Calcineurin Orchestrates Hyphal Growth, Septation, Drug Resistance and Pathogenesis of Aspergillus fumigatus: Where Do We Go from Here?
Abstract
:1. Introduction
2. Calcineurin Is Essential for Hyphal Extension and Septation
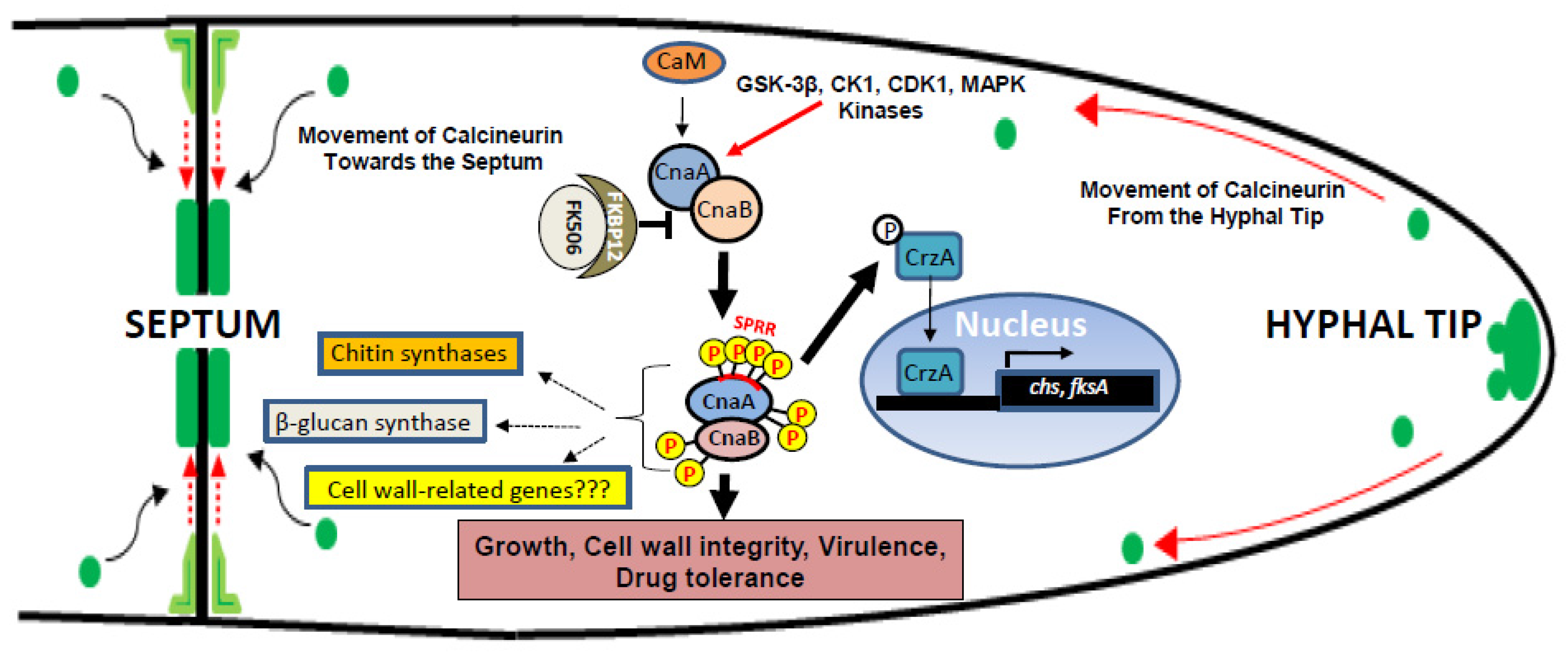
3. Septal Localization: A Key to Calcineurin Function
4. Calcineurin Phosphorylation: A Unique Modification
5. Calcineurin as an Antifungal Drug Target: Future Perspectives
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kontoyiannis, D.P.; Marr, K.A.; Park, B.J.; Alexander, B.D.; Anaissie, E.J.; Walsh, T.J.; Ito, J.; Andes, D.R.; Baddley, J.W.; Brown, J.M.; et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: Overview of the transplant-associated infection surveillance network (transnet) database. Clin. Infect. Dis. 2010, 50, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Hemenway, C.; Heitman, J. Calcineurin. Cell Biochem. Biophys. 1999, 30, 115–151. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.; Clipstone, N.; Timmermann, L.; Northrop, J.; Graef, I.; Fiorentino, D.; Nourse, J.; Crabtree, G.R. The mechanism of action of cyclosporin A and fk506. Clin. Immunol. Immunopathol. 1996, 80, S40–S45. [Google Scholar] [CrossRef] [PubMed]
- Breuder, T.; Hemenway, C.S.; Movva, N.R.; Cardenas, M.E.; Heitman, J. Calcineurin is essential in cyclosporin a- and fk506-sensitive yeast strains. Proc. Natl. Acad. Sci. USA 1994, 91, 5372–5376. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, M.E.; Muir, R.S.; Breuder, T.; Heitman, J. Targets of immunophilin-immunosuppressant complexes are distinct highly conserved regions of calcineurin A. EMBO J. 1995, 14, 2772–2783. [Google Scholar] [PubMed]
- Klee, C.B.; Crouch, T.H.; Krinks, M.H. Calcineurin: A calcium- and calmodulin-binding protein of the nervous system. Proc. Natl. Acad. Sci. USA 1979, 76, 6270–6273. [Google Scholar] [CrossRef] [PubMed]
- Rusnak, F.; Mertz, P. Calcineurin: Form and function. Physiol. Rev. 2000, 80, 1483–1521. [Google Scholar] [PubMed]
- Rumi-Masante, J.; Rusinga, F.I.; Lester, T.E.; Dunlap, T.B.; Williams, T.D.; Dunker, A.K.; Weis, D.D.; Creamer, T.P. Structural basis for activation of calcineurin by calmodulin. J. Mol. Biol. 2012, 415, 307–317. [Google Scholar] [CrossRef] [PubMed]
- King, M.M.; Huang, C.Y. The calmodulin-dependent activation and deactivation of the phosphoprotein phosphatase, calcineurin, and the effect of nucleotides, pyrophosphate, and divalent metal ions. Identification of calcineurin as a Zn and Fe metalloenzyme. J. Biol. Chem. 1984, 259, 8847–8856. [Google Scholar] [PubMed]
- Juvvadi, P.R.; Lamoth, F.; Steinbach, W.J. Calcineurin as a multifunctional regulator: Unraveling novel functions in fungal stress responses, hyphal growth, drug resistance, and pathogenesis. Fungal Biol. Rev. 2014, 28, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Cyert, M.S.; Kunisawa, R.; Kaim, D.; Thorner, J. Yeast has homologs (CNA1 and CNA2 gene products) of mammalian calcineurin, a calmodulin-regulated phosphoprotein phosphatase. Proc. Natl. Acad. Sci. USA 1991, 88, 7376–7380. [Google Scholar] [CrossRef] [PubMed]
- Cyert, M.S. Calcineurin signaling in saccharomyces cerevisiae: How yeast go crazy in response to stress. Biochem. Biophys. Res. Commun. 2003, 311, 1143–1150. [Google Scholar] [CrossRef]
- Matsumoto, T.K.; Ellsmore, A.J.; Cessna, S.G.; Low, P.S.; Pardo, J.M.; Bressan, R.A.; Hasegawa, P.M. An osmotically induced cytosolic Ca2+ transient activates calcineurin signaling to mediate ion homeostasis and salt tolerance of Saccharomyces cerevisiae. J. Biol. Chem. 2002, 277, 33075–33080. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, I.; Quintero, F.J.; Bressan, R.A.; Hasegawa, P.M.; Pardo, J.M. Activated calcineurin confers high tolerance to ion stress and alters the budding pattern and cell morphology of yeast cells. J. Biol. Chem. 1996, 271, 23061–23067. [Google Scholar] [PubMed]
- Mendoza, I.; Rubio, F.; Rodriguez-Navarro, A.; Pardo, J.M. The protein phosphatase calcineurin is essential for nacl tolerance of Saccharomyces cerevisiae. J. Biol. Chem. 1994, 269, 8792–8796. [Google Scholar] [PubMed]
- Sugiura, R.; Sio, S.O.; Shuntoh, H.; Kuno, T. Calcineurin phosphatase in signal transduction: Lessons from fission yeast. Genes Cells 2002, 7, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Toda, T.; Yanagida, M. A calcineurin-like gene ppb1+ in fission yeast: Mutant defects in cytokinesis, cell polarity, mating and spindle pole body positioning. J. Cell Sci. 1994, 107, 1725–1735. [Google Scholar] [PubMed]
- Lu, Y.; Sugiura, R.; Yada, T.; Cheng, H.; Sio, S.O.; Shuntoh, H.; Kuno, T. Calcineurin is implicated in the regulation of the septation initiation network in fission yeast. Genes Cells 2002, 7, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Kume, K.; Koyano, T.; Kanai, M.; Toda, T.; Hirata, D. Calcineurin ensures a link between the DNA replication checkpoint and microtubule-dependent polarized growth. Nat. Cell Biol. 2011, 13, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Kozubowski, L.; Cardenas, M.E.; Heitman, J. On the roles of calcineurin in fungal growth and pathogenesis. Curr. Fungal Infect. Rep. 2010, 4, 244–255. [Google Scholar] [CrossRef]
- Kozubowski, L.; Heitman, J. Profiling a killer, the development of Cryptococcus neoformans. FEMS Microbiol. Rev. 2012, 36, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Sanglard, D.; Ischer, F.; Marchetti, O.; Entenza, J.; Bille, J. Calcineurin a of Candida albicans: Involvement in antifungal tolerance, cell morphogenesis and virulence. Mol. Microbiol. 2003, 48, 959–976. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.C.; Fox, D.S.; Heitman, J. Calcineurin is required for hyphal elongation during mating and haploid fruiting in Cryptococcus neoformans. EMBO J. 2001, 20, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.C.; Goldstein, A.L.; Blankenship, J.R.; del Poeta, M.; Davis, D.; Cardenas, M.E.; Perfect, J.R.; McCusker, J.H.; Heitman, J. Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J. 2002, 21, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Kothe, G.O.; Free, S.J. Calcineurin subunit b is required for normal vegetative growth in Neurospora crassa. Fungal Genet. Biol. 1998, 23, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Prokisch, H.; Yarden, O.; Dieminger, M.; Tropschug, M.; Barthelmess, I.B. Impairment of calcineurin function in Neurospora crassa reveals its essential role in hyphal growth, morphology and maintenance of the apical Ca2+ gradient. Mol. Gen. Genet. 1997, 256, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Juvvadi, P.R.; Arioka, M.; Nakajima, H.; Kitamoto, K. Cloning and sequence analysis of cnaa gene encoding the catalytic subunit of calcineurin from Aspergillus oryzae. FEMS Microbiol. Lett. 2001, 204, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Juvvadi, P.R.; Kuroki, Y.; Arioka, M.; Nakajima, H.; Kitamoto, K. Functional analysis of the calcineurin-encoding gene cnaa from Aspergillus oryzae: Evidence for its putative role in stress adaptation. Arch. Microbiol. 2003, 179, 416–422. [Google Scholar] [PubMed]
- Nanthakumar, N.N.; Dayton, J.S.; Means, A.R. Role of Ca++/calmodulin binding proteins in Aspergillus nidulans cell cycle regulation. Prog. Cell Cycle Res. 1996, 2, 217–228. [Google Scholar] [PubMed]
- Harel, A.; Bercovich, S.; Yarden, O. Calcineurin is required for sclerotial development and pathogenicity of Sclerotinia sclerotiorum in an oxalic acid-independent manner. Mol. Plant Microbe Interact. 2006, 19, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kim, Y.S.; Lee, Y.H. Functional analysis of mcna, a gene encoding a catalytic subunit of calcineurin, in the rice blast fungus Magnaporthe oryzae. J. Microbiol. Biotechnol. 2009, 19, 11–16. [Google Scholar] [PubMed]
- Schumacher, J.; de Larrinoa, I.F.; Tudzynski, B. Calcineurin-responsive zinc finger transcription factor crz1 of Botrytis cinerea is required for growth, development, and full virulence on bean plants. Eukaryot. Cell 2008, 7, 584–601. [Google Scholar] [CrossRef] [PubMed]
- Egan, J.D.; García-Pedrajas, M.D.; Andrews, D.L.; Gold, S.E. Calcineurin is an antagonist to pka protein phosphorylation required for postmating filamentation and virulence, while pp2a is required for viability in Ustilago maydis. Mol. Plant Microbe Interact. 2009, 22, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Chávez, J.A.; Ali, S.; Bakkeren, G. Response to environmental stresses, cell-wall integrity, and virulence are orchestrated through the calcineurin pathway in Ustilago hordei. Mol. Plant Microbe Interact. 2010, 24, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Fox, D.S.; Heitman, J. Good fungi gone bad: The corruption of calcineurin. BioEssays 2002, 24, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.J.; Chang, Y.L.; Chen, Y.L. Calcineurin signaling: Lessons from candida species. FEMS Yeast Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, W.J.; Reedy, J.L.; Cramer, R.A.; Perfect, J.R.; Heitman, J. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat. Rev. Microbiol. 2007, 5, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Li, A.; Calo, S.; Heitman, J. Calcineurin plays key roles in the dimorphic transition and virulence of the human pathogenic zygomycete Mucor circinelloides. PLoS Pathog. 2013, 9, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Li, A.; Calo, S.; Inoue, M.; Tonthat, N.K.; Bain, J.M.; Louw, J.; Shinohara, M.L.; Erwig, L.P.; Schumacher, M.A.; et al. Calcineurin orchestrates dimorphic transitions, antifungal drug responses and host–pathogen interactions of the pathogenic mucoralean fungus Mucor circinelloides. Mol. Microbiol. 2015, 97, 844–865. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, W.J.; Cramer, R.A.; Perfect, B.Z.; Asfaw, Y.G.; Sauer, T.C.; Najvar, L.K.; Kirkpatrick, W.R.; Patterson, T.F.; Benjamin, D.K., Jr.; Heitman, J.; et al. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryot. Cell 2006, 5, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Ferreira, M.E.; Heinekamp, T.; Härtl, A.; Brakhage, A.A.; Semighini, C.P.; Harris, S.D.; Savoldi, M.; de Gouvêa, P.F.; da Silva Goldman, M.H.; Goldman, G.H. Functional characterization of the Aspergillus fumigatus calcineurin. Fungal Genet. Biol. 2007, 44, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Juvvadi, P.R.; Fortwendel, J.R.; Rogg, L.E.; Burns, K.A.; Randell, S.H.; Steinbach, W.J. Localization and activity of the calcineurin catalytic and regulatory subunit complex at the septum is essential for hyphal elongation and proper septation in Aspergillus fumigatus. Mol. Microbiol. 2011, 82, 1235–1259. [Google Scholar] [CrossRef] [PubMed]
- Juvvadi, P.R.; Fortwendel, J.R.; Pinchai, N.; Perfect, B.Z.; Heitman, J.; Steinbach, W.J. Calcineurin localizes to the hyphal septum in Aspergillus fumigatus: Implications for septum formation and conidiophore development. Eukaryot. Cell 2008, 7, 1606–1610. [Google Scholar] [CrossRef] [PubMed]
- Ichinomiya, M.; Yamada, E.; Yamashita, S.; Ohta, A.; Horiuchi, H. Class i and class ii chitin synthases are involved in septum formation in the filamentous fungus Aspergillus nidulans. Eukaryot. Cell 2005, 4, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Yamada, K.; Deoka, K.; Yamashita, S.; Ohta, A.; Horiuchi, H. Class iii chitin synthase chsb of aspergillus nidulans localizes at the sites of polarized cell wall synthesis and is required for conidial development. Eukaryot. Cell 2009, 8, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Muszkieta, L.; Aimanianda, V.; Mellado, E.; Gribaldo, S.; Alcàzar-Fuoli, L.; Szewczyk, E.; Prevost, M.C.; Latgé, J.P. Deciphering the role of the chitin synthase families 1 and 2 in the in vivo and in vitro growth of Aspergillus fumigatus by multiple gene targeting deletion. Cell. Microbiol. 2014, 16, 1784–1805. [Google Scholar] [CrossRef] [PubMed]
- Juvvadi, P.R.; Gehrke, C.; Fortwendel, J.R.; Lamoth, F.; Soderblom, E.J.; Cook, E.C.; Hast, M.A.; Asfaw, Y.G.; Moseley, M.A.; Creamer, T.P.; et al. Phosphorylation of calcineurin at a novel serine-proline rich region orchestrates hyphal growth and virulence in Aspergillus fumigatus. PLoS Pathog. 2013, 9. [Google Scholar] [CrossRef] [PubMed]
- Falloon, K.; Juvvadi, P.R.; Richards, A.D.; Vargas-Muñiz, J.M.; Renshaw, H.; Steinbach, W.J. Characterization of the fkbp12-encoding genes in Aspergillus fumigatus. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Juvvadi, P.R.; Muñoz, A.; Lamoth, F.; Soderblom, E.J.; Moseley, M.A.; Read, N.D.; Steinbach, W.J. Calcium-mediated induction of paradoxical growth following caspofungin treatment is associated with calcineurin activation and phosphorylation in Aspergillus fumigatus. Antimicrob. Agents Chemother. 2015, 59, 4946–4955. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, W.J. Are we there yet? Recent progress in the molecular diagnosis and novel antifungal targeting of Aspergillus fumigatus and invasive aspergillosis. PLoS Pathog. 2013, 9. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Juvvadi, P.R.; Steinbach, W.J. Histone deacetylase inhibition as an alternative strategy against invasive aspergillosis. Front. Microbiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Juvvadi, P.R.; Gehrke, C.; Steinbach, W.J. In vitro activity of calcineurin and heat shock protein 90 inhibitors against Aspergillus fumigatus azole- and echinocandin-resistant strains. Antimicrob. Agents Chemother. 2013, 57, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, W.J.; Schell, W.A.; Blankenship, J.R.; Onyewu, C.; Heitman, J.; Perfect, J.R. In vitro interactions between antifungals and immunosuppressants against Aspergillus fumigatus. Antimicrob. Agents Chemother. 2004, 48, 1664–1669. [Google Scholar] [CrossRef] [PubMed]
- Fortwendel, J.R.; Juvvadi, P.R.; Perfect, B.Z.; Rogg, L.E.; Perfect, J.R.; Steinbach, W.J. Transcriptional regulation of chitin synthases by calcineurin controls paradoxical growth of Aspergillus fumigatus in response to caspofungin. Antimicrob. Agents Chemother. 2010, 54, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Juvvadi, P.R.; Soderblom, E.J.; Moseley, M.A.; Steinbach, W.J. Hsp70 and the cochaperone stia (hop) orchestrate hsp90-mediated caspofungin tolerance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 2015, 59, 4727–4733. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Juvvadi, P.R.; Fortwendel, J.R.; Steinbach, W.J. Heat shock protein 90 is required for conidiation and cell wall integrity in Aspergillus fumigatus. Eukaryot. Cell 2012, 11, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Alexander, B.D.; Juvvadi, P.R.; Steinbach, W.J. Antifungal activity of compounds targeting the hsp90-calcineurin pathway against various mould species. J. Antimicrob. Chemother. 2015, 70, 1408–1411. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juvvadi, P.R.; Steinbach, W.J. Calcineurin Orchestrates Hyphal Growth, Septation, Drug Resistance and Pathogenesis of Aspergillus fumigatus: Where Do We Go from Here? Pathogens 2015, 4, 883-893. https://doi.org/10.3390/pathogens4040883
Juvvadi PR, Steinbach WJ. Calcineurin Orchestrates Hyphal Growth, Septation, Drug Resistance and Pathogenesis of Aspergillus fumigatus: Where Do We Go from Here? Pathogens. 2015; 4(4):883-893. https://doi.org/10.3390/pathogens4040883
Chicago/Turabian StyleJuvvadi, Praveen R, and William J Steinbach. 2015. "Calcineurin Orchestrates Hyphal Growth, Septation, Drug Resistance and Pathogenesis of Aspergillus fumigatus: Where Do We Go from Here?" Pathogens 4, no. 4: 883-893. https://doi.org/10.3390/pathogens4040883
APA StyleJuvvadi, P. R., & Steinbach, W. J. (2015). Calcineurin Orchestrates Hyphal Growth, Septation, Drug Resistance and Pathogenesis of Aspergillus fumigatus: Where Do We Go from Here? Pathogens, 4(4), 883-893. https://doi.org/10.3390/pathogens4040883





