Enumeration of Somatic and F-RNA Phages as an Indicator of Fecal Contamination in Potable Water from Rural Areas of the North West Province
Abstract
:1. Introduction
2. Materials and Methods
2.1. Water Collection Methods
2.2. Enumeration of Bacteriophages from Water Samples
2.2.1. Enumeration of Somatic Coliphages
2.2.2. Enumeration of F-RNA Bacteriophages
3. Results
| Areas SANS 241:2011 | Turbidity (NTU) ≤ 5 | pH ≥ 5 to ≤9.7 | Temperature None |
|---|---|---|---|
| Makgobistad tap | 141.2 | 7.59 | 18.0 |
| Loporung tap | 88.93 | 8.07 | 16.1 |
| Logagane tap 1 | 68.19 | 7.21 | 26.8 |
| Logagane 2 | 1.06 | 7.12 | 20.7 |
| Tshidilamolomo tap 1 | 0.04 | 6.70 | 18.5 |
| Tshidilamolomo tap 2 | 102.9 | 6.70 | 22.9 |
| Tshidilamolomo tap 3 | 0.26 | 6.70 | 21.2 |
| Masamane tap 1 | 0.46 | 7.78 | 27.3 |
| Masamane tap 2 | 1.58 | 6.84 | 17.4 |
| Mabule tap 1 | 1.01 | 7.50 | 24.4 |
| Mabule tap 2 | 2.35 | 7.32 | 18.5 |
| Dingateng 1 | 1.74 | 7.80 | 17.6 |
| Dingateng 2 | 24.25 | 7.81 | 19.9 |
| Dingateng 3 | 64.42 | 7.78 | 17.6 |
| Dingateng 4 | 68.75 | 7.80 | 29.7 |
| Disaneng borehole | 107.7 | 7.08 | 24.1 |
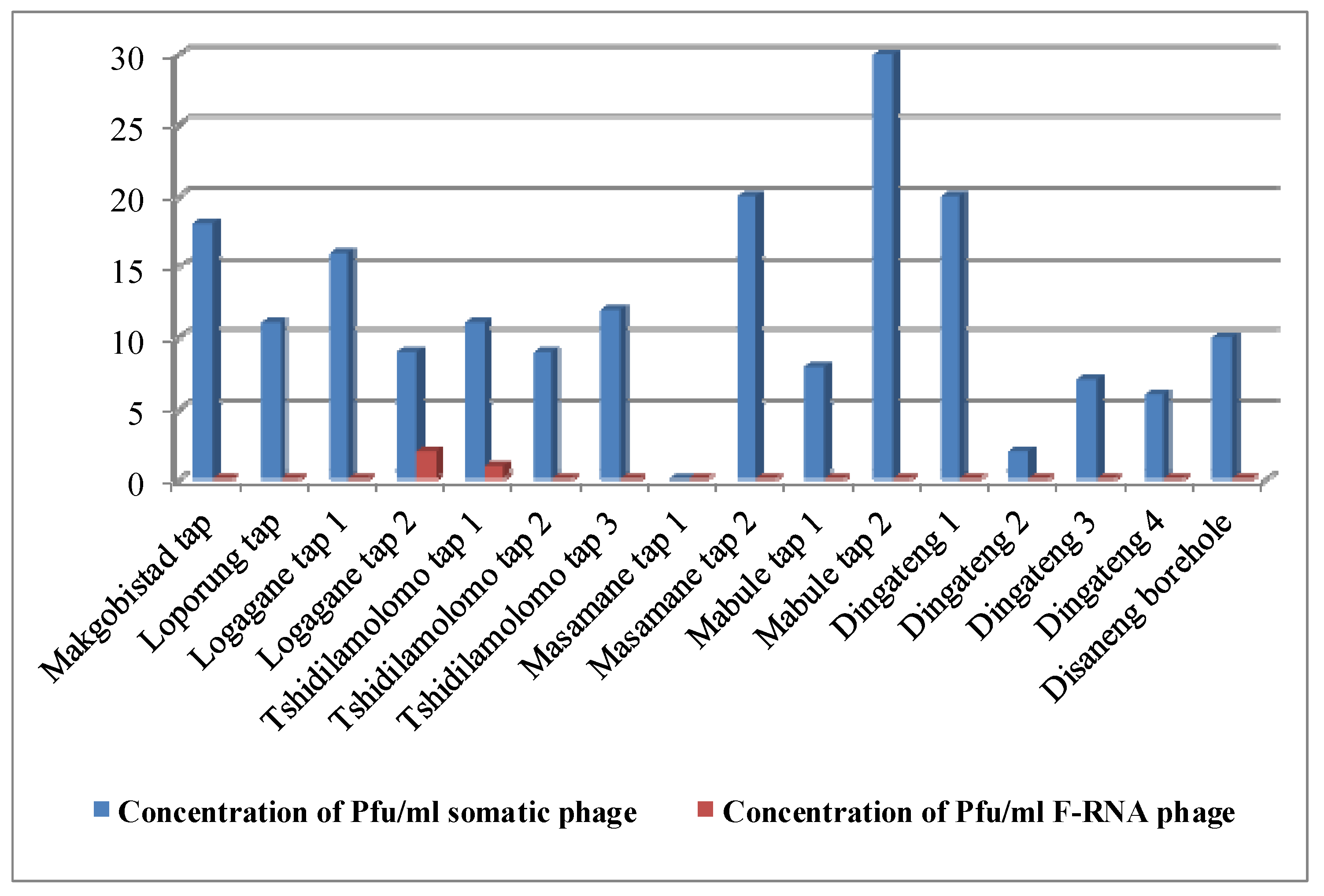
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nevondo, T.S.; Cloete, T.S. Bacterial and chemical quality of water supply in the Dertig village settlement. Water SA. 1999, 25, 215–220. [Google Scholar]
- Mulamattathil, S.G.; Esterhuysen, H.A.; Pretorius, P.J. Antibiotic resistant Gram-negative bacteria in a virtually closed water reticulation system. J. Appl. Microbiol. 2000, 88, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Momba, M.N.B.; Kaleni, P. Regrowth and survival of indicator microorganisms on the surfaces of household containers used for the storage of drinking water in rural communities of South Africa. Water Res. 2002, 36, 3023–3028. [Google Scholar] [CrossRef]
- Van der Walt, I.J.; Winde, F.; Nell, B. Integrated Catchment Management: The Mooi River, North West Province, South Africa as a case study. Caud. d’Investiga. Geogr. 2002, 28, 109–126. [Google Scholar]
- Momba, M.N.B.; Notshe, T.L. The microbiological quality of groundwater-derived drinking water after long storage in household containers in a rural community of South Africa. Aqua. J. Water Supply Res. Technol. 2003, 52, 67–78. [Google Scholar]
- Kalule-Sabiti, M.; Heath, R. Underground water—A key resource and the associated environmental issues in the North-West Province of South-Africa. In Proceedings of the WISA Biennial Conference, Sun City, South Africa, 18–22 May 2008; Available online: http://www.ewisa.co.za/misc/WISAConf/default2008.htm (accessed on 8 December 2010).
- Environment outlook: A Report on the State of the Environment; North West Department of Agriculture, Conservation and Environment (NWDACE): Mmabatho, South Africa, 2008.
- Water Quality Guidelines for South Africa, 1st ed.; Department of Water Affairs and Forestry: Pretoria, South Africa, 1996.
- Pathak, S.P.; Gopal, K. Rapid detection of Escherichia coli as an indicator of faecal pollution in water. Indian J. Microbiol. 2001, 41, 139–151. [Google Scholar]
- Ministry of Health. Drinking-Water Standards for New Zealand; Ministry of Health: Wellington, New Zealand, 2005. [Google Scholar]
- Jagals, P.; Traore, H.N.; Barnard, T.G. Inflammatory Potential Measurement as a Supplement to Health-Related Microbial Water-Quality Assessment; Report No. TT1444/1/06; Water Research Commission: Pretoria, South Africa, 2006. [Google Scholar]
- Katzenelson, E.; Lketter, B.; Schechter, H.; Shuval, H.I. Inactivation of viruses and bacteria by ozone. In Chemistry of Water Supply: Treatment and Distribution; Rubin, A.J., Ed.; Ann Arbor Science: Ann Arbor, Michigan, 1974; pp. 409–421. [Google Scholar]
- Payment, P.; Trudel, M.; Plante, R. Elimination of viruses and indicator bacteria at each step of treatment during preparation of drinking water at seven water treatment plants. Appl. Environ. Microbiol. 1985, 49, 1418–1428. [Google Scholar] [PubMed]
- Payment, P.; Franco, E.; Siemiatycki, J. Absence of relationship between health effects due to tap water consumption and drinking water quality parameters. Water Sci. Technol. 1993, 27, 137–143. [Google Scholar]
- Hilton, M.C.; Stotzky, G. Use of coliphages as indicators of water pollution. Can. J. Microbiol. 1973, 19, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Kott, Y.; Rose, N.; Sperber, S.; Betzer, N. Bacteriophages as viral pollution indicators. Water Res. 1974, 8, 165–171. [Google Scholar] [CrossRef]
- IAWPRC Study Group on Health Related Water Microbiology. Bacteriophages as model viruses in water quality control. Water Res. 1991, 25, 529–545. [Google Scholar]
- Havelaar, A.H.; Hogeboom, W.H. A method for the detection of male specific bacteriophages in sewage. J. Appl. Bacteriol. 1984, 56, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Jofre, J.; Bosch, A.; Lucena, F.; Girones, R.; Tartera, C. Evaluation of Bacteroides. fragilis bacteriophages as indicators of the virological quality of water. Water Sci. Technol. 1986, 18, 167–173. [Google Scholar]
- UNDP: South African Human Development Report 2003; Oxford University Press: Cape Town, Southern Africa, 2004.
- Momba, M.N.B.; Sibewu, M.; Mandeya, A. Survival of somatic and F-RNA Coliphages in treated wastewater effluents and their impact on viral quality of the receiving water bodies in the Eastern Cape Province, South Africa. J. Biol. Sci. 2009, 9, 648–654. [Google Scholar] [CrossRef]
- SANS 241-1. In Drinking Water Part 1: Microbiological, Physical, Aesthetic and Chemical Determinants; South African Bureau of Standards: Pretoria, South Africa, 2011.
- Gersberg, R.M.; Gearheart, R.A.; Ives, M. Pathogen removal in constructed wetlands. In Constructed Wetlands for Wastewater Treatment; Hammer, D.A., Ed.; Lewies Publishers: Chelsea, UK; Minneapolis, MN, USA, 1989; pp. 431–445. [Google Scholar]
- Haile, R.W.; Witte, J.S.; Gold, M.; Cressey, R.; McGee, C.; Millikan, R.C.; Glasser, A.; Harawa, N.; Ervin, C.; Harmon, P.; et al. The health effects of swimming in ocean water contaminated by Storm drain runoff. Epidemiology 1999, 10, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, H.H.; Edberg, S.; Pierzo, V.; Delattre, J.M. Bacteriophages as indicators of enteric viruses and public health risk in groundwaters. J. Appl. Microbiol. 2000, 88, 5–21. [Google Scholar] [CrossRef] [PubMed]
- ISO 10705-1. Water Quality—Detection and Enumeration of Bacteriophages. Part 1: Enumeration of F-Specific RNA Bacteriophages. Available online: https://www.iso.org/obp/ui/#iso:std:18794:en.
- ISO 10705-2. Water Quality—Detection and Enumeration of Bacteriophages. Part 2: Enumeration of Somatic Coliphages. Available online: https://www.iso.org/obp/ui/#iso:std:iso:10705:-2:ed-1:v1:en.
- Jing, X.; Yang, H.; Cao, Y.; Wang, W. Identification of indicators of groundwater quality formation process using a zoning model. J. Hydrol. 2014, 514, 30–40. [Google Scholar] [CrossRef]
- Wang, X.J. Study on evolution and formation of chemical composition of groundwater in Yinchuan Plain. Chan’an University Paper. 2006, 4, P641.12. [Google Scholar]
- Zheng, G.; Wang, R. Research on the bearing capacity of water resources in Yinchuan Plain. J. Ningxia Univ. (Nat. Sci. Ed.) 2006, 27, 80–83. [Google Scholar]
- Zhang, Q.; Zhang, L. Main water environmental problem and its countermeasures in Yinchuan Plain. J. Earth Sci. Environ. 2010, 32, 392–397. [Google Scholar]
- Chung, H.; Jaykus, L.A.; Lovelace, G.; Sobsey, M.D. Bacteriophages and bacteria as indicators of enteric viruses in oysters and their harvest waters. Water Sci. Technol. 1998, 38, 37–44. [Google Scholar] [CrossRef]
- Tufenkji, N.; Emelko, M.B. Fate and Transport of Microbial Contaminants in Groundwater. Encyclopedia Environ. Health 2011, 715–726. [Google Scholar]
- Payment, P.; Affoyon, F.; Trudel, M. Detection of animal and human enteric viruses in water from the Assomption River and its tributaries. Can. J. Microbiol. 1988, 34, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Gantzer, C.; Gillerman, L.; Kuznetsov, M.; Oron, G. Adsorption and survival of faecal coliforms, somatic coliphages and F-specific RNA phages in soil irrigated with wastewater. Water Sci. Technol. 2001, 43, 117–124. [Google Scholar] [PubMed]
- Feng, Y.Y.; Ong, S.L.; Hu, J.Y.; Tan, X.L.; Ng, W.J. Effects of pH and temperature on the survival of coliphages MS2 and Qβ. J. Ind. Microbiol. Biotechnol. 2003, 30, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Breidt, F.; Plengvidhya, V.; Fleming, H.P. Bacteriophage ecology in commercial sauerkraut fermentations. Appl. Environ. Microbiol. 2003, 69, 3192–3202. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, H.W.; Tremblay, D.; Moineau, S. Long-term bacteriophage preservation. WFCC Newsl. 2004, 38, 35–40. [Google Scholar]
- Jepson, C.D.; March, J.B. Bacteriophage lambda is highly stable DNA vaccine delivery vehicle. Vaccine 2004, 22, 3413–1419. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.R.; Axler, R.P.; Hicks, R.E. Effects of freezing and storage temperature on MS2 viability. J. Virol. Methods. 2004, 122, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Tey, B.T.; Ooi, S.T.; Yong, K.C.; tan Ng, M.Y.; Ling, T.C.; Tan, W.S. Production of fusion m13 phage bearing the disulphide constrained peptide sequence (C-WSFFSNI-C) that interacts with hepatitis B core antigen. Afr. J. Biotechnol. 2009, 8, 268–273. [Google Scholar]
- Jonczyk, E.; Klak, M.; Miȩdzybrodzki, R.; Gorski, A. The influence of external factors on bacteriophages- review. Folia Microbiol. 2011, 56, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Griffiths, M. Comparative Persistence of subgroups F+RNA phages in river water. Appl. Environ. Microbiol. 2013, 79, 4564–4567. [Google Scholar] [CrossRef] [PubMed]
- WHO/UNICEF. Global Water Supply and Sanitation Assessment; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Greendrop Report. In Introduction to Green Drop PAT 2012/National Overview; Department of Water Affairs: Pretoria, South Africa, 2012.
- Momba, M.N.B.; Mfenyana, C. Inadequate treatment of wastewater: A source of Coliform bacteria in receiving surface water bodies in developing countries-case study: Eastern Cape Province of South Africa. In Water Encyclopedia-Domestic, Municipality and Industrial Water Supply and Waste Disposal; Lehr, J.H., Keeley, J., Lehr, J., Eds.; John Wiley and Son Inc: New York, NY, USA, 2005; pp. 661–667. [Google Scholar]
- Egboka, B.C.; Nwankwor, G.I.; Orajaka, I.P.; Ejiofor, A.O. Principles and problems of environmental pollution of groundwater resources with case examples from developing countries. Environ. Health Perspect. 1989, 83, 39–68. [Google Scholar] [CrossRef] [PubMed]
- Pedley, S.; Howard, G. The public health implications of microbiological contamination of groundwater. Q. J. Eng. Geol. Hydrogeol. 1997, 30, 179–188. [Google Scholar] [CrossRef]
- Lodder, W.J.; van der Berg, H.H.; Rutjes, S.A.; de Roda Husman, A.M. Presence of enteric viruses in source waters for drinking water production in the Netherlands. Appl. Environ. Microbiol. 2010, 76, 5965–5971. [Google Scholar] [CrossRef] [PubMed]
- Kwenamore, K.M.; Bezuidenhout, C.C. Characteristics of faecal coliforms and enterococci isolated from ground-drinking water sources within Ditsobotla and Molopo districts, North-West Province, South Africa. In Proceedings of the WISA Biennial Conference, Sun City, South Africa, 18–22 May 2008.
- Ferreira, S.L. Microbial and physico-chemical quality of groundwater in the North-West Province, South Africa. Master’s Thesis, North West University, Potchefstroom, South Africa, 2011. [Google Scholar]
- Phokela, P.T.; Ateba, C.N.; Kawadza, T.D. Assessing antibiotic resistance profiles in Escherichia coli and Salmonella species from groundwater in the Mafikeng area, South Africa. Afr. J. Microbiol. Res. 2011, 5, 5902–5909. [Google Scholar]
- Mpenyana-Monyatsi, L.; Momba, M.N.B. Assessment of groundwater quality in rural areas of the North West Province, South Africa. Sci. Res. Essays 2012, 7, 903–914. [Google Scholar] [CrossRef]
- Williamson, S.J.; Houchin, L.A.; McDaniel, L.; Paul, J. Seasonal Variation in Lysogeny as Depicted by Prophage Induction in Tampa Bay, Florida. Appl. Environ. Microbiol. 2000, 68, 4307–4314. [Google Scholar] [CrossRef]
- Sundram, A.; Donnelly, L.; Ehlers, M.M.; Vrey, A.; Grabow, W.O.K.; Bailey, I.W. Evaluation of F-RNA coliphages as indicators of viruses and the source of faecal pollution. In Proceedings of the Biennial Conference of the Water Institute of Southern Africa (WISA), Durban, South Africa, 19–23 May 2002.
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nkwe, K.I.; Ateba, C.N.; Sithebe, N.P.; Bezuidenhout, C.C. Enumeration of Somatic and F-RNA Phages as an Indicator of Fecal Contamination in Potable Water from Rural Areas of the North West Province. Pathogens 2015, 4, 503-512. https://doi.org/10.3390/pathogens4030503
Nkwe KI, Ateba CN, Sithebe NP, Bezuidenhout CC. Enumeration of Somatic and F-RNA Phages as an Indicator of Fecal Contamination in Potable Water from Rural Areas of the North West Province. Pathogens. 2015; 4(3):503-512. https://doi.org/10.3390/pathogens4030503
Chicago/Turabian StyleNkwe, Keitumetse Idah, Collins Njie Ateba, Nomathamsanqa Patricia Sithebe, and Cornelius Carlos Bezuidenhout. 2015. "Enumeration of Somatic and F-RNA Phages as an Indicator of Fecal Contamination in Potable Water from Rural Areas of the North West Province" Pathogens 4, no. 3: 503-512. https://doi.org/10.3390/pathogens4030503
APA StyleNkwe, K. I., Ateba, C. N., Sithebe, N. P., & Bezuidenhout, C. C. (2015). Enumeration of Somatic and F-RNA Phages as an Indicator of Fecal Contamination in Potable Water from Rural Areas of the North West Province. Pathogens, 4(3), 503-512. https://doi.org/10.3390/pathogens4030503






