Immune Response to Human Metapneumovirus Infection: What We Have Learned from the Mouse Model
Abstract
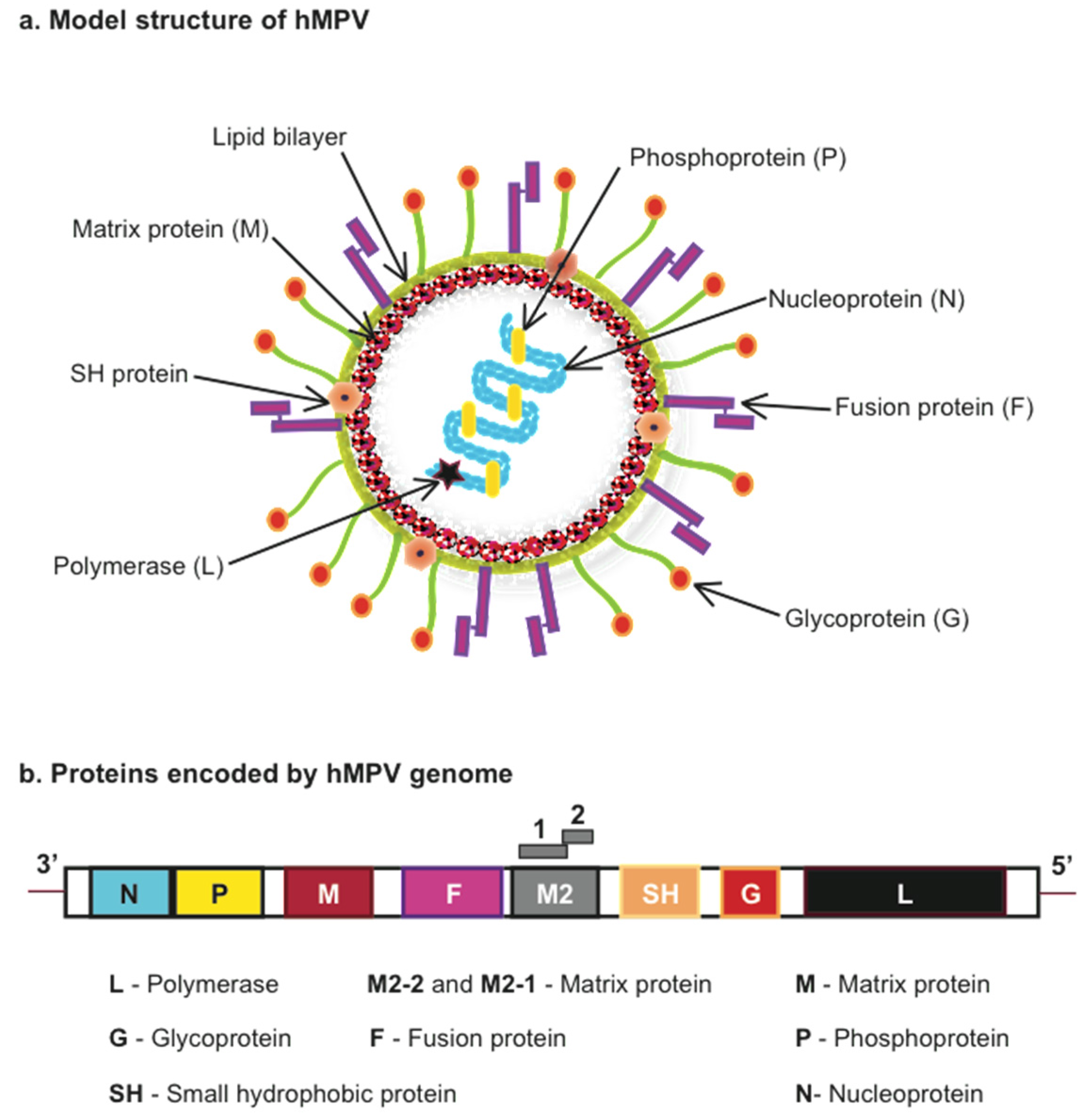
:1. Introduction
| Mice Strain | Mice Age | (Group) Strain | Virus Dose | Refs. |
|---|---|---|---|---|
| BALB/c | F 6–8 week-old | (A) NL 00-01 | 3.3 × 105 PFU | [25] |
| BALB/c | F 4–6-week-old | (A) C-85473 | 1.5 × 105–108 TCID50 | [16,26,27,28,29,30,31,32] |
| BALB/c | F 6–8-week-old | (A) C4-CJP05 | 106 PFU | [33] |
| BALB/c | F 4–6-week-old | (B) CAN98-75 | 0.8–1 × 106 PFU | [29,34,35] |
| BALB/c | F 5–7 week-old | (A) NL/1/00 | 106–107 PFU | [20,36] |
| BALB/c | F 6–7 week-old | (B) NL/1/99 | 107 PFU | [36] |
| BALB/c | F 6–10 week-old | (A) CAN97-83 | 106–107 PFU/TCID50 | [30,37,38,39,40,41] |
| BALB/c | F 5–6 week-old | (A) CZ0107 | 106 PFU | [42] |
| BALB/c | M 19 month-old | (A) CAN97-83 | 2 × 107 geq | [43] |
| BALB/c | F 8–10 week-old | (A) D03-574 | 2 × 105 PFU | [44] |
| C57BL/6 | 6–10 week-old | (A) CAN97-83 | 106–107 PFU | [38,45,46,47,48,49] |
| C57BL/6 | F 6–12 week-old | (A) TN/94-49 | 0.6–1.5 × 106 PFU | [50,51,52,53] |
| DBA/2 | 5–6 week-old | (A) TN/94-49 | 105.9 PFU | [17] |
| SCID | F 6–8 week-old | (A) NL/1/00 | 6.5 × 106 PFU | [54] |
2. hMPV Infection in Mice
| Mice Strain | Virus Strain | Virus Inoculum | Peak Viral Titer | Ref. |
|---|---|---|---|---|
| BALB/c | NL/1/00 | 3.3 × 105 PFU | Day 4 (Log10 2.37 PFU/g) | [25] |
| BALB/c | CAN97-83 | 107 TCID50 | Day 4 (105 TCID50/g) | [41] |
| BALB/c | C85473 | 1.5 × 105 TCID50 | Day 6 (~104 TCID50/lung) | [26] |
| BALB/c | C85473 | 1 × 108 TCID50 | Day 5 (7 × 106 TCID50/lung) | [30] |
| BALB/c | C85473 | 1 × 108 TCID50 | Day 5 (1.92 × 107 TCID50/g) | [16] |
| BALB/c | C85473 | 5.8 × 105 TCID50 | Day 5 (~105 TCID50/g) | [32] |
| BALB/c | NL/1/00 | 1.5 × 105 PFU | Day 5 (5.1 × 105 PFU/g) | [55] |
| BALB/c | D03-574 | 2 × 105 PFU | Day 4 (~103.6 PFU/lung) | [44] |
| C57BL/6 | CAN97-83 | 5 × 106 PFU | Day 5 (104.9 PFU/g) | [46] |
| C57BL/6 | TN/94-49 | 1 × 106 PFU | Day 5 (~4.7 Log10 PFU/g) | [53] |
| C57BL/6 | CAN97-83 | 1 × 107 PFU | Day 5 (~4.1 Log10 PFU/g) | [47] |
| C57BL/6 | TN/94-49 | 6 × 105 PFU | Day 5 (~4.2 Log10 PFU/g) | [51] |
3. Lung Antiviral and Inflammatory Responses
3.1. Innate Immunity
3.1.1. Pattern Recognition Receptors and Signaling Pathways
3.1.2. Cytokine Production
3.1.3. Dendritic Cells
3.1.4. Alveolar Macrophages
3.1.5. Natural Killer Cells
3.2. Adaptive Immunity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Feuillet, F.; Lina, B.; Rosa-Calatrava, M.; Boivin, G. Ten years of human metapneumovirus research. J. Clin. Virol. 2012, 53, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Van den Hoogen, B.G.; de Jong, J.C.; Groen, J.; Kuiken, T.; de Groot, R.; Fouchier, R.A.; Osterhaus, A.D. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 2001, 7, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Boivin, G.; de Serres, G.; Cote, S.; Gilca, R.; Abed, Y.; Rochette, L.; Bergeron, M.G.; Dery, P. Human metapneumovirus infections in hospitalized children. Emerg. Infect. Dis. 2003, 9, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Caracciolo, S.; Minini, C.; Colombrita, D.; Rossi, D.; Miglietti, N.; Vettore, E.; Caruso, A.; Fiorentini, S. Human metapneumovirus infection in young children hospitalized with acute respiratory tract disease: Virologic and clinical features. Pediatr. Infect. Dis. J. 2008, 27, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Crowe, J.E., Jr. Human metapneumovirus as a major cause of human respiratory tract disease. Pediatr. Infect. Dis. J. 2004, 23, S215–S221. [Google Scholar] [CrossRef] [PubMed]
- Kahn, J.S. Epidemiology of human metapneumovirus. Clin. Microbiol. Rev. 2006, 19, 546–557. [Google Scholar] [CrossRef]
- Mullins, J.A.; Erdman, D.D.; Weinberg, G.A.; Edwards, K.; Hall, C.B.; Walker, F.J.; Iwane, M.; Anderson, L.J. Human metapneumovirus infection among children hospitalized with acute respiratory illness. Emerg. Infect. Dis. 2004, 10, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Esper, F.; Boucher, D.; Weibel, C.; Martinello, R.A.; Kahn, J.S. Human metapneumovirus infection in the United States: Clinical manifestations associated with a newly emerging respiratory infection in children. Pediatrics 2003, 111, 1407–1410. [Google Scholar] [CrossRef] [PubMed]
- Kahn, J.S. Human metapneumovirus, a newly emerging respiratory virus. Pediatr. Infect. Dis. J. 2003, 22, 923–924. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.V.; Wang, C.K.; Yang, C.F.; Tollefson, S.J.; House, F.S.; Heck, J.M.; Chu, M.; Brown, J.B.; Lintao, L.D.; Quinto, J.D.; et al. The role of human metapneumovirus in upper respiratory tract infections in children: A 20-year experience. J. Infect. Dis. 2006, 193, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.L.; Crowe, J. Respiratory Syncytial Virus and Metapneumovirus. In Fileds Virology, 5th ed.; Knipe, D.M., Howley, P.M., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2007; Volume 2, pp. 1601–1646. [Google Scholar]
- Van den Hoogen, B.G.; Herfst, S.; Sprong, L.; Cane, P.A.; Forleo-Neto, E.; de Swart, R.L.; Osterhaus, A.D.; Fouchier, R.A. Antigenic and genetic variability of human metapneumoviruses. Emerg. Infect. Dis. 2004, 10, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Biacchesi, S.; Skiadopoulos, M.H.; Boivin, G.; Hanson, C.T.; Murphy, B.R.; Collins, P.L.; Buchholz, U.J. Genetic diversity between human metapneumovirus subgroups. Virology 2003, 315, 1–9. [Google Scholar] [CrossRef]
- Mackay, I.M.; Jacob, K.C.; Woolhouse, D.; Waller, K.; Syrmis, M.W.; Whiley, D.M.; Siebert, D.J.; Nissen, M.; Sloots, T.P. Molecular assays for detection of human metapneumovirus. J. Clin. Microbiol. 2003, 41, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Easton, A.J.; Domachowske, J.B.; Rosenberg, H.F. Animal pneumoviruses: Molecular genetics and pathogenesis. Clin. Microbiol. Rev. 2004, 17, 390–412. [Google Scholar] [CrossRef] [PubMed]
- Hamelin, M.E.; Yim, K.; Kuhn, K.H.; Cragin, R.P.; Boukhvalova, M.; Blanco, J.C.; Prince, G.A.; Boivin, G. Pathogenesis of human metapneumovirus lung infection in BALB/c mice and cotton rats. J. Virol. 2005, 79, 8894–8903. [Google Scholar] [CrossRef] [PubMed]
- Mok, H.; Tollefson, S.J.; Podsiad, A.B.; Shepherd, B.E.; Polosukhin, V.V.; Johnston, R.E.; Williams, J.V.; Crowe, J.E., Jr. An alphavirus replicon-based human metapneumovirus vaccine is immunogenic and protective in mice and cotton rats. J. Virol. 2008, 82, 11410–11418. [Google Scholar] [CrossRef] [PubMed]
- Green, M.G.; Huey, D.; Niewiesk, S. The cotton rat (Sigmodon hispidus) as an animal model for respiratory tract infections with human pathogens. Lab Animal 2013, 42, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Boukhvalova, M.S.; Prince, G.A.; Blanco, J.C. The cotton rat model of respiratory viral infections. Biologicals 2009, 37, 152–159. [Google Scholar] [CrossRef] [PubMed]
- MacPhail, M.; Schickli, J.H.; Tang, R.S.; Kaur, J.; Robinson, C.; Fouchier, R.A.; Osterhaus, A.D.; Spaete, R.R.; Haller, A.A. Identification of small-animal and primate models for evaluation of vaccine candidates for human metapneumovirus (hMPV) and implications for hMPV vaccine design. J. Gen. Virol. 2004, 85, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Herfst, S.; de Graaf, M.; Schrauwen, E.J.; Sprong, L.; Hussain, K.; van den Hoogen, B.G.; Osterhaus, A.D.; Fouchier, R.A. Generation of temperature-sensitive human metapneumovirus strains that provide protective immunity in hamsters. J. Gen. Virol. 2008, 89, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Schickli, J.H.; Kaur, J.; Macphail, M.; Guzzetta, J.M.; Spaete, R.R.; Tang, R.S. Deletion of human metapneumovirus M2-2 increases mutation frequency and attenuates growth in hamsters. Virol. J. 2008. [Google Scholar] [CrossRef] [PubMed]
- Biacchesi, S.; Pham, Q.N.; Skiadopoulos, M.H.; Murphy, B.R.; Collins, P.L.; Buchholz, U.J. Infection of nonhuman primates with recombinant human metapneumovirus lacking the SH, G, or M2-2 protein categorizes each as a nonessential accessory protein and identifies vaccine candidates. J. Virol. 2005, 79, 12608–12613. [Google Scholar] [CrossRef] [PubMed]
- Biacchesi, S.; Pham, Q.N.; Skiadopoulos, M.H.; Murphy, B.R.; Collins, P.L.; Buchholz, U.J. Modification of the trypsin-dependent cleavage activation site of the human metapneumovirus fusion protein to be trypsin independent does not increase replication or spread in rodents or nonhuman primates. J. Virol. 2006, 80, 5798–5806. [Google Scholar] [CrossRef] [PubMed]
- Darniot, M.; Petrella, T.; Aho, S.; Pothier, P.; Manoha, C. Immune response and alteration of pulmonary function after primary human metapneumovirus (hMPV) infection of BALB/c mice. Vaccine 2005, 23, 4473–4480. [Google Scholar] [CrossRef] [PubMed]
- Kukavica-Ibrulj, I.; Hamelin, M.E.; Prince, G.A.; Gagnon, C.; Bergeron, Y.; Bergeron, M.G.; Boivin, G. Infection with human metapneumovirus predisposes mice to severe pneumococcal pneumonia. J. Virol. 2009, 83, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Hamelin, M.E.; Prince, G.A.; Boivin, G. Effect of ribavirin and glucocorticoid treatment in a mouse model of human metapneumovirus infection. Antimicrob. Agents Chemother. 2006, 50, 774–777. [Google Scholar] [CrossRef] [PubMed]
- Hamelin, M.E.; Couture, C.; Sackett, M.K.; Boivin, G. Enhanced lung disease and Th2 response following human metapneumovirus infection in mice immunized with the inactivated virus. J. Gen. Virol. 2007, 88, 3391–3400. [Google Scholar] [CrossRef] [PubMed]
- Levy, C.; Aerts, L.; Hamelin, M.E.; Granier, C.; Szecsi, J.; Lavillette, D.; Boivin, G.; Cosset, F.L. Virus-like particle vaccine induces cross-protection against human metapneumovirus infections in mice. Vaccine 2013, 31, 2778–2785. [Google Scholar] [CrossRef] [PubMed]
- Hamelin, M.E.; Prince, G.A.; Gomez, A.M.; Kinkead, R.; Boivin, G. Human metapneumovirus infection induces long-term pulmonary inflammation associated with airway obstruction and hyperresponsiveness in mice. J. Infect. Dis. 2006, 193, 1634–1642. [Google Scholar] [CrossRef] [PubMed]
- Ludewick, H.P.; Aerts, L.; Hamelin, M.E.; Boivin, G. Long-term impairment of Streptococcus pneumoniae lung clearance is observed after initial infection with influenza A virus but not human metapneumovirus in mice. J. Gen. Virol. 2011, 92, 1662–1665. [Google Scholar] [CrossRef] [PubMed]
- Aerts, L.; Hamelin, M.E.; Rheaume, C.; Lavigne, S.; Couture, C.; Kim, W.; Susan-Resiga, D.; Prat, A.; Seidah, N.G.; Vergnolle, N.; et al. Modulation of protease activated receptor 1 influences human metapneumovirus disease severity in a mouse model. PLoS ONE 2013. [Google Scholar] [CrossRef] [PubMed]
- Darniot, M.; Schildgen, V.; Schildgen, O.; Sproat, B.; Kleines, M.; Ditt, V.; Pitoiset, C.; Pothier, P.; Manoha, C. RNA interference in vitro and in vivo using DsiRNA targeting the nucleocapsid N mRNA of human metapneumovirus. Antiviral Res. 2012, 93, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.; Harrod, K.S.; Shieh, W.J.; Zaki, S.; Tripp, R.A. Human metapneumovirus persists in BALB/c mice despite the presence of neutralizing antibodies. J. Virol. 2004, 78, 14003–14011. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.; Tripp, R.A. The immune response to human metapneumovirus is associated with aberrant immunity and impaired virus clearance in BALB/c mice. J. Virol. 2005, 79, 5971–5978. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Shu, Z.; Qin, X.; Dou, Y.; Zhao, Y.; Zhao, X. A live attenuated human metapneumovirus vaccine strain provides complete protection against homologous viral infection and cross-protection against heterologous viral infection in BALB/c mice. Clin. Vaccine Immunol. 2013, 20, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Plata, A.; Casola, A.; Garofalo, R.P. Human metapneumovirus induces a profile of lung cytokines distinct from that of respiratory syncytial virus. J. Virol. 2005, 79, 14992–14997. [Google Scholar] [CrossRef] [PubMed]
- Herd, K.A.; Mahalingam, S.; Mackay, I.M.; Nissen, M.; Sloots, T.P.; Tindle, R.W. Cytotoxic T-lymphocyte epitope vaccination protects against human metapneumovirus infection and disease in mice. J. Virol. 2006, 80, 2034–2044. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Plata, A.; Baron, S.; Poast, J.S.; Adegboyega, P.A.; Casola, A.; Garofalo, R.P. Activity and regulation of alpha interferon in respiratory syncytial virus and human metapneumovirus experimental infections. J. Virol. 2005, 79, 10190–10199. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Plata, A.; Kolli, D.; Hong, C.; Casola, A.; Garofalo, R.P. Subversion of pulmonary dendritic cell function by paramyxovirus infections. J. Immunol. 2009, 182, 3072–3083. [Google Scholar] [CrossRef] [PubMed]
- Kolli, D.; Bataki, E.L.; Spetch, L.; Guerrero-Plata, A.; Jewell, A.M.; Piedra, P.A.; Milligan, G.N.; Garofalo, R.P.; Casola, A. T lymphocytes contribute to antiviral immunity and pathogenesis in experimental human metapneumovirus infection. J. Virol. 2008, 82, 8560–8569. [Google Scholar] [CrossRef] [PubMed]
- Palavecino, C.E.; Cespedes, P.F.; Gomez, R.S.; Kalergis, A.M.; Bueno, S.M. Immunization with a recombinant bacillus Calmette-Guerin strain confers protective Th1 immunity against the human metapneumovirus. J. Immunol. 2014, 192, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Ditt, V.; Lusebrink, J.; Tillmann, R.L.; Schildgen, V.; Schildgen, O. Respiratory infections by HMPV and RSV are clinically indistinguishable but induce different host response in aged individuals. PLoS ONE 2011. [Google Scholar] [CrossRef] [PubMed]
- Huck, B.; Neumann-Haefelin, D.; Schmitt-Graeff, A.; Weckmann, M.; Mattes, J.; Ehl, S.; Falcone, V. Human metapneumovirus induces more severe disease and stronger innate immune response in BALB/c mice as compared with respiratory syncytial virus. Respir. Res. 2007. [Google Scholar] [CrossRef] [PubMed]
- Banos-Lara Mdel, R.; Ghosh, A.; Guerrero-Plata, A. Critical role of MDA5 in the interferon response induced by human metapneumovirus infection in dendritic cells and in vivo. J. Virol. 2013, 87, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Zhou, Z.; Wakamatsu, N.; Guerrero-Plata, A. Interleukin-12p40 modulates human metapneumovirus-induced pulmonary disease in an acute mouse model of infection. PLoS ONE 2012. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Kolli, D.; Deng, J.; Fang, R.; Gong, B.; Xue, M.; Casola, A.; Garofalo, R.P.; Wang, T.; Bao, X. MyD88 controls human metapneumovirus-induced pulmonary immune responses and disease pathogenesis. Virus Res. 2013, 176, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Velayutham, T.S.; Kolli, D.; Ivanciuc, T.; Garofalo, R.P.; Casola, A. Critical role of TLR4 in human metapneumovirus mediated innate immune responses and disease pathogenesis. PLoS ONE 2013. [Google Scholar] [CrossRef] [PubMed]
- Banos-Lara Mdel, R.; Harvey, L.; Mendoza, A.; Simms, D.; Chouljenko, V.N.; Wakamatsu, N.; Kousoulas, K.G.; Guerrero-Plata, A. Impact and regulation of lambda interferon response in human metapneumovirus infection. J. Virol. 2015, 89, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.G.; Erickson, J.J.; Hastings, A.K.; Becker, J.C.; Johnson, M.; Craven, R.E.; Tollefson, S.J.; Boyd, K.L.; Williams, J.V. Human metapneumovirus virus-like particles induce protective B and T cell responses in a mouse model. J. Virol. 2014, 88, 6368–6379. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.C.; Tollefson, S.J.; Johnson, M.; Gilchuk, P.; Boyd, K.L.; Shepherd, B.; Joyce, S.; Williams, J.V. Acute clearance of human metapneumovirus occurs independently of natural killer cells. J. Virol. 2014, 88, 10963–10969. [Google Scholar] [CrossRef] [PubMed]
- Erickson, J.J.; Rogers, M.C.; Hastings, A.K.; Tollefson, S.J.; Williams, J.V. Programmed death-1 impairs secondary effector lung CD8(+) T cells during respiratory virus reinfection. J. Immunol. 2014, 193, 5108–5117. [Google Scholar] [CrossRef] [PubMed]
- Hastings, A.K.; Erickson, J.J.; Schuster, J.E.; Boyd, K.L.; Tollefson, S.J.; Johnson, M.; Gilchuk, P.; Joyce, S.; Williams, J.V. Role of type I interferon signaling in human metapneumovirus pathogenesis and control of viral replication. J. Virol. 2015, 89, 4405–4420. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.M.; Li, R.P.; Chen, X.; Liu, P.; Zhao, X.D. Replication and pathogenicity of attenuated human metapneumovirus F mutants in severe combined immunodeficiency mice. Vaccine 2012, 30, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dou, Y.; Wu, J.; She, W.; Luo, L.; Zhao, Y.; Liu, P.; Zhao, X. Effects of N-linked glycosylation of the fusion protein on replication of human metapneumovirus in vitro and in mouse lungs. J. Gen. Virol. 2011, 92, 1666–1675. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Reikine, S.; Nguyen, J.B.; Modis, Y. Pattern Recognition and Signaling Mechanisms of RIG-I and MDA5. Front. Immunol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Spann, K.M.; Loh, Z.; Lynch, J.P.; Ullah, A.; Zhang, V.; Baturcam, E.; Werder, R.B.; Khajornjiraphan, N.; Rudd, P.; Loo, Y.M.; et al. IRF-3, IRF-7, and IPS-1 promote host defense against acute human metapneumovirus infection in neonatal mice. Am. J. Pathol. 2014, 184, 1795–1806. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Chen, Y.; Liu, G.; Ren, J.; Go, C.; Ivanciuc, T.; Deepthi, K.; Casola, A.; Garofalo, R.P.; Bao, X. MAVS plays an essential role in host immunity against human metapneumovirus. J. Gen. Virol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Laham, F.R.; Israele, V.; Casellas, J.M.; Garcia, A.M.; Lac Prugent, C.M.; Hoffman, S.J.; Hauer, D.; Thumar, B.; Name, M.I.; Pascual, A.; et al. Differential production of inflammatory cytokines in primary infection with human metapneumovirus and with other common respiratory viruses of infancy. J. Infect. Dis. 2004, 189, 2047–2056. [Google Scholar] [CrossRef] [PubMed]
- Aherne, W.; Bird, T.; Court, S.D.; Gardner, P.S.; McQuillin, J. Pathological changes in virus infections of the lower respiratory tract in children. J. Clin. Pathol. 1970, 23, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Darniot, M.; Pitoiset, C.; Petrella, T.; Aho, S.; Pothier, P.; Manoha, C. Age-associated aggravation of clinical disease after primary metapneumovirus infection of BALB/c mice. J. Virol. 2009, 83, 3323–3332. [Google Scholar] [CrossRef] [PubMed]
- Stumbles, P.A.; Upham, J.W.; Holt, P.G. Airway dendritic cells: Co-ordinators of immunological homeostasis and immunity in the respiratory tract. APMIS 2003, 111, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Plata, A.; Casola, A.; Suarez, G.; Yu, X.; Spetch, L.; Peeples, M.E.; Garofalo, R.P. Differential response of dendritic cells to human metapneumovirus and respiratory syncytial virus. Am. J. Respir. Cell Mol. Biol. 2006, 34, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Plata, A. Dendritic cells in human Pneumovirus and Metapneumovirus infections. Viruses 2013, 5, 1553–1570. [Google Scholar] [CrossRef] [PubMed]
- Le Nouen, C.; Munir, S.; Losq, S.; Winter, C.C.; McCarty, T.; Stephany, D.A.; Holmes, K.L.; Bukreyev, A.; Rabin, R.L.; Collins, P.L.; et al. Infection and maturation of monocyte-derived human dendritic cells by human respiratory syncytial virus, human metapneumovirus, and human parainfluenza virus type 3. Virology 2009, 385, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Pribul, P.K.; Harker, J.; Wang, B.; Wang, H.; Tregoning, J.S.; Schwarze, J.; Openshaw, P.J. Alveolar macrophages are a major determinant of early responses to viral lung infection but do not influence subsequent disease development. J. Virol. 2008, 82, 4441–4448. [Google Scholar] [CrossRef] [PubMed]
- Lohmann-Matthes, M.L.; Steinmuller, C.; Franke-Ullmann, G. Pulmonary macrophages. Eur. Respir. J. 1994, 7, 1678–1689. [Google Scholar] [CrossRef] [PubMed]
- Kolli, D.; Gupta, M.R.; Sbrana, E.; Velayutham, T.S.; Hong, C.; Casola, A.; Garofalo, R.P. Alveolar Macrophages Contribute to the Pathogenesis of hMPV Infection While Protecting Against RSV Infection. Am. J. Respir. Cell Mol. Biol. 2014, 51, 502–515. [Google Scholar] [CrossRef] [PubMed]
- Benoit, A.; Huang, Y.; Proctor, J.; Rowden, G.; Anderson, R. Effects of alveolar macrophage depletion on liposomal vaccine protection against respiratory syncytial virus (RSV). Clin. Exp. Immunol. 2006, 145, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Herd, K.A.; Nelson, M.; Mahalingam, S.; Tindle, R.W. Pulmonary infection of mice with human metapneumovirus induces local cytotoxic T-cell and immunoregulatory cytokine responses similar to those seen with human respiratory syncytial virus. J. Gen. Virol. 2010, 91, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Erickson, J.J.; Gilchuk, P.; Hastings, A.K.; Tollefson, S.J.; Johnson, M.; Downing, M.B.; Boyd, K.L.; Johnson, J.E.; Kim, A.S.; Joyce, S.; et al. Viral acute lower respiratory infections impair CD8+ T cells through PD-1. J. Clin. Investig. 2012, 122, 2967–2982. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.C.; Schuster, J.E.; Gilchuk, P.; Boyd, K.L.; Joyce, S.; Williams, J.V. Lung CD8+ T cell impairment occurs during human metapneumovirus infection despite Virus-Like Particle (VLP) induction of functional CD8+ T cells. J. Virol. 2015, 89, 8713–8726. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheemarla, N.R.; Guerrero-Plata, A. Immune Response to Human Metapneumovirus Infection: What We Have Learned from the Mouse Model. Pathogens 2015, 4, 682-696. https://doi.org/10.3390/pathogens4030682
Cheemarla NR, Guerrero-Plata A. Immune Response to Human Metapneumovirus Infection: What We Have Learned from the Mouse Model. Pathogens. 2015; 4(3):682-696. https://doi.org/10.3390/pathogens4030682
Chicago/Turabian StyleCheemarla, Nagarjuna R., and Antonieta Guerrero-Plata. 2015. "Immune Response to Human Metapneumovirus Infection: What We Have Learned from the Mouse Model" Pathogens 4, no. 3: 682-696. https://doi.org/10.3390/pathogens4030682
APA StyleCheemarla, N. R., & Guerrero-Plata, A. (2015). Immune Response to Human Metapneumovirus Infection: What We Have Learned from the Mouse Model. Pathogens, 4(3), 682-696. https://doi.org/10.3390/pathogens4030682






