Defensin Production by Human Limbo-Corneal Fibroblasts Infected with Mycobacteria
Abstract
:1. Introduction
2. Results
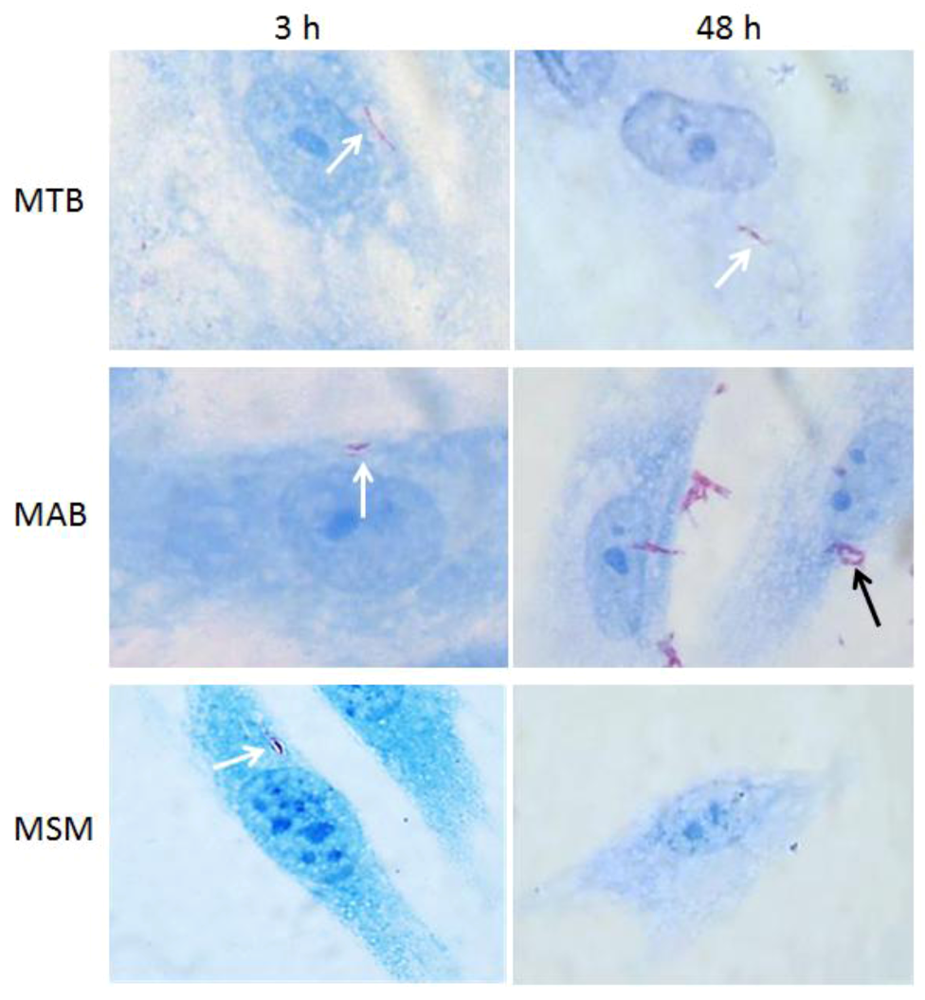
2.1. Intracellular Replication of the Mycobacteria in Human Corneal Fibroblasts

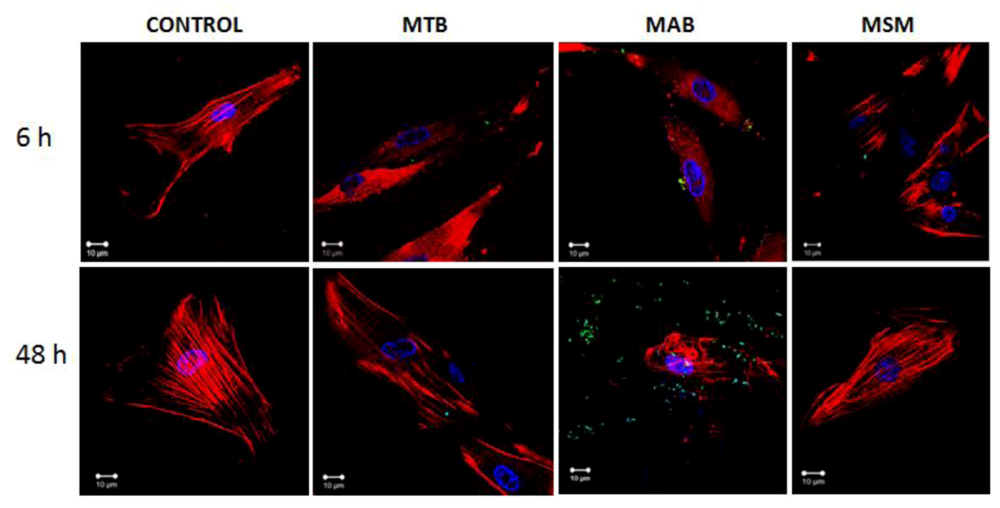
2.2. M. abscessus and M. tuberculosis Stimulate the Redistribution of the Actin Filaments
2.3. Mycobacteria Induce the Expression of hBD1 and LL-37 mRNAs in Human Limbo-Corneal Fibroblasts
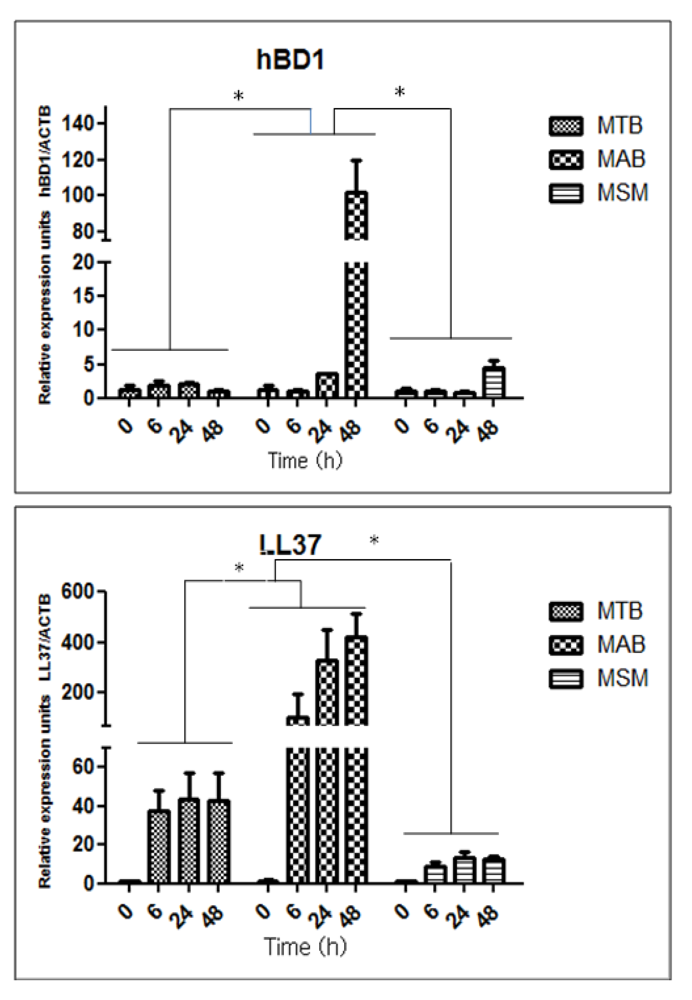
2.4. The Production of Antimicrobial Peptides in the Limbo-Corneal Fibroblasts Infected with Mycobacteria
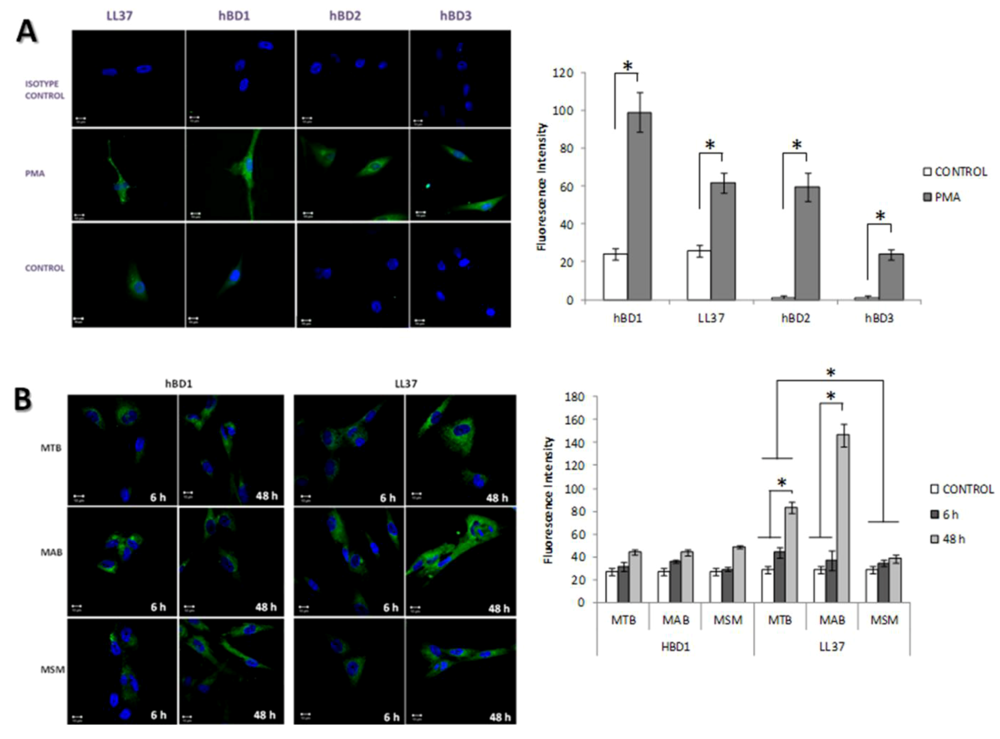
2.5. The Limbo-Corneal Fibroblasts Produce IL-6 in Response to Infection by Mycobacteria
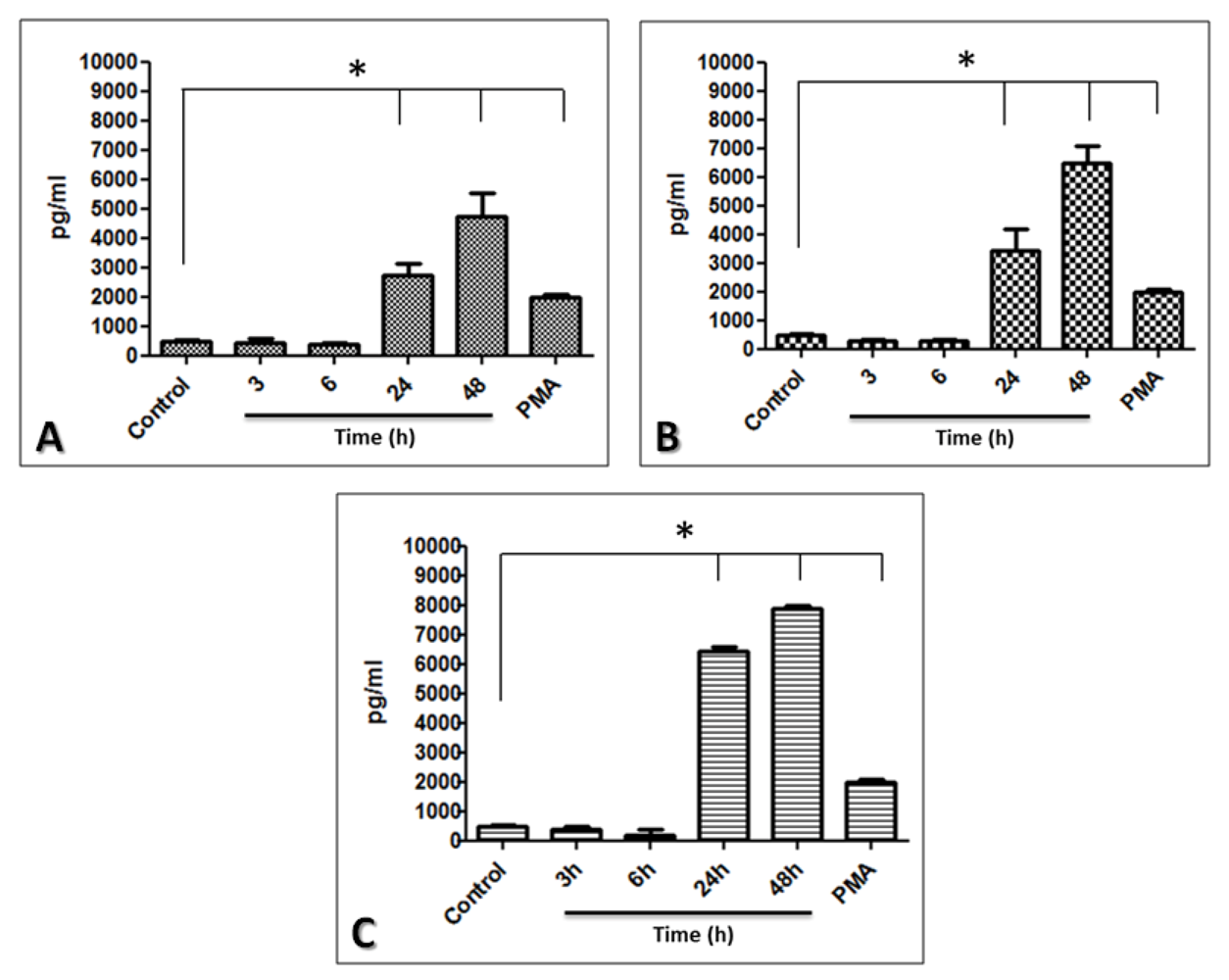
3. Discussion
4. Experimental Section
4.1. Bacteria
4.2. Primary Culture of Limbo-Corneal Fibroblasts
4.3. Cellular Infection Assays
4.4. Ziehl-Neelsen Stain
4.5. Intracellular Replication Assays
4.6. Isolation of RNA and RT-qPCR (Reverse Transcription Real-Time PCR) for the Preparation of Beta-Defensin and Cathelicidin LL-37 Gene Fragments
4.7. Confocal Microscopy
4.8. Determination of the Soluble Cytokines
4.9. Statistical Analysis
5. Conclusions
Conflict of Interest
Acknowledgements
References
- Sharma, S. Antibiotic resistance in ocular bacterial pathogens. Indian. J. Med. Microbiol. 2011, 29, 218–222. [Google Scholar] [CrossRef]
- Ahmad, S. Pathogenesis, immunology and diagnosis of latent Mycobacterium tuberculosis infection. Clin. Dev. Immunol. 2011, 2011, 814–943. [Google Scholar]
- Gupta, V.; Gupta, A.; Rao, N.A. Intraocular tuberculosis--an update. Surv. Ophthalmol. 2007, 52, 561–587. [Google Scholar]
- Höfling-Lima, A.L.; de Freitas, D.; Sampaio, J.L.; Leão, S.C.; Contarini, P. In vitro activity of fluoroquinolones against Mycobacterium abscessus and Mycobacterium chelonae causing infectious keratitis after LASIK in Brazil. Cornea 2005, 24, 730–734. [Google Scholar]
- McNamara, N.A. Innate defense of the ocular surface. Eye Contact. Lens. 2003, 29, S10–S13. [Google Scholar] [CrossRef]
- Qu, X.D.; Lehrer, R.I. Secretory phospholipase A2 is the principal bactericide for staphylococci and other Gram-positive bacteria in human tears. Infect. Immun. 1998, 66, 2791–2797. [Google Scholar]
- Garreis, F.; Schlorf, T.; Worlitzsch, D.; Steven, P.; Bräuer, L.; Jäger, K.; Paulsen, F.P. Roles of human beta-defensins in innate immune defense at the ocular surface: arming and alarming corneal and conjunctival epithelial cells. Histochem. Cell. Biol. 2010, 134, 59–73. [Google Scholar] [CrossRef]
- Frohm, M.; Agerberth, B.; Ahangari, G.; Stâhle-Bäckdahl, M.; Lidén, S.; Wigzell, H.; Gudmundsson, G.H. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J. Biol. Chem. 1997, 272, 15258–15263. [Google Scholar]
- Gropp, R.; Frye, M.; Wagner, T.O.; Bargon, J. Epithelial defensins impair adenoviral infection: implication for adenovirus-mediated gene therapy. Hum. Gene. Ther. 1999, 10, 957–964. [Google Scholar] [CrossRef]
- Harder, J.; Bartels, J.; Christophers, E.; Schröder, J.M. A peptide antibiotic from human skin. Nature 1997, 387, 861. [Google Scholar]
- Shin, D.M.; Jo, E.K. Antimicrobial Peptides in Innate Immunity against Mycobacteria. Immune. Netw. 2011, 11, 245–252. [Google Scholar]
- White, S.H.; Wimley, W.C.; Selsted, M.E. Structure, function, and membrane integration of defensins. Curr. Opin. Struct. Biol. 1995, 5, 521–527. [Google Scholar] [CrossRef]
- Hoover, D.M.; Rajashankar, K.R.; Blumenthal, R.; Puri, A.; Oppenheim, J.J. Chertov, O.; Lubkowski, J. The structure of human beta-defensin-2 shows evidence of higher order oligomerization. J. Biol. Chem. 2000, 275, 32911–32918. [Google Scholar]
- Gordon, Y.J.; Huang, L.C.; Romanowski, E.G.; Yates, K.A.; Proske, R.J.; McDermott, A.M. Human cathelicidin (LL-37), a multifunctional peptide, is expressed by ocular surface epithelia and has potent antibacterial and antiviral activity. Curr. Eye. Res. 2005, 30, 385–394. [Google Scholar]
- Hattenbach, L.O.; Gümbel, H.; Kippenberger, S. Identification of beta-defensins in human conjunctiva. Antimicrob. Agents. Chemother. 1998, 42, 3332. [Google Scholar]
- Haynes, R.J.; Tighe, P.J.; Dua, H.S. Antimicrobial defensin peptides of the human ocular surface. Br. J. Ophthalmol. 1999, 83, 737–741. [Google Scholar] [CrossRef]
- McDermott, A.M.; Redfern, R.L.; Zhang, B.; Pei, Y.; Huang, L.; Proske, R.J. Defensin expression by the cornea: multiple signaling pathways mediate IL-1 beta stimulation of hBD-2 expression by human corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 2003, 44, 1859–1865. [Google Scholar]
- McNamara, N.A.; Van, R.; Tuchin, O.S.; Fleiszig, S.M. Ocular surface epithelia express mRNA for human beta defensin-2. Exp. Eye Res. 1999, 69, 483–490. [Google Scholar] [CrossRef]
- Sack, R.A.; Conradi, L.; Krumholz, D.; Beaton, A.; Sathe, S.; Morris, C. Membrane array characterization of 80 chemokines, cytokines, and growth factors in open- and closed-eye tears: angiogenin and other defense system constituents. Invest. Ophthalmol. Vis. Sci. 2005, 46, 1228–1238. [Google Scholar] [CrossRef]
- Huang, L.C.; Jean, D.; Proske, R.J.; Reins, R.Y.; McDermott, A.M. Ocular surface expression and in vitro activity of antimicrobial peptides. Curr. Eye Res. 2007, 32, 595–609. [Google Scholar] [CrossRef]
- Haniffa, M.A.; Collin, M.P.; Buckley, C.D.; Dazzi, F. Mesenchymal stem cells: the fibroblasts’ new clothes? Haematologica 2009, 94, 258–263. [Google Scholar] [CrossRef]
- Fries, K.M.; Blieden, T.; Looney, R.J.; Sempowski, G.D.; Silvera, M.R.; Willis, R.A.; Phipps, R.P. Evidence of fibroblast heterogeneity and the role of fibroblast subpopulations in fibrosis. Clin. Immunol. Immunopathol. 1994, 72, 283–292. [Google Scholar]
- Serhan, C.N.; Brain, S.D.; Buckley, C.D.; Gilroy, D.W.; Haslett, C.; O'Neill, L.A.; Perretti, M.; Rossi, A.G.; Wallace, J.L. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007, 21, 325–332. [Google Scholar] [CrossRef]
- Le, J.M.; Vilcek, J. Accessory function of human fibroblasts in mitogen-stimulated interferon-gamma production by T lymphocytes. Inhibition by interleukin 1 and tumor necrosis factor. J. Immunol. 1987, 139, 3330–3337. [Google Scholar]
- Shimabukuro, Y.; Murakami, S.; Okada, H. Interferon-gamma-dependent immunosuppressive effects of human gingival fibroblasts. Immunology 1992, 76, 344–347. [Google Scholar]
- Strieter, R.M.; Gomperts, B.N.; Keane, M.P. The role of CXC chemokines in pulmonary fibrosis. J. Clin. Invest. 2007, 117, 549–556. [Google Scholar]
- Fukuda, K. Role of corneal fibroblasts in the pathogenesis of ocular allergic diseases. Nippon. Ganka. Gakkai. Zasshi. 2005, 109, 717–726. [Google Scholar]
- Sempowski, G.D.; Chess, P.R.; Moretti, A.J.; Padilla, J.; Phipps, R.P.; Blieden, T.M. CD40 mediated activation of gingival and periodontal ligament fibroblasts. J. Periodontol. 1997, 68, 284–292. [Google Scholar]
- Hong, J.W.; Liu, J.J.; Lee, J.S.; Mohan, R.R.; Mohan, R.R.; Woods, D.J.; He, Y.G.; Wilson, S.E. Proinflammatory chemokine induction in keratocytes and inflammatory cell infiltration into the cornea. Invest. Ophthalmol. Vis. Sci. 2001, 42, 2795–2803. [Google Scholar]
- Bourcier, T.; Sauer, A.; Letscher-Bru, V.; Candolfi, E. Fungal keratitis. J. Fr. Ophtalmol. 2011, 34, 563–567. [Google Scholar] [CrossRef]
- Chee, S.P.; Jap, A. Immune ring formation associated with cytomegalovirus endotheliitis. Am. J. Ophthalmol. 2011, 152, 449–453. [Google Scholar] [CrossRef]
- Gao, N.; Kumar, A.; Guo, H.; Wu, X.; Wheater, M.; Yu, F.S. Topical flagellin-mediated innate defense against Candida albicans keratitis. Invest. Ophthalmol. Vis. Sci. 2011, 52, 3074–3082. [Google Scholar] [CrossRef]
- Kuruba, S.L.; Prabhakaran, V.C.; Nagarajappa, A.H.; Biligi, D.S. Orbital aspergillus infection diagnosed by FNAC. Diagn. Cytopathol. 2011, 39, 523–526. [Google Scholar] [CrossRef]
- Miller, K.V.; Eisley, K.M.; Shanks, R.M.; Lahr, R.M.; Lathrop, K.L.; Kowalski, R.P.; Noecker, R.J. Recurrent enterococcal endophthalmitis seeded by an intraocular lens biofilm. J. Cataract. Refract. Surg. 2011, 37, 1355–1359. [Google Scholar] [CrossRef]
- García-Pérez, B.E.; Villagomez-Palatto, D.A.; Castañeda-Sánchez, J.I.; Coral-vázquez, R.M.; Ramírez-Sánchez, I.; Ordoñez-Razo, R.M.; Luna-Herrera, J. Innate response of human endothelial cells infected with mycobacteria. Immunobiology 2011, 216, 925–935. [Google Scholar]
- Sanguinetti, M.; Ardito, F.; Fiscarelli, E.; La Sorda, M.; D'Argenio, P.; Ricciotti, G.; Fadda, G. Fatal pulmonary infection due to multidrug-resistant Mycobacterium abscessus in a patient with cystic fibrosis. J. Clin. Microbiol. 2001, 39, 816–819. [Google Scholar] [CrossRef]
- Ham, H.; Sreelatha, A.; Orth, K. Manipulation of host membranes by bacterial effectors. Nat. Rev. Microbiol. 2011, 9, 635–646. [Google Scholar] [CrossRef]
- Dickinson, J.M.; Mitchison, D.A. Experimental models to explain the high sterilizing activity of rifampin in the chemotherapy of tuberculosis. Am. Rev. Respir. Dis. 1981, 123, 367–371. [Google Scholar]
- Arriaga, A.K.; Orozco, E.H.; Aguilar, L.D.; Rook, G.A. Hernández Pando R. Immunological and pathological comparative analysis between experimental latent tuberculous infection and progressive pulmonary tuberculosis. Clin. Exp. Immunol. 2002, 128, 229–237. [Google Scholar] [CrossRef]
- Cutrufello, N.J.; Karakousis, P.C.; Fishler, J.; Albini, T.A. Intraocular tuberculosis. Ocul. Immunol. Inflamm. 2010, 18, 281–291. [Google Scholar]
- DiLoreto, D.A., Jr.; Rao, N.A. Solitary nonreactive choroidal tuberculoma in a patient with acquired immune deficiency syndrome. Am. J. Ophthalmol. 2001, 131, 138–140. [Google Scholar] [CrossRef]
- Redfern, R.L.; Reins, R.Y.; McDermott, A.M. Toll-like receptor activation modulates antimicrobial peptide expression by ocular surface cells. Exp. Eye Res. 2011, 92, 209–220. [Google Scholar] [CrossRef]
- Yuan, X.; Hua, X.; Wilhelmus, K.R. The corneal expression of antimicrobial peptides during experimental fungal keratitis. Curr. Eye Res. 2010, 35, 872–879. [Google Scholar]
- Gallo, R.L.; Murakami, M.; Ohtake, T.; Zaiou, M. Biology and clinical relevance of naturally occurring antimicrobial peptides. J. Allergy Clin. Immunol. 2002, 110, 823–831. [Google Scholar]
- Rivas-Santiago, B.; Contreras, J.C.; Sada, E.; Hernandez-Pando, R. The potential role of lung epithelial cells and beta-defensins in experimental latent tuberculosis. Scand. J. Immunol. 2008, 67, 448–452. [Google Scholar]
- Rivas-Santiago, B.; Sada, E.; Tsutsumi, V.; Aguilar-Leon, D.; Contreras, J.L.; Hernandez-Pando, R. Beta-Defensin gene expression during the course of experimental tuberculosis infection. J. Infect. Dis. 2006, 194, 697–703. [Google Scholar]
- Medzhitov, R. 2009. Damage control in host-pathogen interactions. Proc. Natl. Acad. Sci. USA 2009, 106, 15525–15526. [Google Scholar] [CrossRef]
- Ladel, C.H.; Blum, C.; Dreher, A.; Reifenberg, K.; Kopf, M.; Kaufmann, S.H. Lethal tuberculosis in interleukin-6-deficient mutant mice. Infect. Immun. 1997, 65, 4843–4849. [Google Scholar]
- Leal, I.S.; Smedegârd, B.; Andersen, P.; Appelberg, R. Interleukin-6 and interleukin-12 participate in induction of a type 1 protective T-cell response during vaccination with a tuberculosis subunit vaccine. Infect. Immun. 1999, 67, 5747–5754. [Google Scholar]
- Dutta, R.K.; Kathania, M.; Raje, M.; Majumdar, S. IL-6 inhibits IFN-γ induced autophagy in Mycobacterium tuberculosis H37Rv infected macrophages. Int. J. Biochem. Cell. Biol. 2012, 44, 942–954. [Google Scholar]
- Tamai, R.; Sugamata, M.; Kiyoura, Y. Candida albicans enhances invasion of human gingival epithelial cells and gingival fibroblasts by Porphyromonas gingivalis. Microb. Pathog. 2011, 51, 250–254. [Google Scholar] [CrossRef]
- Li, J.; Raghunath, M.; Tan, D.; Lareu, R.R.; Chen, Z.C.; Beuerman, R.W. Defensins HNP1 and HBD2 stimulation of wound-associated responses in human conjunctival fibroblasts. Invest. Ophthalmol. Vis. Sci. 2006, 47, 3811–3819. [Google Scholar]
- Li, J.; Zhu, H.Y.; Beuerman, R.W. Stimulation of specific cytokines in human conjunctival epithelial cells by defensins HNP1, HBD2 and HBD3. Invest. Ophthalmol. Vis. Sci. 2009, 50, 644–653. [Google Scholar]
- Larrick, J.; Hirata, M.; Balint, R.; Lee, J.; Zhong, J.; Wright, S.C. Human CAP 18: a novel antimicrobial lipopolysaccharide-binding protein. Infect. Immun. 1995, 63, 1291–1297. [Google Scholar]
- García-Pérez, B.E.; Hernández-González, J.C.; García-Nieto, S.; Luna-Herrera, J. Internalization of a non-pathogenic mycobacteria by macropinocytosis in human alveolar epithelial A549 cells. Microb. Pathog. 2008, 45, 1–6. [Google Scholar] [CrossRef]
- Sonawane, A.; Santos, J.C.; Mishra, B.B.; Jena, P.; Progida, C.; Sorensen, O.E.; Gallo, R.; Appelberg, R.; Griffiths, G. Cathelicidin is involved in the intracellular killing of mycobacteria in macrophages. Cell. Microbiol. 2011, 13, 1601–6017. [Google Scholar] [CrossRef]
- Chaurasia, S.S.; Kaur, H.; de Medeiros, F.W.; Smith, S.D.; Wilson, S.E. Dynamics of the expression of intermediate filaments vimentin and desmin during myofibroblast differentiation after corneal injury. Exp. Eye Res. 2009, 89, 133–139. [Google Scholar] [CrossRef]
- Liu, J.; Song, G.; Wang, Z.; Huang, B.; Gao, Q.; Liu, B.; Xu, Y.; Liang, X.; Ma, P.; Gao, N.; Ge, J. Establishment of a corneal epithelial cell line spontaneously derived from human limbal cells. Exp. Eye Res. 2007, 84, 599–609. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Castañeda-Sánchez, J.I.; García-Pérez, B.E.; Muñoz-Duarte, A.R.; Baltierra-Uribe, S.L.; Mejia-López, H.; López-López, C.; Bautista-De Lucio, V.M.; Robles-Contreras, A.; Luna-Herrera, J. Defensin Production by Human Limbo-Corneal Fibroblasts Infected with Mycobacteria. Pathogens 2013, 2, 13-32. https://doi.org/10.3390/pathogens2010013
Castañeda-Sánchez JI, García-Pérez BE, Muñoz-Duarte AR, Baltierra-Uribe SL, Mejia-López H, López-López C, Bautista-De Lucio VM, Robles-Contreras A, Luna-Herrera J. Defensin Production by Human Limbo-Corneal Fibroblasts Infected with Mycobacteria. Pathogens. 2013; 2(1):13-32. https://doi.org/10.3390/pathogens2010013
Chicago/Turabian StyleCastañeda-Sánchez, Jorge I., Blanca E. García-Pérez, Ana R. Muñoz-Duarte, Shantal L. Baltierra-Uribe, Herlinda Mejia-López, Carlos López-López, Victor M. Bautista-De Lucio, Atzín Robles-Contreras, and Julieta Luna-Herrera. 2013. "Defensin Production by Human Limbo-Corneal Fibroblasts Infected with Mycobacteria" Pathogens 2, no. 1: 13-32. https://doi.org/10.3390/pathogens2010013
APA StyleCastañeda-Sánchez, J. I., García-Pérez, B. E., Muñoz-Duarte, A. R., Baltierra-Uribe, S. L., Mejia-López, H., López-López, C., Bautista-De Lucio, V. M., Robles-Contreras, A., & Luna-Herrera, J. (2013). Defensin Production by Human Limbo-Corneal Fibroblasts Infected with Mycobacteria. Pathogens, 2(1), 13-32. https://doi.org/10.3390/pathogens2010013