2.1. Identification of Pathogenic S. marcescens Strains
A search for entomopathogenic bacteria was conducted taking samples from the hemocoel of dead third-instar larvae presenting previous disease symptoms as darker color, softer appearance and decrements in the amount of food consumed compared to healthy larvae. Ninety-six bacterial isolates, coming from a total of 116 larvae, had been obtained previously [
5]. Twelve bacterial isolates were selected based on the fact that the original hemocoel samples contained high bacterial titers (1 × 10
9 bacteria mL
−1) and each sample presented bacteria with a similar colony morphology. The other 84 hemocoel samples presented a mixture of bacteria with different colony morphology and showed lower bacterial titers (1 × 10
2 bacteria mL
−1). Only five
S. marcescens strains were found among the 12 isolates. The five strains were isolated from
Phyllophaga ravida larvae.
Oral bioassays were done to test the ability of the bacterial isolates to cause pathogenic symptoms: antifeeding effect, change in color and mortality. Healthy larvae of
P. blanchardi were fed with small pieces of carrot containing the
S. marcescens isolates, as indicated in the Materials and Methods section. Larvae in the control treatment group were fed with un-inoculated carrot. A second negative control was done with larvae fed with the non-pathogenic bacteria
S. plymuthica ATCC 15928. As indicated in
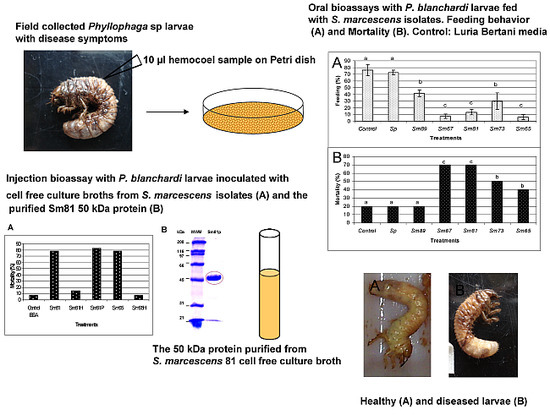
Figure 1, the bacterial isolates of
S. marcescens caused different degrees of antifeeding effects, since the larvae showed a significant decrement in the amount of food consumed compared to the control larvae (
p < 0.05) during the 6 days of oral inoculation. The larvae fed with the isolates of
S. marcescens 67 (Sm67),
S. marcescens 81 (Sm81) and
S. marcescens 65 (Sm65) consumed only a small proportion of the available food, showing a food consumption of 7.51%, 13.46% and 6.06% respectively; the strains
S. marcescens 89 (Sm89) and
S. marcescens 73 (Sm73) consumed only 41.84% and 29.89% of their food, respectively. Larvae from the control groups, fed with un-inoculated carrot and with the nonpathogenic bacteria
S. plymuthica consumed 76.19% and 72.33% of the available food, respectively. After the inoculation period of 6 days, all the larvae were fed with inoculated carrot. The larvae treated with the different isolates of
S. marcescens maintained low levels of food consumption compared to control larvae, even after the pathogenic bacteria was separated from the food; the larvae in this control group consumed about 75% of the available carrot, and the average of food consumption in the larvae fed with the
S. marcescens isolates was 25%.
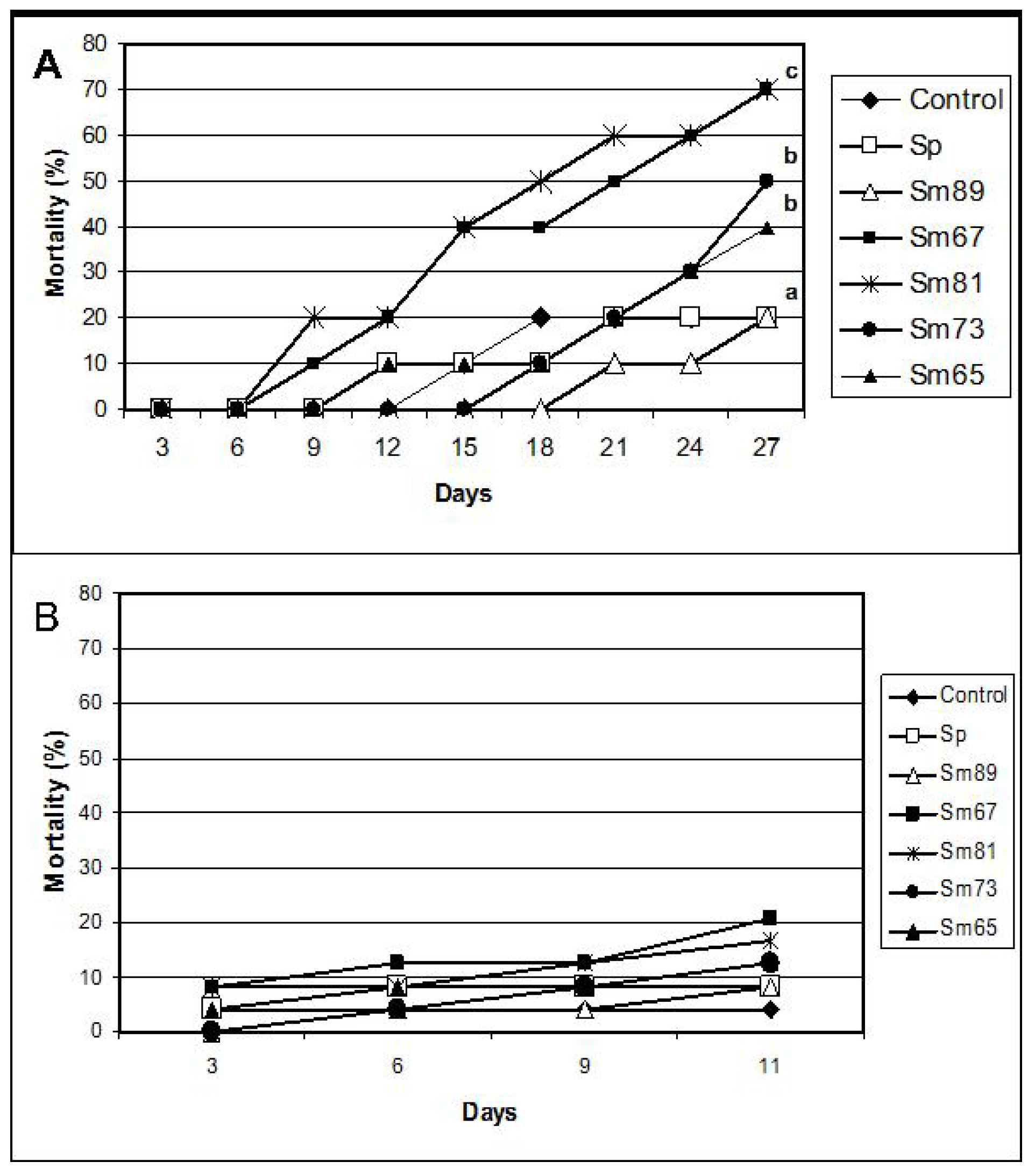
Significant mortality (
p < 0.01) compared with control larvae was observed for the larvae inoculated with Sm67, Sm81, Sm73 and Sm65 after 27 days from the beginning of the test (
Figure 2). The observed mortality for the control larvae was 20% and the observed mortality in the group fed with the Sm89 isolate was also 20%. On the contrary, the larvae inoculated with the other four
S. marcescens isolates showed a mortality of 70% for both Sm67 and Sm81 and mortalities of 50% and 40% for Sm73 and Sm65, respectively. Larvae inoculated with the different
S. marcescens isolates developed a slightly brown color compared with the beige appearance of healthy larvae observed in the control groups.
Figure 1.
Feeding behavior of S. marcescens isolates towards larvae of P. blanchardi by oral inoculation. The percentage of feeding represents an average of food ingested during 6 days of oral inoculation. Control groups were fed with uncoated pieces of carrot and also carrot coated with the non-pathogenic bacterium Serratia plymuthica ATCC15928. A lack of significant differences is indicated by the same letter above the bars (ANOVA, p < 0.001); n = 10.
Figure 1.
Feeding behavior of S. marcescens isolates towards larvae of P. blanchardi by oral inoculation. The percentage of feeding represents an average of food ingested during 6 days of oral inoculation. Control groups were fed with uncoated pieces of carrot and also carrot coated with the non-pathogenic bacterium Serratia plymuthica ATCC15928. A lack of significant differences is indicated by the same letter above the bars (ANOVA, p < 0.001); n = 10.
To evaluate the potential insecticidal activity of the S. marcescens isolates for P. blanchardi by intracoelomic inoculation, bioassays were done by injecting 103 bacteria per larva. All five S. marcescens isolates caused death of 100% of larvae in a time period of 48 h to 96 h from inoculation. The strain Sm81 killed all larvae after 48 h and Sm73, Sm67 and Sm65 after 72 h from inoculation. The strain Sm89 killed 100% of the larvae after 96h. The same amounts of E. coli Top10 and E. coli Epi300 injected into the larvae did not cause significant mortality (0%–20%; p <0.001) after 96 h from inoculation. Attempts to determine the median lethal dose were not successful, since no differences in larvae mortality were observed at bacterial concentrations higher than 103 bacteria per larva, that is, a concentration between 103 to 106 bacteria per larva caused a mortality of 100% after 48–96 h depending on the strain. Bacterial concentrations lower than 103 bacteria per larva produced no significant mortality, even after 20 days from inoculation. It is possible that once the insect immune response is surpassed by the bacterial infection, larval death occurs very fast.
To test the ability of the
S. marcescens isolates to kill larvae belonging to a different insect orders, oral bioassays were performed with larvae of
Spodoptera frugiperda (Lepidoptera: Noctuidae) fed with the five
S. marcescens isolates. No significant mortality was observed in this bioassay (
Figure 2).
2.3. Identification of Toxic Activity in Sm81 Culture Supernatant
It has been suggested that some extracellular toxin proteins might be involved in
Serratia spp pathogenicity [
4]. To test the ability of the Sm81 extracellular proteins to produce mortality, oral bioassays were done with pieces of carrot containing cell-free supernatant from the Sm81 isolate grown for 24 h using 2 µg of protein per larva. To investigate the nature of the potential toxin-like compounds, a sample of the Sm81 culture broth was also evaluated after being boiled for 5 min. Since proteins are denatured by heat, a decrement in the toxic activity would suggest that a protein would be involved in the toxic activity. Carrot containing nutrient broth with 2 µg of bovine-serum albumin (BSA) was used to feed control larvae. The samples were fed to
P. blanchardi larvae as indicated in Materials and Methods.
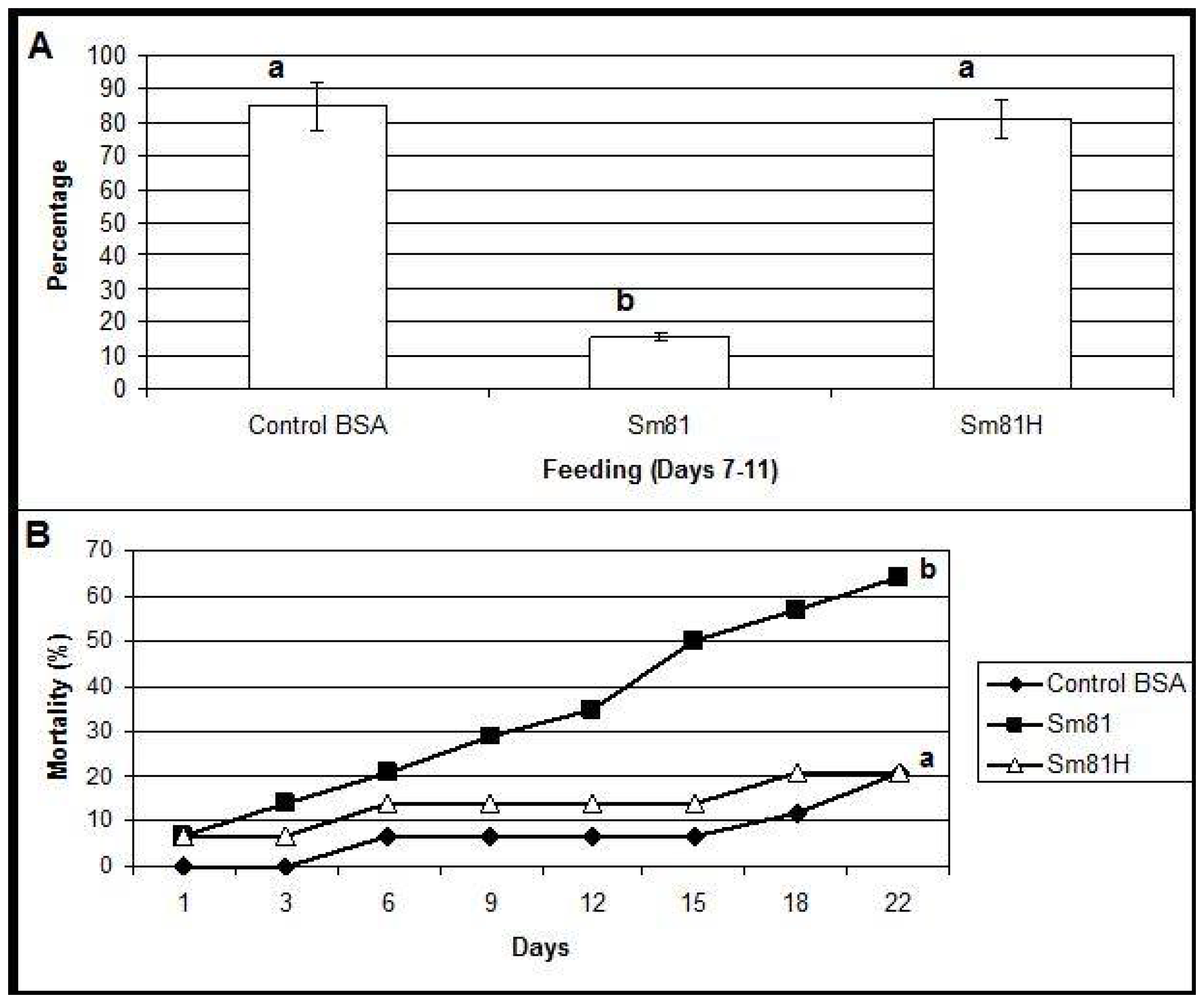
During the first time period analyzed (inoculation period), from the beginning of the test to Day 6, control larvae and those fed with the boiled sample consumed about 94% and 90% of their food, respectively. A slight but significant decrement (18%, compared to the control 94%) in food consumption was observed in the larvae fed with the Sm81 broth (76%). During the second period analyzed, from Days 7–11, the control larvae showed a food consumption of 85 % (
Figure 3). On the other hand, the larvae fed with Sm81 broth consumed only about 15% (
p < 0.001) of the available food; thereby showing an antifeeding effect. This effect was not observed when the sample was boiled, since larvae showed a food consumption of 81%, which was not significant when compared to the control larvae (85%,
p > 0.05), but it was significant when compared with the larvae fed with non-boiled broth (15%,
p < 0.01). The Sm81 broth sample also caused a significant mortality rate of 64% (
p > 0.05) after 22 days from the beginning of the test. No significant mortality was observed for control larvae and those fed with boiled broth (20%).
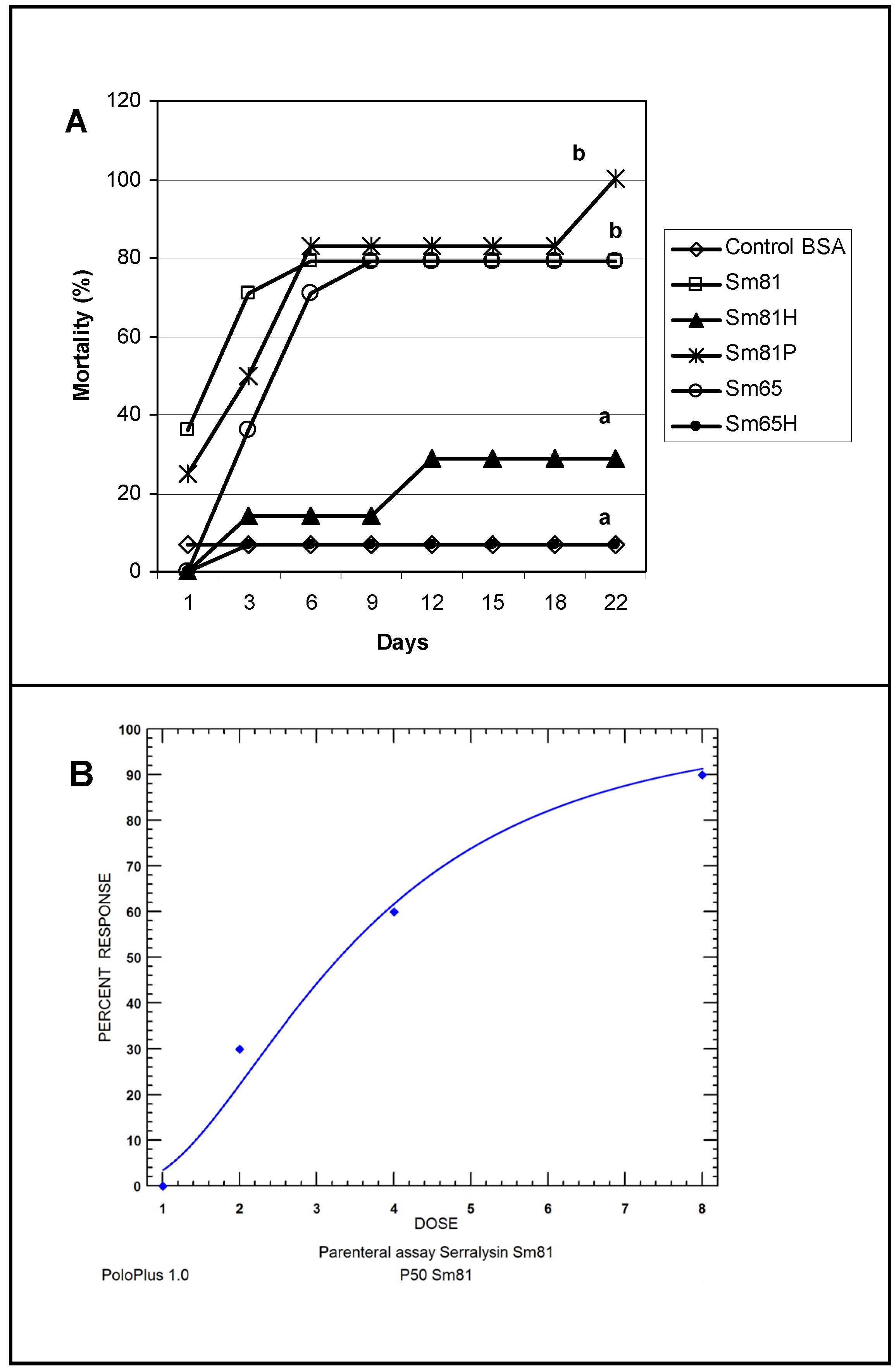
To test the ability of the
S. marcescens extracellular proteins to produce mortality by intracoelomic inoculation, a bioassay by injection was performed. Cell-free culture supernatant from the isolates Sm81 and Sm65 grown for 24 h was injected into healthy
P. blanchardi larvae. As observed in
Figure 4, control larvae injected with nutrient broth containing BSA cause no significant mortality. On the other hand, larvae injected with Sm81 and Sm65 cell-free culture supernatant showed a significant mortality of about 78% (
p < 0.0001). No significant mortality was observed when the culture broth from both isolates was boiled and injected into the larvae.
Figure 3.
Oral bioassay showing the antifeeding effect (Panel A) and mortality (Panel B) of cell-free culture supernatants from Sm81 isolate towards P. blanchardi larvae. The control group was fed with carrot containing nutrient broth plus BSA. The feeding activity of larvae was evaluated during Days 7–11 after an inoculation period of 6 days. The average of food ingestion during the period is shown. Mortality was evaluated during 22 days from the beginning of bioassay. Differences among the groups of larvae shown by the letters above the bars or lines were evaluated by ANOVA for feeding activity and χ2 for mortality. Sm81H represents a treatment where the cell-free culture supernatant from the isolate Sm81 were boiled for 5 min. The groups of larvae evaluated are shown by different letters above the lines; n = 14.
Figure 3.
Oral bioassay showing the antifeeding effect (Panel A) and mortality (Panel B) of cell-free culture supernatants from Sm81 isolate towards P. blanchardi larvae. The control group was fed with carrot containing nutrient broth plus BSA. The feeding activity of larvae was evaluated during Days 7–11 after an inoculation period of 6 days. The average of food ingestion during the period is shown. Mortality was evaluated during 22 days from the beginning of bioassay. Differences among the groups of larvae shown by the letters above the bars or lines were evaluated by ANOVA for feeding activity and χ2 for mortality. Sm81H represents a treatment where the cell-free culture supernatant from the isolate Sm81 were boiled for 5 min. The groups of larvae evaluated are shown by different letters above the lines; n = 14.
Figure 4.
Injection bioassay showing insecticidal activity in the Sm81 and Sm65 cell-free culture supernatant and Sm81 50 kDa protein. Panel A. Supernatant from 24 h culture from the S. marcescens 81 and S. marcescens 65 (2 µg·mL−1) was injected into the larvae. Sm81H and Sm65H represent the same culture supernatant from Sm81 and Sm65 but boiled for 5 min. Sm81P represents the mortality observed for the larvae injected with the 50 kDa protein (2 µg·mL−1) purified from the cell-free culture supernatant from Sm81. The control represents a nutrient broth containing BSA (2 µg mL−1). Mortality was recorded for 22 days after injection. Differences among the groups of larvae shown by the letters above the lines were evaluated using χ2 (p < 0.0001; n = 14). Panel B. Dose-response bioassay showing dose dependency of the Sm81P50 insect killing activity. Dose-response curve for the injection bioassay of Sm81P50 (1 µg to 8 µg) against P. blanchardi larvae. Ten larvae were used for each dose.
Figure 4.
Injection bioassay showing insecticidal activity in the Sm81 and Sm65 cell-free culture supernatant and Sm81 50 kDa protein. Panel A. Supernatant from 24 h culture from the S. marcescens 81 and S. marcescens 65 (2 µg·mL−1) was injected into the larvae. Sm81H and Sm65H represent the same culture supernatant from Sm81 and Sm65 but boiled for 5 min. Sm81P represents the mortality observed for the larvae injected with the 50 kDa protein (2 µg·mL−1) purified from the cell-free culture supernatant from Sm81. The control represents a nutrient broth containing BSA (2 µg mL−1). Mortality was recorded for 22 days after injection. Differences among the groups of larvae shown by the letters above the lines were evaluated using χ2 (p < 0.0001; n = 14). Panel B. Dose-response bioassay showing dose dependency of the Sm81P50 insect killing activity. Dose-response curve for the injection bioassay of Sm81P50 (1 µg to 8 µg) against P. blanchardi larvae. Ten larvae were used for each dose.
2.4. A 50 kDa Serralysin-Like Protein Is Responsible for Insecticidal Activity in Sm81
It has been reported that a 61 kDa protease from
S. marcescens HR3 culture broth has insecticidal activity towards the grassland locust
Myrneleotettix palpalis Zub [
9]. A characteristic of the genus
Serratia is the production of extracellular proteases [
6], hence, it was hypothesized that the Sm81 protease was the causative agent for the insecticidal activity observed for the cell-free culture supernatants. The
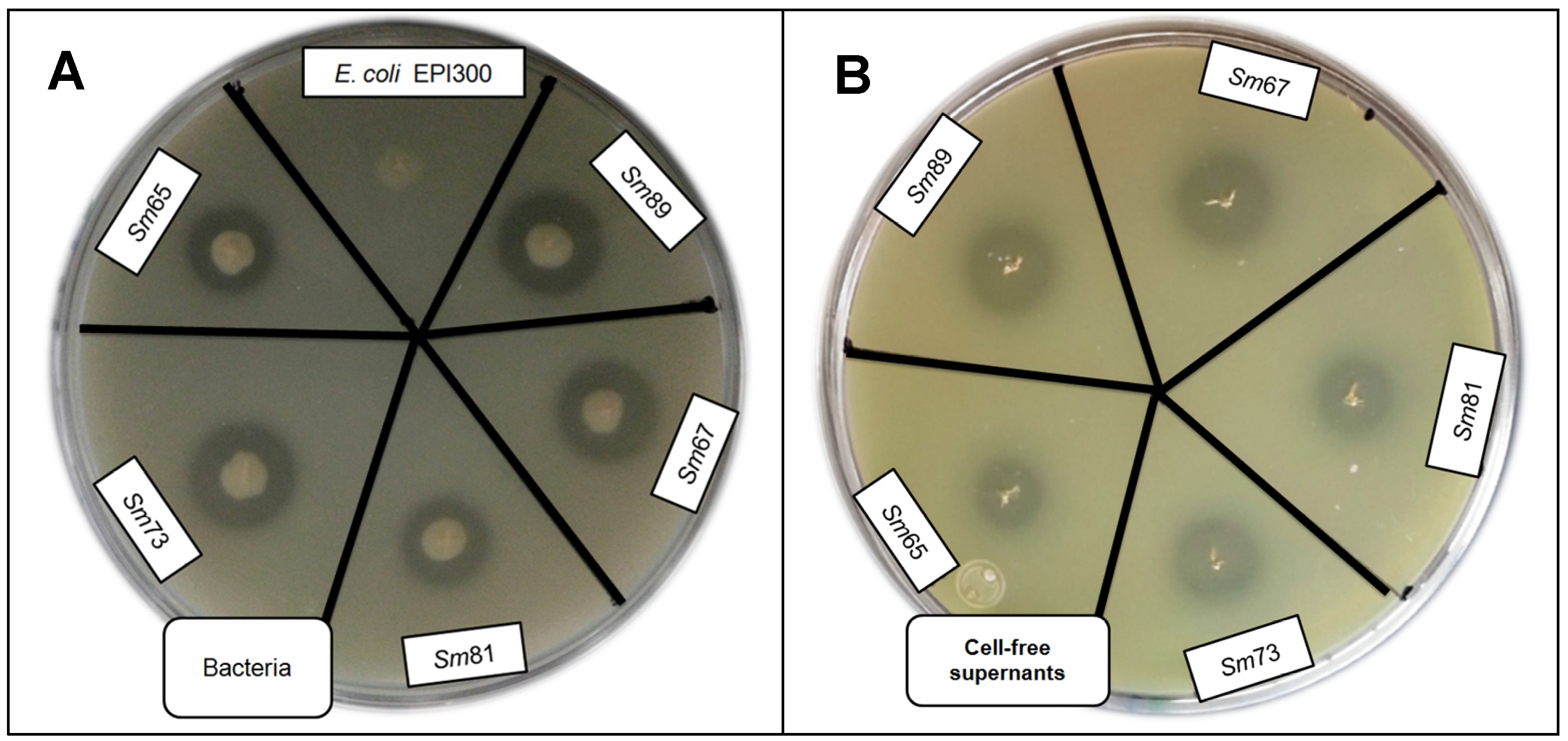
S. marcescens isolates and cell-free supernatants were screened for extracellular protease activity on agar-milk plates as reported [
10]. Milk clearing zones were produced by the five isolates as expected (
Figure 5) demonstrating proteolytic activity. It was observed that the halo diameter sizes of one colony grown for 24 h were about 10–12 mm (
Table 1).
Figure 5.
Skim milk plate assay to evaluate protease activity by S. marcescens strains and cell-free culture supernatants. Panel A. Protease activity of bacteria. Panel B. Protease activity of cell-free culture supernatants.
Figure 5.
Skim milk plate assay to evaluate protease activity by S. marcescens strains and cell-free culture supernatants. Panel A. Protease activity of bacteria. Panel B. Protease activity of cell-free culture supernatants.
Table 1.
Protease activity of S. marcescens bacteria and supernatants.
Table 1.
Protease activity of S. marcescens bacteria and supernatants.
| | Bacteria Halo (mm) | Supernatant Halo (mm) |
|---|
| Strain | Mean | SD | Confidence limits | mean | SD | Confidence limits |
| Sm89 | 12 a | ±0.7071 | (11.22–12.88) | 10.8 a | ±1.095 | (9.44–12.16) |
| Sm67 | 12 a | ±0.7071 | (11.22–12.88) | 11.2 a | ±0.4472 | (10.64–11.75) |
| Sm65 | 10 b | ±0.70.71 | (9.12–10.88) | 8.8 b | ±0.4472 | (8.24–9.35) |
| Sm81 | 10.8 b | ±0.4472 | (10.24–10.87) | 8.6 b | ±0.5477 | (7.92–9.28) |
| Sm73 | 12.2 a | ±0.4472 | (11.64–12.75) | 9.8 a | ±0.8367 | (8.76–10.84) |
The halo diameter observed for Sm65 and Sm81 were smaller (10 and 10.8 mm) than the halo diameter observed for Sm89, Sm67 and Sm73 (12 mm). Similar results were observed for the halo diameter of the cell-free supernatants. Five µL of cell-free supernatant produced a halo diameter of about 8–11 mm, and the halos observed for Sm65 and Sm81 were smaller (8.8 and 8.6 mm) than the ones of Sm89, Sm67 and Sm73.
In a next step, in order to study whether the
S. marcescens isolates produced one or several protease(s) of similar or different molecular size, the
S. marcescens cell-free culture supernatants were analyzed by
in gel zymography as reported [
11]. It was observed in the zymography (
Figure 6) that the five
S. marcescens isolates produced a single band with protease activity, corresponding to an extracellular protease of about 50 kDa (
Figure 6, Panel C). No other protein with protease activity was detected by this system.
Figure 6.
Electrophoretic analysis of S. marcescens 81 cell-free culture supernatants. Panel A. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (12%) analysis of cell-free culture supernatant of S. marcescens 81 strain. Cell-free culture supernatant was concentrated and adjusted to 30 µL per line. Panel B. SDS-PAGE analysis of the Sm81 50 kDa protein purified by HiTrap Q FF ion exchange column. Twenty µg of Sm81P50 was charged in the gel line. Panel C. Zymography showing the 50 kDa protease from the S. marcescens strains cell-free culture supernatants. Gelatin-SDS-PAGE stained with coomassie brilliant blue. The protein concentration was adjusted to 6 µg per line. For Sm65 the protein concentration was 0.6 µg per line.
Figure 6.
Electrophoretic analysis of S. marcescens 81 cell-free culture supernatants. Panel A. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (12%) analysis of cell-free culture supernatant of S. marcescens 81 strain. Cell-free culture supernatant was concentrated and adjusted to 30 µL per line. Panel B. SDS-PAGE analysis of the Sm81 50 kDa protein purified by HiTrap Q FF ion exchange column. Twenty µg of Sm81P50 was charged in the gel line. Panel C. Zymography showing the 50 kDa protease from the S. marcescens strains cell-free culture supernatants. Gelatin-SDS-PAGE stained with coomassie brilliant blue. The protein concentration was adjusted to 6 µg per line. For Sm65 the protein concentration was 0.6 µg per line.
In order to determine whether the protease isolated from the Sm81 strain presented toxic activity towards
P. blanchardi, the 50 kDa protein showing protease activity was purified from the Sm81 cell-free culture supernatant (
Figure 6, Panel B) as indicated in Materials and Methods and tested for toxic activity by injection bioassays. As indicated in
Figure 4 (Panel A), the 50 kDa protein showed strong insecticidal activity, causing a mortality of 83% in
P. blanchardi larvae. The mortality observed was comparable to the mortality observed for the Sm81 and Sm65 supernatants (79%) from Day 5 to Day 18 after injection. At Day 22 after injection, 100% mortality was observed in the larvae group injected with Sm81P50, contrary to a mortality of 79% observed for Sm81 and Sm65 supernatants at Day 22. It was expected that the purified toxin might cause higher mortality than the mixture of proteins present in the cell-free supernatants. This expectation was only evident at Day 22 after injection.
To determine whether the mortality caused by Sm81P50 was dose-dependant, an injection bioassay administrating several Sm81P50 doses to
P. blanchardi larvae was done. As observed in
Figure 4, Panel B, larvae mortality shows Sm81P50 dose-dependency. The results indicates that mortality increases according to increments in the concentration of purified Sm81P50 administrated to the larvae and also that Sm81P50 is able to kill a high percentage of
P. blanchardi larvae (90%) after a single injection of 8 µg protein per larva. A similar amount of BSA injected into the larvae used as control for this experiment did not cause mortality, showing that Sm81P50 was responsible for the observed mortality. The LD
50 value calculated by probit analysis (
Figure 4, Panel B) was 3.291 µg (2.292 to 4.806; 95% confidence limit).
Analysis of the insecticidal protein by tandem- mass spectrometry (LC-MS/MS) showed a similarity to a Serralysin-like protein from the
Serratia spp protein group accession A8G880; [
12]. Analysis of the tryptic digest of Sm81P50 identified 22 peptides that covered 65.2% of the homolog protein Serralysin (Accession V9NCZ4), with a molecular weight of 50.2 kDa, including peptides from the central region of the protein and the C termini (
Figure 7).
Figure 7.
Amino acid sequence of the Serralysin protein (Accession V9NCZ4) showing homology to 22 peptides from Sm81P50. Amino acids shaded in green represent identical peptides that were identified by mass spectrometry. Amino acids shaded in yellow represent peptides also identified by mass spectrometry but not identical to the V9NCZ4 Serralysin.
Figure 7.
Amino acid sequence of the Serralysin protein (Accession V9NCZ4) showing homology to 22 peptides from Sm81P50. Amino acids shaded in green represent identical peptides that were identified by mass spectrometry. Amino acids shaded in yellow represent peptides also identified by mass spectrometry but not identical to the V9NCZ4 Serralysin.
A blast homology [
8] search of the putative Sm81P50 Serralysin (V9NCZ4) revealed a high degree of homology with other
S. marcescens serralysin-like proteins. There were about 63 entries with sequences producing significant alignments with identities above 50%. Seven entries were associated with insecticidal proteins, although four of them were partial sequences and were not compared. The insecticidal proteins led to identities of 99%, 99% and 97%, respectively to Serralysin-like proteases from
S. marcescens strain HR-3 (Sequence ID: gb|ABK55613.1|),
S. marcescens strain Zhen Jiang (Sequence ID: gb|ACH90152.1|) and
S. marcescens strain DOAB 216-82 (Sequence ID: gb|AGG14207.1|) corresponding to proteins of 487 amino acids (E-value 0.0) (S2.
Supplementary material). Serralysins are Zinc-metalloproteases, which are common in pathogenic bacteria, and are often attributed roles in virulence. These virulence roles include direct toxin activity, processing of other enzymes and degradation of host connective tissues [
13]. Complete genomes of the
S. marcescens strains Db11, WW4, SM39 and FG194 have been announced (National Center for Biotechnology Information [
8]), and the 50 kDa protein also leads to significant alignments with Serralysin-like sequences from Db11, WW4, SM39 strains (S.3.
Supplementary material). Contrarily, no significant alignment was observed for Serralysin (Accession NC_020064.1) from
S. marcescens FG194. Alignment of putative Sm81P50
versus Db11 Serralysin (Accession WP_038629638.1) led to 56% identity (199 out of 350 amino acids; 2e-127), Sm81P50
versus WW4 Serralysin (Accession WP_015377863) led to 63% identity (231 out of 365 amino acids; 7e-160) and Sm81P50
versus SM39 Serralysin (Accession WP_041035877) produced 60% identity (196 out of 329 amino acids; 5e-126).