates human natural killer cells to clear the intestinal protozoan Cryptosporidium. J. Infect. Dis. 2005, 192, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- McDonald, V.; Korbel, D.S.; Barakat, F.M.; Choudhry, N.; Petry, F. Innate immune responses against Cryptosporidium parvum infection. Parasite Immunol. 2013, 35, 55–64. [Google Scholar] [CrossRef] [PubMed]
- McDonald, V. Host cell-mediated responses to infection with Cryptosporidium. Parasite Immunol. 2000, 22, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Bedi, B.; Mead, J.R. Cryptosporidium parvum antigens induce mouse and human dendritic cells to generate Th1-enhancing cytokines. Parasite Immunol. 2012, 34, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.P.; Travers, P.; Walport, M.; Janeway, C. Janeway’s Immunobiology, 7th ed.; Garland Science: New York, NY, USA, 2008; 887p, ISBN 0815341237. [Google Scholar]
- Barakat, F.M.; McDonald, V.; Foster, G.R.; Tovey, M.G.; Korbel, D.S. Cryptosporidium parvum infection rapidly induces a protective innate immune response involving type I interferon. J. Infect. Dis. 2009, 200, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.F., Jr.; Fayer, R.; Chen, S.J.; Gause, W.C.; Gately, M.K.; Finkelman, F.D. IL-12 protects immunocompetent and immunodeficient neonatal mice against infection with Cryptosporidium parvum. J. Immunol. 1996, 156, 263–268. [Google Scholar] [PubMed]
- McDonald, V.; Pollok, R.C.; Dhaliwal, W.; Naik, S.; Farthing, M.J.; Bajaj-Elliott, M. A potential role for interleukin-18 in inhibition of the development of Cryptosporidium parvum. Clin. Exp. Immunol. 2006, 145, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Von Oettingen, J.; Nath-Chowdhury, M.; Ward, B.J.; Rodloff, A.C.; Arrowood, M.J.; Ndao, M. High-yield amplification of Cryptosporidium parvum in interferon gamma receptor knockout mice. Parasitology 2008, 135, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Ndao, M.; Nath-Chowdhury, M.; Sajid, M.; Marcus, V.; Mashiyama, S.T.; Sakanari, J.; Chow, E.; Mackey, Z.; Land, K.M.; Jacobson, M.P.; et al. A cysteine protease inhibitor rescues mice from a lethal Cryptosporidium parvum infection. Antimicrob. Agents Chemother. 2013, 57, 6063–6073. [Google Scholar] [CrossRef] [PubMed]
- Sonzogni-Desautels, K.; Renteria, A.E.; Camargo, F.V.; Di Lenardo, T.Z.; Mikhail, A.; Arrowood, M.J.; Fortin, A.; Ndao, M. Oleylphosphocholine (OlPC) arrests Cryptosporidium parvum growth in vitro and prevents lethal infection in interferon gamma receptor knock-out mice. Front. Microbiol. 2015, 6, 973. [Google Scholar] [CrossRef] [PubMed]
- Lean, I.S.; McDonald, V.; Pollok, R.C. The role of cytokines in the pathogenesis of Cryptosporidium infection. Curr. Opin. Infect. Dis. 2002, 15, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Tessema, T.S.; Schwamb, B.; Lochner, M.; Forster, I.; Jakobi, V.; Petry, F. Dynamics of gut mucosal and systemic Th1/Th2 cytokine responses in interferon-gamma and interleukin-12p40 knock out mice during primary and challenge Cryptosporidium parvum infection. Immunobiology 2009, 214, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Jakobi, V.; Petry, F. Humoral immune response in IL-12 and IFN-gamma deficient mice after infection with Cryptosporidium parvum. Parasite Immunol. 2008, 30, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Forchielli, M.; Walker, A.W. The role of gut-associated lymphoid tissues and mucosal defence. Br. J. Nutr. 2005, 93, S41–S48. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Koboziev, I.; Karlsson, F.; Grisham, M.B. Gut-associated lymphoid tissue, T cell trafficking, and chronic intestinal inflammation. Ann. N. Y. Acad. Sci. 2010, 1207, E86–E93. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F.; Finotto, S.; Glimcher, L.H.; Neurath, M.F.; Finotto, S.; Glimcher, L.H. The role of Th1/Th2 polarization in mucosal immunity. Nat. Med. 2002, 8, 567–583. [Google Scholar] [CrossRef] [PubMed]
- Hunter, P.R.; Nichols, G. Epidemiology and clinical features of Cryptosporidium infection in immunocompromised patients. Clin. Microbiol. Rev. 2002, 15, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Nsagha, D.S.; Njunda, A.L.; Assob, N.J.; Ayima, C.W.; Tanue, E.A.; Kibu, O.D.; Kwenti, T.E. Intestinal parasitic infections in relation to CD4(+) T cell counts and diarrhea in HIV/AIDS patients with or without antiretroviral therapy in Cameroon. BMC Infect. Dis. 2016, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Pozio, E.; Rezza, G.; Boschini, A.; Pezzotti, P.; Tamburrini, A.; Rossi, P.; Fine, D.M.; Smacchia, C.; Schiesari, A.; Gattei, E.; et al. Clinical cryptosporidiosis and human immunodeficiency virus (HIV)-induced immunosuppression: Findings from a longitudinal study of HIV-positive and HIV-negative former injection drug users. J. Infect. Dis. 1997, 176, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, K.S.; Ravi Kumar, K.L. Intestinal cryptosporidiosis and the profile of the CD4 counts in a cohort of HIV infected patients. J. Clin. Diagn. Res. 2013, 7, 1016–1020. [Google Scholar] [CrossRef]
- Gedle, D.; Kumera, G.; Eshete, T.; Ketema, K.; Adugna, H.; Feyera, F. Intestinal parasitic infections and its association with undernutrition and CD4 T cell levels among HIV/AIDS patients on HAART in Butajira, Ethiopia. J. Health Popul. Nutr. 2017, 36, 15. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.J.; Rutala, W.A. Cryptosporidiosis. N. Engl. J. Med. 2002, 347, 1287. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.B.; White, C.A. An updated review on Cryptosporidium and Giardia. Gastroenterol. Clin. N. Am. 2006, 35, 291–314. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.H.; Fang, Y.Q.; Ryan, U.; Guo, Y.X.; Wu, F.; Du, S.Z.; Chen, D.K.; Lin, Q. Dynamics of Th17 associating cytokines in Cryptosporidium parvum-infected mice. Parasitol. Res. 2016, 115, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Kindt, T.J.; Goldsby, R.A.; Osborne, B.A.; Kuby, J. Kuby Immunology, 6th ed.; W.H. Freeman: New York, NY, USA, 2007; 693p, ISBN 9781429202114. [Google Scholar]
- Marchi, L.F.; Sesti-Costa, R.; Ignacchiti, M.D.; Chedraoui-Silva, S.; Mantovani, B. In vitro activation of mouse neutrophils by recombinant human interferon-gamma: Increased phagocytosis and release of reactive oxygen species and pro-inflammatory cytokines. Int. Immunopharmacol. 2014, 18, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Diamond, R.D.; Lyman, C.A.; Wysong, D.R. Disparate effects of interferon-gamma and tumor necrosis factor-alpha on early neutrophil respiratory burst and fungicidal responses to Candida albicans hyphae in vitro. J. Clin. Investig. 1991, 87, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Maródi, L.; Káposzta, R.; Nemes, E. Survival of group B streptococcus type III in mononuclear phagocytes: Differential regulation of bacterial killing in cord macrophages by human recombinant gamma interferon and granulocyte-macrophage colony-stimulating factor. Infect. Immun. 2000, 68, 2167–2170. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, S.A.; Perryman, L.E.; Davis, W.C.; McGuire, T.C. IL-4 protects adult C57BL/6 mice from prolonged Cryptosporidium parvum infection: Analysis of CD4 + alpha beta + IFN-gamma + and CD4 + alpha beta + IL-4 + lymphocytes in gut-associated lymphoid tissue during resolution of infection. J. Immunol. 1998, 161, 1891–1900. [Google Scholar] [PubMed]
- McDonald, S.A.; O’Grady, J.E.; Bajaj-Elliott, M.; Notley, C.A.; Alexander, J.; Brombacher, F.; McDonald, V. Protection against the early acute phase of Cryptosporidium parvum infection conferred by interleukin-4-induced expression of T helper 1 cytokines. J. Infect. Dis. 2004, 190, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, T.; Zimmermann, S.; Himmelrich, H.; Gumy, A.; Egeter, O.; Sakrauski, A.K.; Seegmuller, I.; Voigt, H.; Launois, P.; Levine, A.D.; et al. IL-4 instructs TH1 responses and resistance to Leishmania major in susceptible BALB/c mice. Nat. Immunol. 2001, 2, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Pantenburg, B.; Castellanos-Gonzalez, A.; Dann, S.M.; Connelly, R.L.; Lewis, D.E.; Ward, H.D.; White, A.C. Human CD8(+) T cells clear Cryptosporidium parvum from infected intestinal epithelial cells. Am. J. Trop. Med. Hyg. 2010, 82, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Harp, J.A.; Harmsen, A.G. Requirements for CD4+ cells and gamma interferon in resolution of established Cryptosporidium parvum infection in mice. Infect. Immun. 1993, 61, 3928–3932. [Google Scholar] [PubMed]
- McDonald, V.; Robinson, H.A.; Kelly, J.P.; Bancroft, G.J. Immunity to Cryptosporidium muris infection in mice is expressed through gut CD4+ intraepithelial lymphocytes. Infect. Immun. 1996, 64, 2556–2562. [Google Scholar] [PubMed]
- McDonald, V.; Robinson, H.A.; Kelly, J.P.; Bancroft, G.J. Cryptosporidium muris in adult mice: Adoptive transfer of immunity and protective roles of CD4 versus CD8 cells. Infect. Immun. 1994, 62, 2289–2294. [Google Scholar] [PubMed]
- Brandtzaeg, P. Mucosal immunity: Induction, dissemination, and effector functions. Scand. J. Immunol. 2009, 70, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Wesemann, D.R.; Portuguese, A.J.; Meyers, R.M.; Gallagher, M.P.; Cluff-Jones, K.; Magee, J.M.; Panchakshari, R.A.; Rodig, S.J.; Kepler, T.B.; Alt, F.W. Microbial colonization influences early B-lineage development in the gut lamina propria. Nature 2013, 501, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, A.J.; Geuking, M.B.; Slack, E.; Hapfelmeier, S.; McCoy, K.D. The habitat, double life, citizenship, and forgetfulness of IgA. Immunol. Rev. 2012, 245, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Kassa, M.; Comby, E.; Lemeteil, D.; Brasseur, P.; Ballet, J.J. Characterization of anti-Cryptosporidium IgA antibodies in sera from immunocompetent individuals and HIV-infected patients. J. Protozool. 1991, 38, 179S–180S. [Google Scholar] [PubMed]
- Allison, G.M.; Rogers, K.A.; Borad, A.; Ahmed, S.; Karim, M.; Kane, A.V.; Hibberd, P.L.; Naumova, E.N.; Calderwood, S.B.; Ryan, E.T.; et al. Antibody responses to the immunodominant Cryptosporidium gp15 antigen and gp15 polymorphisms in a case–control study of cryptosporidiosis in children in Bangladesh. Am. J. Trop. Med. Hyg. 2011, 85, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Borad, A.J.; Allison, G.M.; Wang, D.; Ahmed, S.; Karim, M.M.; Kane, A.V.; Moy, J.; Hibberd, P.L.; Ajjampur, S.; Kang, G.; et al. Systemic antibody responses to the immunodominant p23 antigen and p23 polymorphisms in children with cryptosporidiosis in Bangladesh. Am. J. Trop. Med. Hyg. 2012, 86, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Mead, J.R. Prospects for immunotherapy and vaccines against Cryptosporidium. Hum. Vaccines Immunother. 2014, 10, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.; Sponseller, J.; Schmidt, D.; Cohen, O.; Tzipori, S. Hyperimmune bovine colostrum for treatment of GI infections. Hum. Vaccines Immunother. 2013, 9, 1565–1568. [Google Scholar] [CrossRef] [PubMed]
- Kramski, M.; Center, R.J.; Wheatley, A.K.; Jacobson, J.C.; Alexander, M.R.; Rawlin, G.; Purcell, D.F. Hyperimmune bovine colostrum as a low-cost, large-scale source of antibodies with broad neutralizing activity for HIV-1 envelope with potential use in microbicides. Antimicrob. Agents Chemother. 2012, 56, 4310–4319. [Google Scholar] [CrossRef] [PubMed]
- Naciri, M.; Mancassola, R.; Reperant, J.M.; Canivez, O.; Quinque, B.; Yvore, P. Treatment of experimental ovine cryptosporidiosis with ovine or bovine hyperimmune colostrum. Vet. Parasitol. 1994, 53, 173–190. [Google Scholar] [CrossRef]
- Wyatt, C.R.; Brackett, E.J.; Savidge, J. Evidence for the emergence of a type-1-like immune response in intestinal mucosa of calves recovering from cryptosporidiosis. J. Parasitol. 2001, 87, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, C.R.; Barrett, W.J.; Brackett, E.J.; Schaefer, D.A.; Riggs, M.W. Association of IL-10 expression by mucosal lymphocytes with increased expression of Cryptosporidium parvum epitopes in infected epithelium. J. Parasitol. 2002, 88, 281–286. [Google Scholar] [CrossRef]
- Wyatt, C.R.; Lindahl, S.; Austin, K.; Kapil, S.; Branch, J. Response of T lymphocytes from previously infected calves to recombinant Cryptosporidium parvum P23 vaccine antigen. J. Parasitol. 2005, 91, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Whitmire, W.M.; Harp, J.A. Characterization of bovine cellular and serum antibody responses during infection by Cryptosporidium parvum. Infect. Immun. 1991, 59, 990–995. [Google Scholar] [PubMed]
- De Graaf, D.C.; Peeters, J.E. Specific interferon-gamma, IgA and IgM responses after experimental infection of neonatal calves with Cryptosporidium parvum. Int. J. Parasitol. 1997, 27, 131–134. [Google Scholar] [CrossRef]
- Peeters, J.E.; Villacorta, I.; Vanopdenbosch, E.; Vandergheynst, D.; Naciri, M.; Ares-Mazas, E.; Yvore, P. Cryptosporidium parvum in calves: Kinetics and immunoblot analysis of specific serum and local antibody responses (immunoglobulin A [IgA], IgG, and IgM) after natural and experimental infections. Infect. Immun. 1992, 60, 2309–2316. [Google Scholar] [PubMed]
- Wyatt, C.R.; Brackett, E.J.; Mason, P.H.; Savidge, J.; Perryman, L.E. Excretion patterns of mucosally delivered antibodies to p23 in Cryptosporidium parvum infected calves. Vet. Immunol. Immunopathol. 2000, 76, 309–317. [Google Scholar] [CrossRef]
- Wyatt, C.R.; Perryman, L.E. Detection of mucosally delivered antibody to Cryptosporidium parvum p23 in infected calves. Ann. N. Y. Acad. Sci. 2000, 916, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.F.; Swain, J.B.; Besser, T.E.; Jasmer, D.; Wyatt, C.R. Detection of antibodies to a recombinant Cryptosporidium parvum p23 in serum and feces from neonatal calves. J. Parasitol. 2003, 89, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.M.; Smith, G.W. Pathophysiology of diarrhea in calves. Vet. Clin. N. Am. Food Anim. Pract. 2009, 25, 13–36. [Google Scholar] [CrossRef] [PubMed]
- Perryman, L.E.; Kapil, S.J.; Jones, M.L.; Hunt, E.L. Protection of calves against cryptosporidiosis with immune bovine colostrum induced by a Cryptosporidium parvum recombinant protein. Vaccine 1999, 17, 2142–2149. [Google Scholar] [CrossRef]
- Askari, N.; Shayan, P.; Mokhber-Dezfouli, M.R.; Ebrahimzadeh, E.; Lotfollahzadeh, S.; Rostami, A.; Amininia, N.; Ragh, M.J. Evaluation of recombinant P23 protein as a vaccine for passive immunization of newborn calves against Cryptosporidium parvum. Parasite Immunol. 2016, 38, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Tatalick, L.M.; Perryman, L.E. Attempts to protect severe combined immunodeficient (SCID) mice with antibody enriched for reactivity to Cryptosporidium parvum surface antigen-1. Vet. Parasitol. 1995, 58, 281–290. [Google Scholar] [CrossRef]
- Perryman, L.E.; Kegerris, K.A.; Mason, P.H. Effect of orally administered monoclonal antibody on persistent Cryptosporidium parvum infection in scid mice. Infect. Immun. 1993, 61, 4906–4908. [Google Scholar] [PubMed]
- Riggs, M.W.; Schaefer, D.A.; Kapil, S.J.; Barley-Maloney, L.; Perryman, L.E. Efficacy of monoclonal antibodies against defined antigens for passive immunotherapy of chronic gastrointestinal cryptosporidiosis. Antimicrob. Agents Chemother. 2002, 46, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Enriquez, F.J.; Riggs, M.W. Role of immunoglobulin A monoclonal antibodies against P23 in controlling murine Cryptosporidium parvum infection. Infect. Immun. 1998, 66, 4469–4473. [Google Scholar] [PubMed]
- Kobayashi, C.; Yokoyama, H.; Nguyen, S.V.; Kodama, Y.; Kimata, T.; Izeki, M. Effect of egg yolk antibody on experimental Cryptosporidium parvum infection in scid mice. Vaccine 2004, 23, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Riggs, M.W.; Cama, V.A.; Leary, H.L., Jr.; Sterling, C.R. Bovine antibody against Cryptosporidium parvum elicits a circumsporozoite precipitate-like reaction and has immunotherapeutic effect against persistent cryptosporidiosis in SCID mice. Infect. Immun. 1994, 62, 1927–1939. [Google Scholar] [PubMed]
- Martin-Gomez, S.; Alvarez-Sanchez, M.A.; Rojo-Vazquez, F.A. Oral administration of hyperimmune anti-Cryptosporidium parvum ovine colostral whey confers a high level of protection against cryptosporidiosis in newborn NMRI mice. J. Parasitol. 2005, 91, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Adjei, A.A.; Jones, J.T.; Riggs, M.W.; Enriquez, F.J. Evidence of thymus-independent local and systemic antibody responses to Cryptosporidium parvum infection in nude mice. Infect. Immun. 1999, 67, 3947–3951. [Google Scholar] [PubMed]
- Cozon, G.; Biron, F.; Jeannin, M.; Cannella, D.; Revillard, J.P. Secretory IgA antibodies to Cryptosporidium parvum in AIDS patients with chronic cryptosporidiosis. J. Infect. Dis. 1994, 169, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Tzipori, S.; Roberton, D.; Chapman, C. Remission of diarrhoea due to cryptosporidiosis in an immunodeficient child treated with hyperimmune bovine colostrum. Br. Med. J. (Clin. Res. Ed.) 1986, 293, 1276–1277. [Google Scholar] [CrossRef]
- Plettenberg, A.; Stoehr, A.; Stellbrink, H.J.; Albrecht, H.; Meigel, W. A preparation from bovine colostrum in the treatment of HIV-positive patients with chronic diarrhea. Clin. Investig. 1993, 71, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, P.D.; Cello, J.P. Treatment of severe diarrhea caused by Cryptosporidium parvum with oral bovine immunoglobulin concentrate in patients with AIDS. J. Acquir. Immune Defic. Syndr. 1996, 13, 348–354. [Google Scholar] [CrossRef]
- Nord, J.; Ma, P.; DiJohn, D.; Tzipori, S.; Tacket, C.O. Treatment with bovine hyperimmune colostrum of cryptosporidial diarrhea in AIDS patients. AIDS 1990, 4, 581–584. [Google Scholar] [CrossRef]
- Abubakar, I.; Aliyu, S.H.; Arumugam, C.; Hunter, P.R.; Usman, N.K. Prevention and treatment of cryptosporidiosis in immunocompromised patients. Cochrane Database Syst. Rev. 2007, CD004932. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S. History of vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 12283–12287. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Ahmed, R. Immunological mechanisms of vaccination. Nat. Immunol. 2011, 12, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Li, J.; Zhang, X.; Gong, P.; Zhang, G.; Li, S.; Wang, H. Induction of immune responses in mice by a DNA vaccine encoding Cryptosporidium parvum Cp12 and Cp21 and its effect against homologous oocyst challenge. Vet. Parasitol. 2010, 172, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Luo, J.; Amer, S.; Guo, Y.; Hu, Y.; Lu, Y.; Wang, H.; Duan, M.; He, H. Multivalent DNA vaccine induces protective immune responses and enhanced resistance against Cryptosporidium parvum infection. Vaccine 2010, 29, 323–328. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zhao, B.; Liu, L.; Zhou, K.; Qin, X.; Zhang, Q.; Li, X.; Zheng, C.; Duan, M. The humoral and cellular immune responses in mice induced by DNA vaccine expressing the sporozoite surface protein of Cryptosporidium parvum. DNA Cell Biol. 2004, 23, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Boulter-Bitzer, J.I.; Lee, H.; Trevors, J.T. Molecular targets for detection and immunotherapy in Cryptosporidium parvum. Biotechnol. Adv. 2007, 25, 13–44. [Google Scholar] [CrossRef] [PubMed]
- Burton, A.J.; Nydam, D.V.; Jones, G.; Zambriski, J.A.; Linden, T.C.; Cox, G.; Davis, R.; Brown, A.; Bowman, D.D. Antibody responses following administration of a Cryptosporidium parvum rCP15/60 vaccine to pregnant cattle. Vet. Parasitol. 2011, 175, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zai, D.; Zhang, D.; Wei, Q.; Han, G.; Gao, H.; Huang, B. Divalent Cp15-23 vaccine enhances immune responses and protection against Cryptosporidium parvum infection. Parasite Immunol. 2010, 32, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, S.L.; Billingsley, P.F.; James, E.; Richman, A.; Loyevsky, M.; Li, T.; Chakravarty, S.; Gunasekera, A.; Chattopadhyay, R.; Li, M.; et al. Development of a metabolically active, non-replicating sporozoite vaccine to prevent Plasmodium falciparum malaria. Hum. Vaccines 2010, 6, 97–106. [Google Scholar] [CrossRef]
- Williams, R.B. Fifty years of anticoccidial vaccines for poultry (1952–2002). Avian Dis. 2002, 46, 775–802. [Google Scholar] [CrossRef]
- McDonald, V.; Shirley, M.W. Past and future: Vaccination against Eimeria. Parasitology 2009, 136, 1477–1489. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, M.C.; Chute, M.B.; Danforth, H.D. Protection against coccidiosis in outbred chickens elicited by gamma-irradiated Eimeria maxima. Avian Dis. 1997, 41, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, M.F.; O’Connell, E.; Te Punga, W.A. Toxoplasmosis in sheep III. Further evaluation of the ability of a live Toxoplasma gondii vaccine to prevent lamb losses and reduce congenital infection following experimental oral challenge. N. Z. Vet. J. 1988, 36, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Buxton, D.; Thomson, K.; Maley, S.; Wright, S.; Bos, H. Experimental challenge of sheep 18 months after vaccination with a live (S48) Toxoplasma gondii vaccine. Vet. Rec. 1993, 133, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Dunning, N. Leishmania vaccines: From leishmanization to the era of DNA technology. Biosci. Horiz. Int. J. Stud. Res. 2009, 2, 73–82. [Google Scholar] [CrossRef]
- Breton, M.; Tremblay, M.J.; Ouellette, M.; Papadopoulou, B. Live nonpathogenic parasitic vector as a candidate vaccine against visceral leishmaniasis. Infect. Immun. 2005, 73, 6372–6382. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Dey, R.; Dagur, P.K.; Kruhlak, M.; Ismail, N.; Debrabant, A.; Joshi, A.B.; Akue, A.; Kukuruga, M.; Takeda, K.; et al. Genetically modified live attenuated Leishmania donovani parasites induce innate immunity through classical activation of macrophages that direct the Th1 response in mice. Infect. Immun. 2015, 83, 3800–3815. [Google Scholar] [CrossRef] [PubMed]
- Arrowood, M.J. In vitro cultivation of cryptosporidium species. Clin. Microbiol. Rev. 2002, 15, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.R.; Park, W.Y. The effect of gamma-irradiation on the viability of Cryptosporidium parvum. J. Parasitol. 2003, 89, 639–642. [Google Scholar] [CrossRef]
- Jenkins, M.; Higgins, J.; Kniel, K.; Trout, J.; Fayer, R. Protection of calves against cryptosporiosis by oral inoculation with gamma-irradiated Cryptosporidium parvum oocysts. J. Parasitol. 2004, 90, 1178–1180. [Google Scholar] [CrossRef] [PubMed]
- Schafer, R.; Portnoy, D.A.; Brassell, S.A.; Paterson, Y. Induction of a cellular immune response to a foreign antigen by a recombinant Listeria monocytogenes vaccine. J. Immunol. 1992, 149, 53–59. [Google Scholar] [PubMed]
- Guy, B.; Guirakhoo, F.; Barban, V.; Higgs, S.; Monath, T.P.; Lang, J. Preclinical and clinical development of YFV 17D-based chimeric vaccines against dengue, West Nile and Japanese encephalitis viruses. Vaccine 2010, 28, 632–649. [Google Scholar] [CrossRef] [PubMed]
- Galen, J.E.; Pasetti, M.F.; Tennant, S.; Ruiz-Olvera, P.; Sztein, M.B.; Levine, M.M. Salmonella enterica serovar Typhi live vector vaccines finally come of age. Immunol. Cell Biol. 2009, 87, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Hathaway, L.J.; Kraehenbuhl, J.P. The role of M cells in mucosal immunity. Cell. Mol. Life Sci. 2000, 57, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Manque, P.A.; Tenjo, F.; Woehlbier, U.; Lara, A.M.; Serrano, M.G.; Xu, P.; Alves, J.M.; Smeltz, R.B.; Conrad, D.H.; Buck, G.A. Identification and immunological characterization of three potential vaccinogens against Cryptosporidium species. Clin. Vaccine Immunol. 2011, 18, 1796–1802. [Google Scholar] [CrossRef] [PubMed]
- Benitez, A.J.; McNair, N.; Mead, J.R. Oral immunization with attenuated Salmonella enterica serovar Typhimurium encoding Cryptosporidium parvum Cp23 and Cp40 antigens induces a specific immune response in mice. Clin. Vaccine Immunol. 2009, 16, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.K.; Rojo, A.L.; Costa, L.B.; Smeltz, R.; Manque, P.; Woehlbier, U.; Bartelt, L.; Galen, J.; Buck, G.; Guerrant, R.L. Intranasal vaccination in mice with an attenuated Salmonella enterica Serovar 908htr A expressing Cp15 of Cryptosporidium: Impact of malnutrition with preservation of cytokine secretion. Vaccine 2013, 31, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Carleton, H.A. Pathogenic bacteria as vaccine vectors: Teaching old bugs new tricks. Yale J. Biol. Med. 2010, 83, 217–222. [Google Scholar] [PubMed]
- Pei, Z.; Jiang, X.; Yang, Z.; Ren, X.; Gong, H.; Reeves, M.; Sheng, J.; Wang, Y.; Pan, Z.; Liu, F.; et al. Oral delivery of a novel attenuated Salmonella vaccine expressing Influenza A virus proteins protects mice against H5N1 and H1N1 viral infection. PLoS ONE 2015, 10, e0129276. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, X.; Bi, K.; Sun, X.; Yang, J.; Gu, Y.; Huang, J.; Zhan, B.; Zhu, X. Oral vaccination with attenuated Salmonella typhimurium-delivered TsPmy DNA vaccine elicits protective immunity against Trichinella spiralis in BALB/c mice. PLoS Negl. Trop. Dis. 2016, 10, e0004952. [Google Scholar] [CrossRef] [PubMed]
- Chinchilla, M.; Pasetti, M.F.; Medina-Moreno, S.; Wang, J.; Gomez-Duarte, O.G.; Stout, R.; Levine, M.M.; Galen, J.E. Enhanced immunity to Plasmodium falciparum circumsporozoite protein (PfCSP) by using Salmonella enterica serovar Typhi expressing PfCSP and a PfCSP-encoding DNA vaccine in a heterologous prime-boost strategy. Infect. Immun. 2007, 75, 3769–3779. [Google Scholar] [CrossRef] [PubMed]
- Shahabi, V.; Maciag, P.C.; Rivera, S.; Wallecha, A. Live, attenuated strains of Listeria and Salmonella as vaccine vectors in cancer treatment. Bioeng. Bugs 2010, 1, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, W.; Xu, X.; Metelitsa, L.; Hensel, M. Evaluation of Salmonella enterica type III secretion system effector proteins as carriers for heterologous vaccine antigens. Infect. Immun. 2012, 80, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Shirafuji, H.; Xuan, X.; Kimata, I.; Takashima, Y.; Fukumoto, S.; Otsuka, H.; Nagasawa, H.; Suzuki, H. Expression of P23 of Cryptosporidium parvum in Toxoplasma gondii and evaluation of its protective effects. J. Parasitol. 2005, 91, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Stevens, T.L.; Bossie, A.; Sanders, V.M.; Fernandez-Botran, R.; Coffman, R.L.; Mosmann, T.R.; Vitetta, E.S. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature 1988, 334, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Kwok, L.Y.; Wang, L.; Zhang, J.; Guo, Z.; Zhang, H. A pilot study on the effect of Lactobacillus casei Zhang on intestinal microbiota parameters in Chinese subjects of different age. Benef. Microbes 2014, 5, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Geriletu; Xu, R.; Jia, H.; Terkawi, M.A.; Xuan, X.; Zhang, H. Immunogenicity of orally administrated recombinant Lactobacillus casei Zhang expressing Cryptosporidium parvum surface adhesion protein P23 in mice. Curr. Microbiol. 2011, 62, 1573–1580. [Google Scholar] [CrossRef]
- Lin, I.Y.C.; Van, T.; Smooker, P.M. Live-attenuated bacterial vectors: Tools for vaccine and therapeutic agent delivery. Vaccines 2015, 3, 940–972. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Iwasaki, A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 2012, 491, 463–467. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemieux, M.W.; Sonzogni-Desautels, K.; Ndao, M. Lessons Learned from Protective Immune Responses to Optimize Vaccines against Cryptosporidiosis. Pathogens 2018, 7, 2. https://doi.org/10.3390/pathogens7010002
Lemieux MW, Sonzogni-Desautels K, Ndao M. Lessons Learned from Protective Immune Responses to Optimize Vaccines against Cryptosporidiosis. Pathogens. 2018; 7(1):2. https://doi.org/10.3390/pathogens7010002
Chicago/Turabian StyleLemieux, Maxime W., Karine Sonzogni-Desautels, and Momar Ndao. 2018. "Lessons Learned from Protective Immune Responses to Optimize Vaccines against Cryptosporidiosis" Pathogens 7, no. 1: 2. https://doi.org/10.3390/pathogens7010002
APA StyleLemieux, M. W., Sonzogni-Desautels, K., & Ndao, M. (2018). Lessons Learned from Protective Immune Responses to Optimize Vaccines against Cryptosporidiosis. Pathogens, 7(1), 2. https://doi.org/10.3390/pathogens7010002



