Structure and Functions of Actin and Actin-Binding Proteins in Leishmania
Abstract
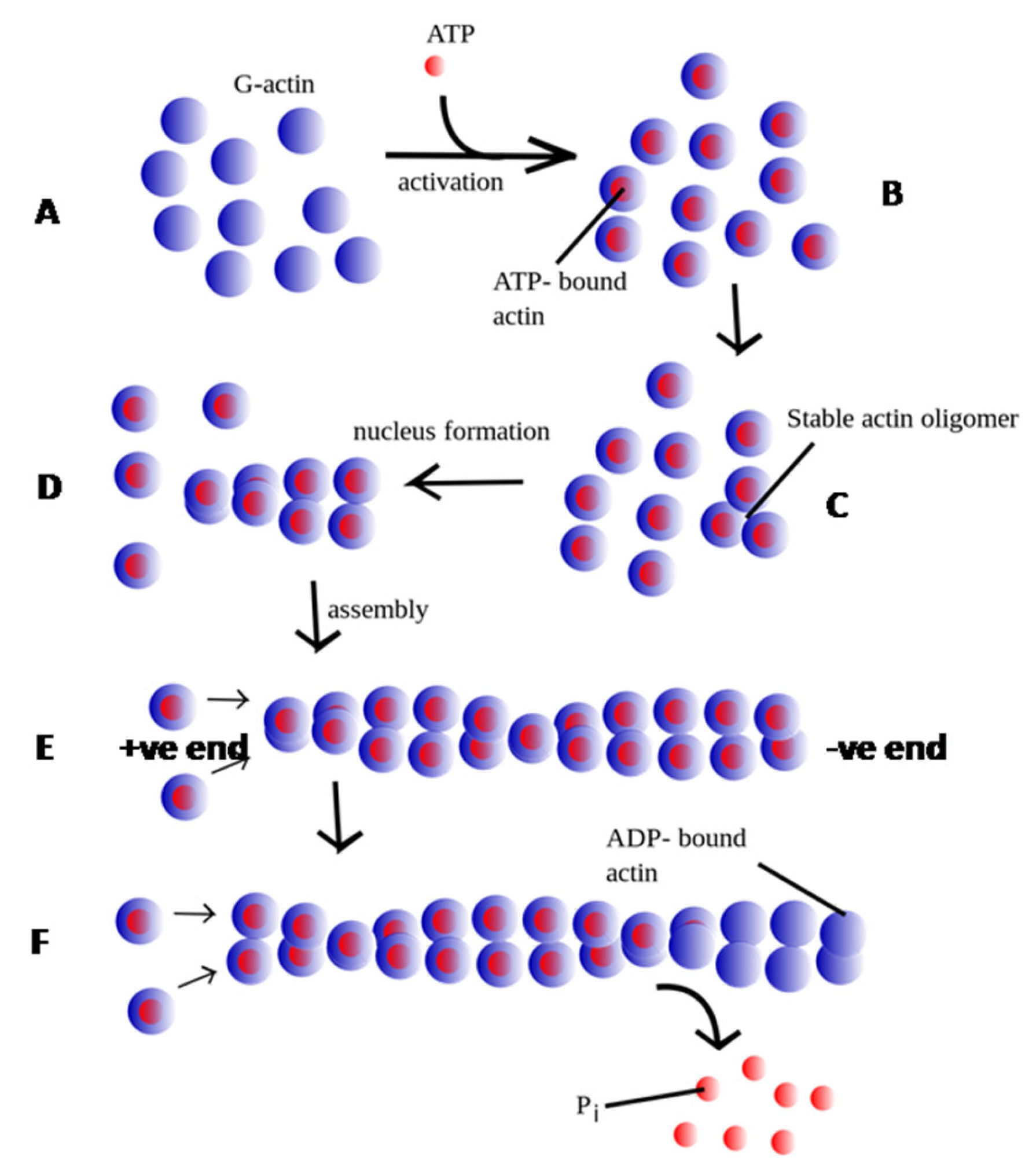
1. Introduction
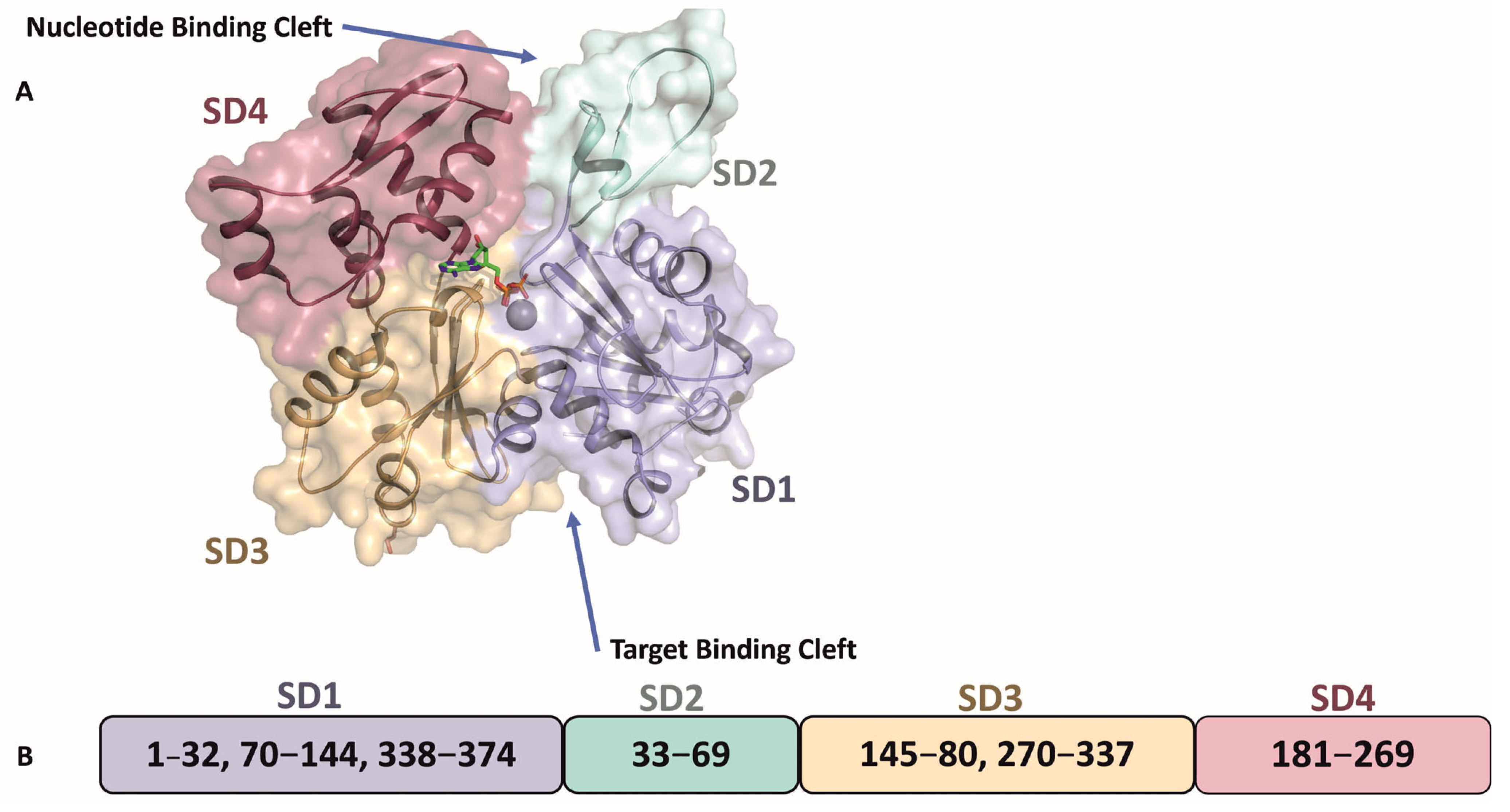
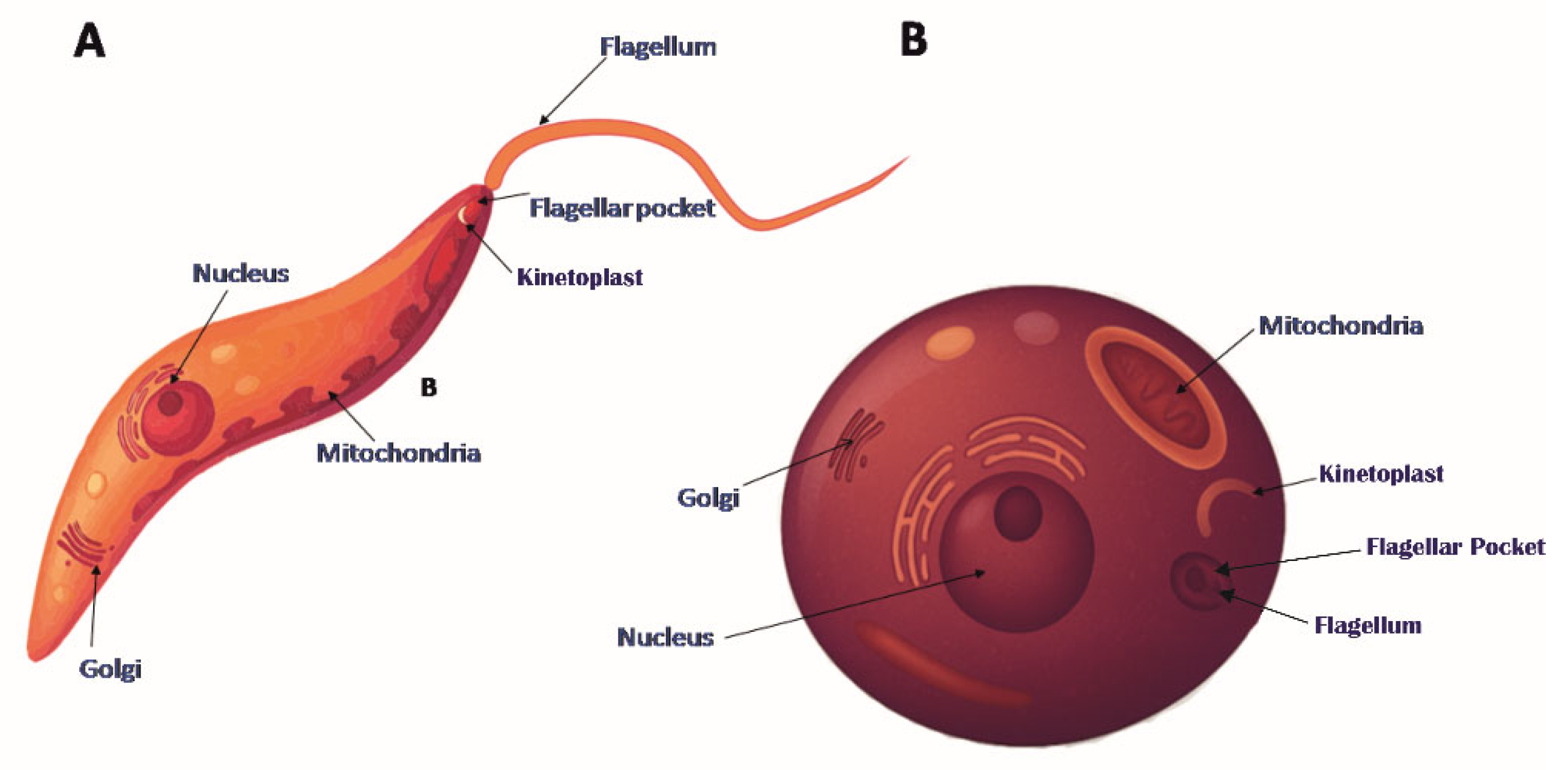
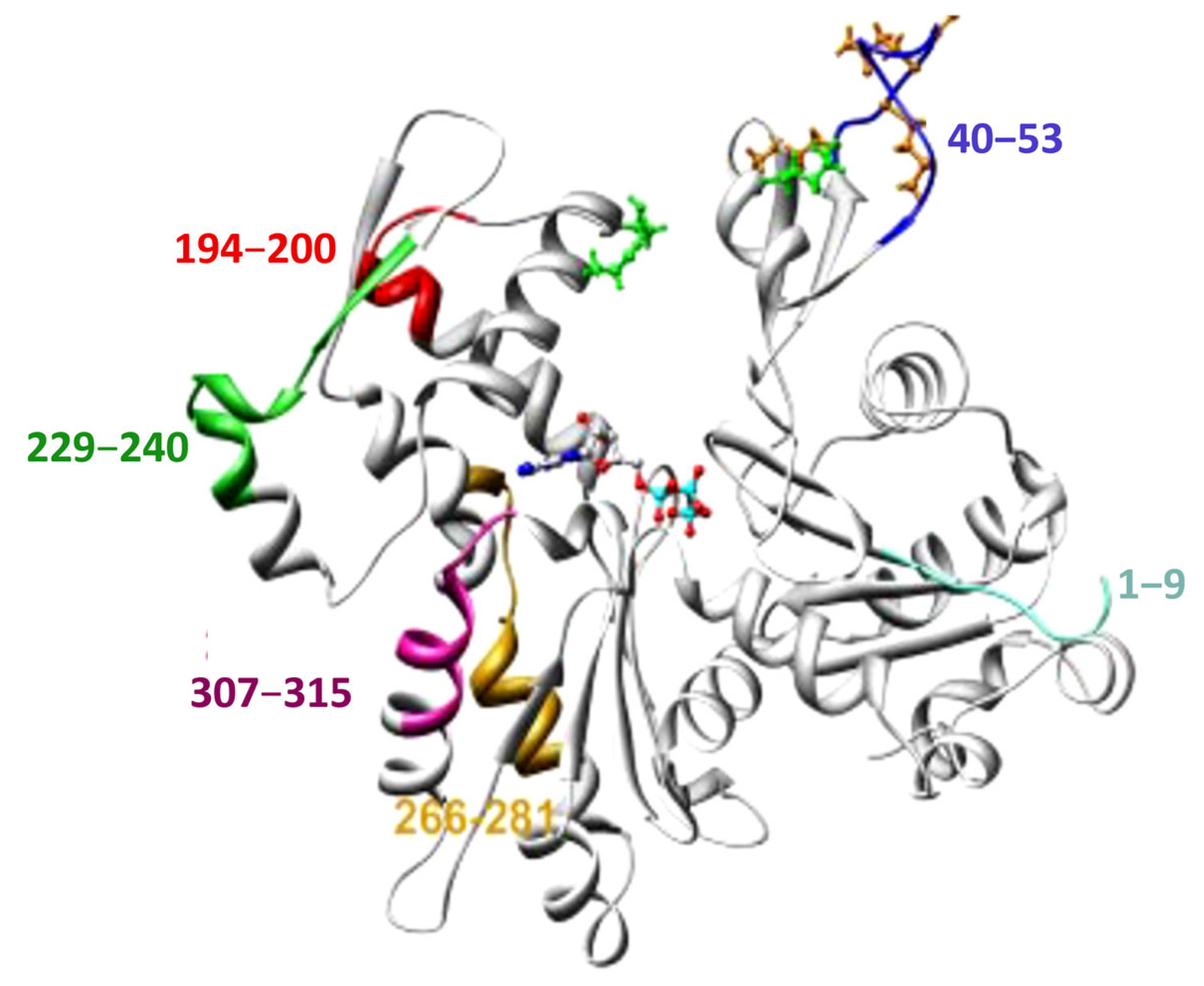
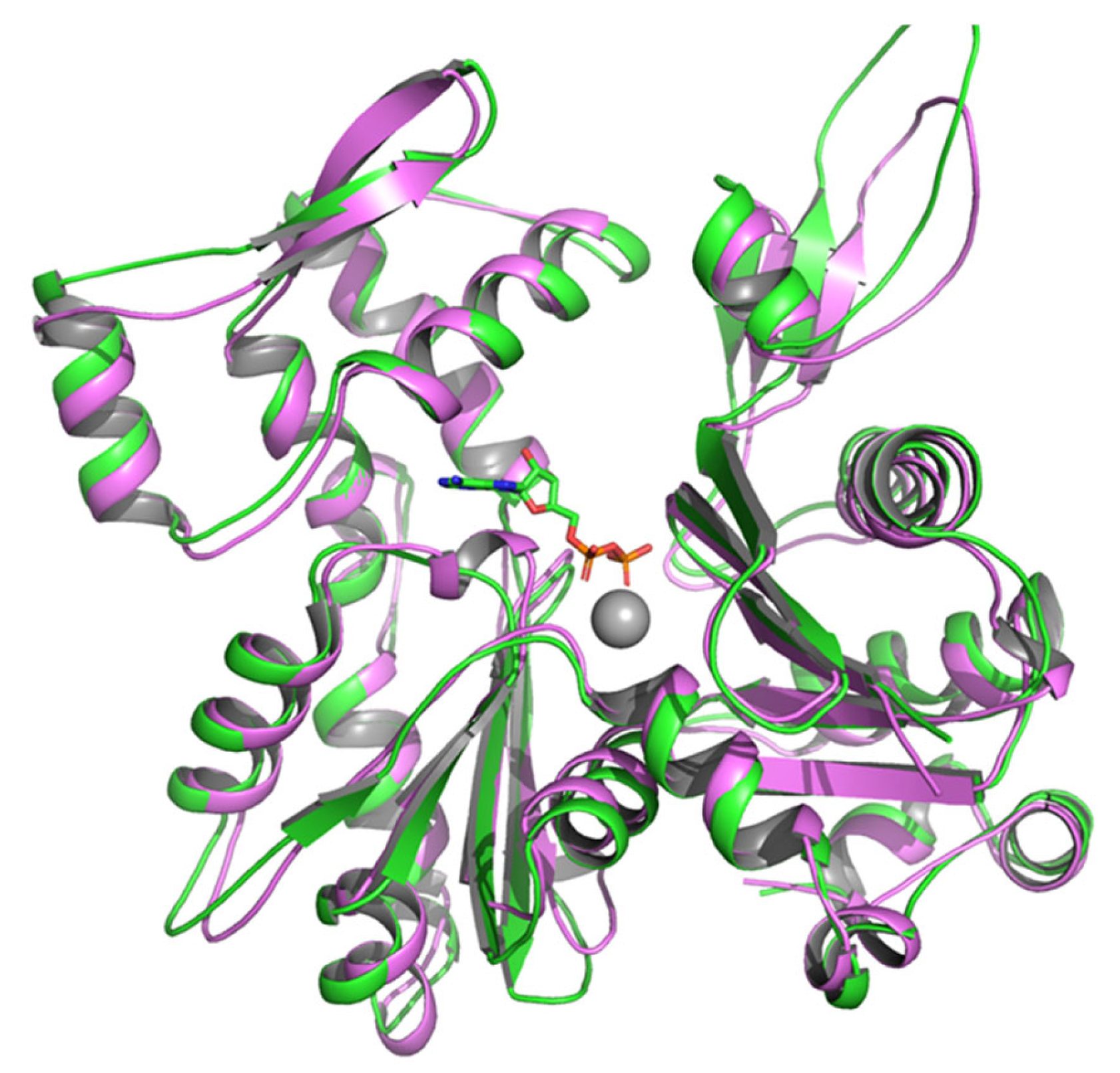
2. Leishmania Actin
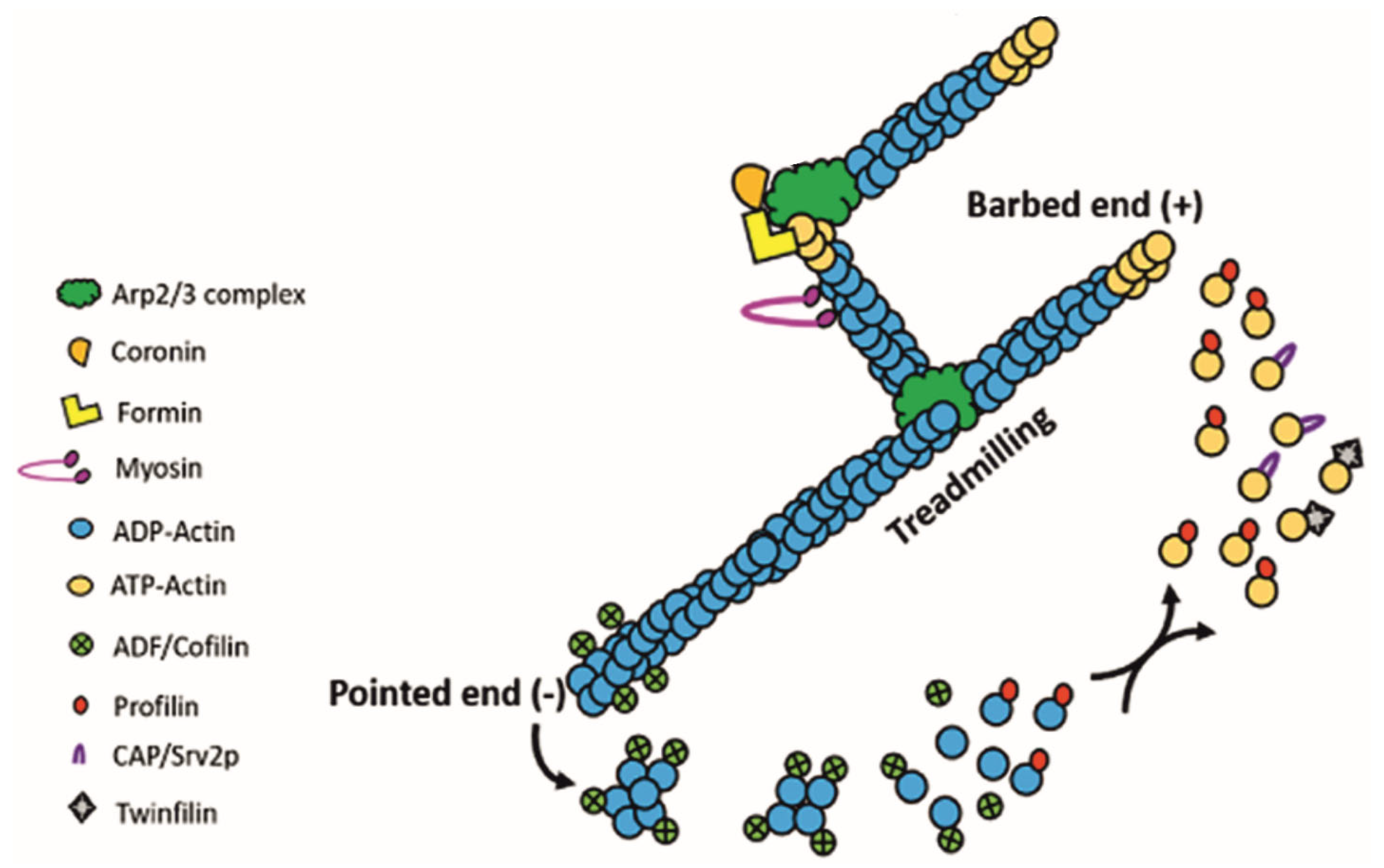
3. Leishmania Actin-Binding Proteins (ABPs)
3.1. The Arp2/3 Complex
3.2. Formins
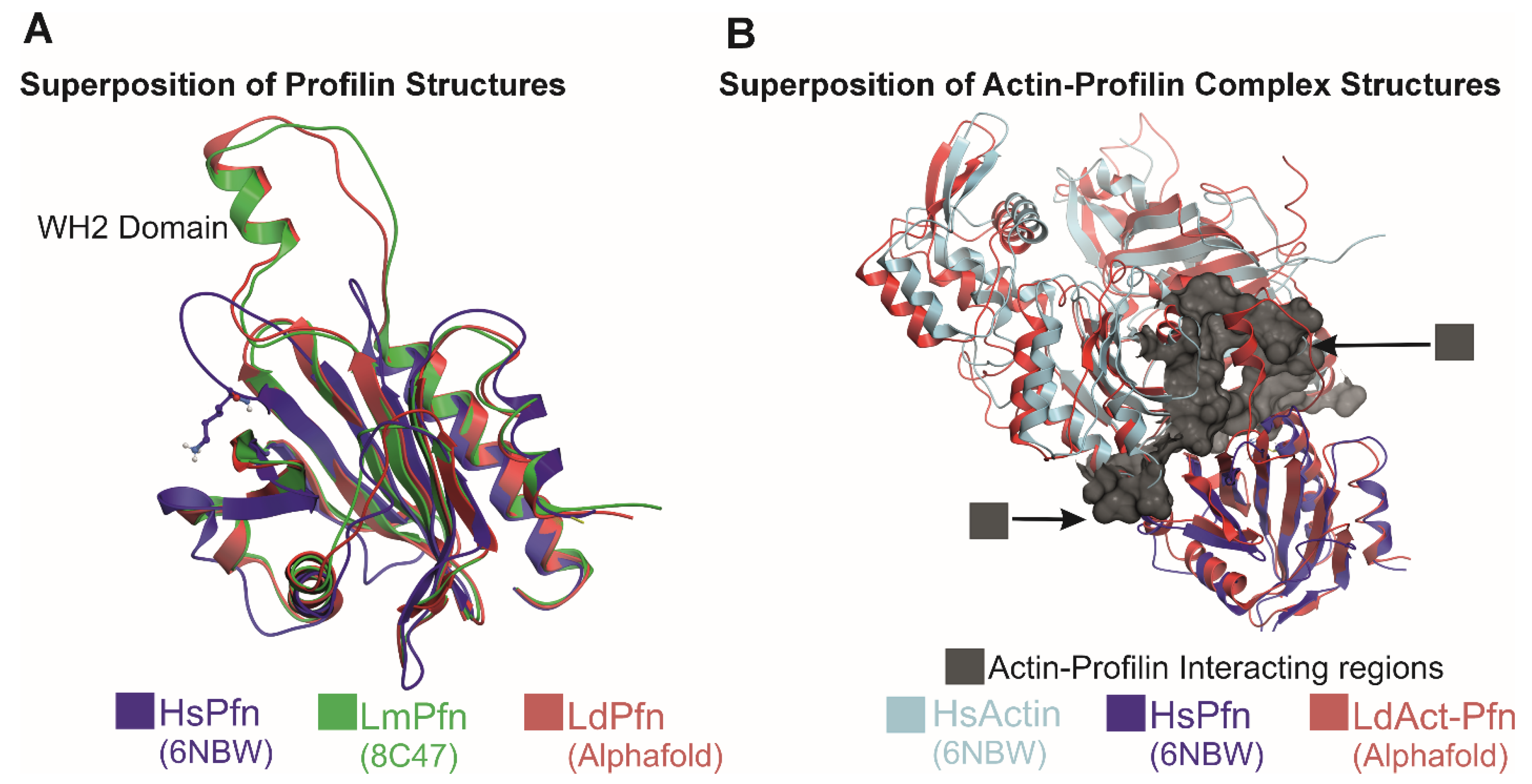
3.3. Profilin
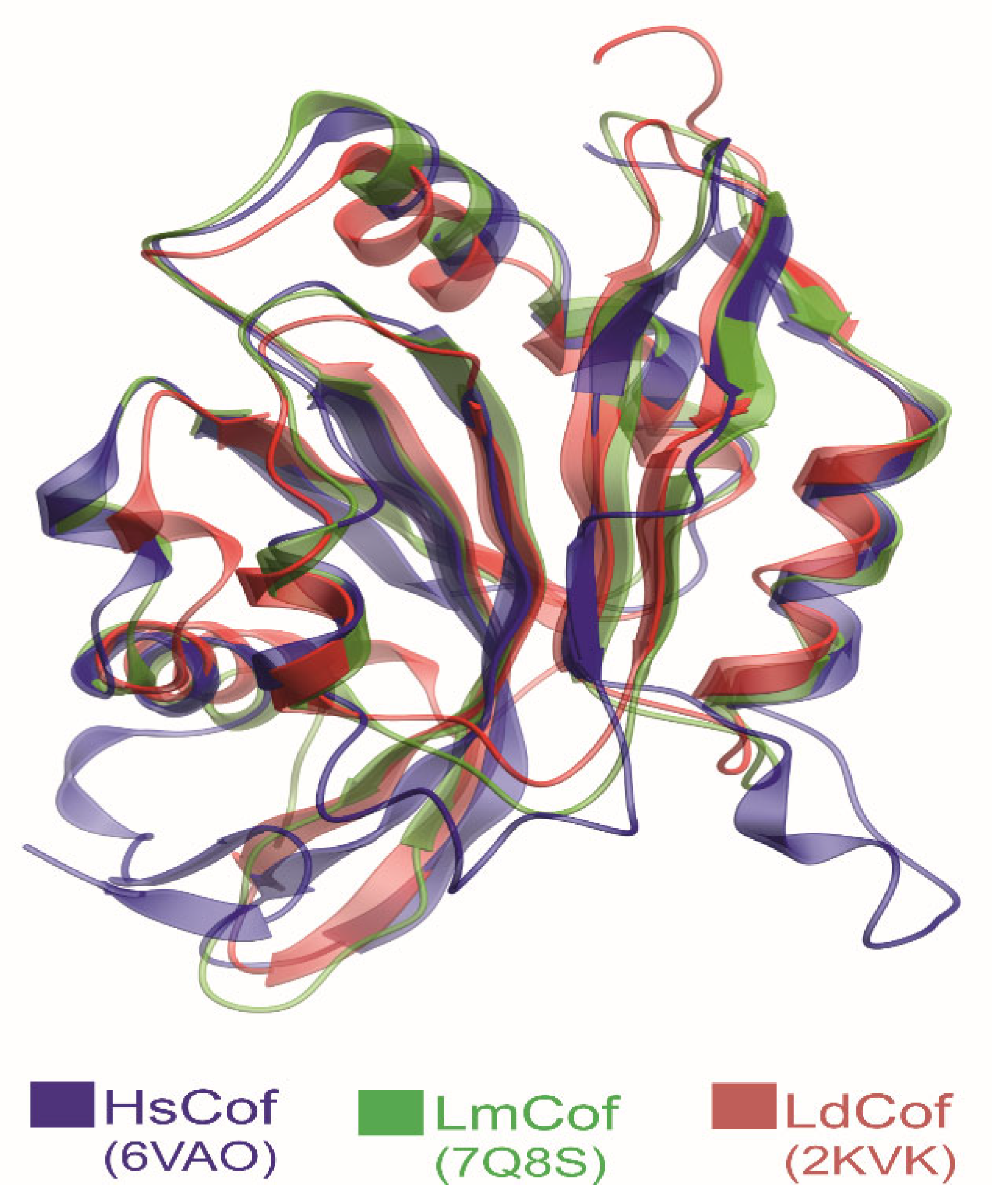
3.4. ADF/Cofilin
3.5. Coronin
3.6. Twinfilin
3.7. Cyclase-Associated Protein (CAP)
3.8. Myosins
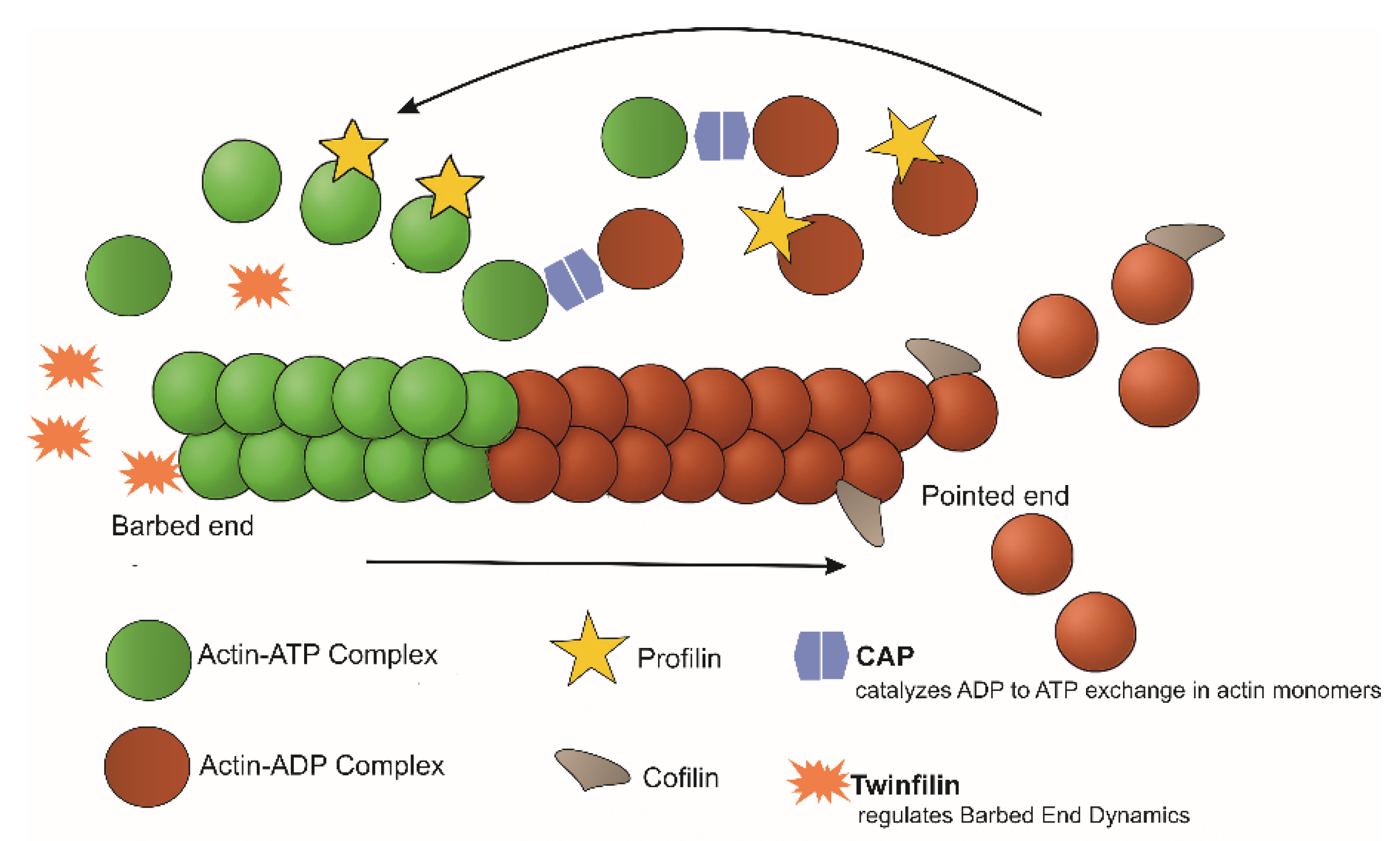
4. Regulation of Actin Dynamics in Leishmania
5. Potential Roles of Actin and Actin-Binding Proteins During Differentiation and Development of Leishmania Parasites
6. Leishmania Actin and Actin-Binding Proteins as Potential Drug Targets
7. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Velle, K.B.; Swafford, A.J.M.; Garner, E.; Fritz-Laylin, L.K. Actin network evolution as a key driver of eukaryotic diversification. J. Cell Sci. 2024, 137, jcs261660. [Google Scholar] [CrossRef]
- Pollard, T.D.; Borisy, G.G. Cellular motility driven by assembly and disassembly of actin filaments. Cell 2003, 112, 453–465. [Google Scholar] [CrossRef]
- Pollard, T.D. Nine unanswered questions about cytokinesis. J. Cell Biol. 2008, 216, 3007–3016. [Google Scholar] [CrossRef] [PubMed]
- Khaitlina, S.Y. Intracellular transport based on actin polymerization. Biochemistry 2014, 79, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Skruzny, M. The endocytic protein machinery as an actin-driven membrane-remodeling machine. Eur. J. Cell Biol. 2022, 101, 151267. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.S.; Chakrabarti, R.; Higgs, H.N. The multiple links between actin and mitochondria. Nat. Rev. Mol. Cell Biol. 2023, 24, 651–667. [Google Scholar] [CrossRef] [PubMed]
- Rambold, R.S.; Lippincott-Schwartz, J. Mechanisms of mitochondria and autophagy crosstalk. Cell Cycle 2011, 10, 4032–4038. [Google Scholar] [CrossRef]
- Caridi, C.P.; D’Agostino, C.; Ryu, T.; Zapotoczny, G.; Delabaere, L.; Li, X.; Khodaverdian, V.Y.; Amaral, N.; Lin, E.; Rau, A.R.; et al. Nuclear F-actin and myosins drive relocalization of heterochromatic breaks. Nature 2018, 559, 54–60. [Google Scholar] [CrossRef]
- Baarlink, C.; Plessner, M.; Sherrard, A.; Morita, K.; Misu, S.; Virant, D.; Kleinschnitz, E.-M.; Harniman, R.; Alibhai, D.; Baumeister, S.; et al. A transient pool of nuclear F-actin at mitotic exit controls chromatin organization. Nat. Cell Biol. 2017, 19, 1389–1399. [Google Scholar] [CrossRef]
- Yoo, Y.; Wu, X.; Guan, J.-L. A novel role of the actin-nucleating Arp2/3 complex in the regulation of RNA polymerase II-dependent transcription. J. Biol. Chem. 2007, 282, 7616–7623. [Google Scholar] [CrossRef]
- Merino, F.; Pospich, S.; Raunser, S. Towards a structural understanding of the remodeling of the actin cytoskeleton. Semin. Cell Dev. Biol. 2020, 102, 51–64. [Google Scholar] [CrossRef] [PubMed]
- dos Remedios, C.G.; Chhabra, D.; Kekic, M.; Dedova, I.V.; Tsubakihara, M.; Berry, D.A.; Nosworthy, N.J. Actin binding proteins: Regulation of cytoskeletal microfilaments. Physiol. Rev. 2003, 83, 433–473. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D. Actin and actin-binding proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a018226. [Google Scholar] [CrossRef] [PubMed]
- Mackay, D.J.; Hall, A. Rho GTPases. J. Biol. Chem. 1998, 273, 20685–20688. [Google Scholar] [CrossRef]
- Heimsath, E.G.; Higgs, H.N. The C-terminus of formin FMNL3 accelerates actin polymerization and contains a WH2 domain-like sequence that binds both monomers and filament barbed ends. J. Biol. Chem. 2012, 287, 3087–3098. [Google Scholar] [CrossRef]
- Mullins, R.D.; Heuser, J.A.; Pollard, T.D. The interaction of Arp2/3 complex with actin: Nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc. Natl. Acad. Sci. USA 1998, 95, 6181–6186. [Google Scholar] [CrossRef]
- Carlier, M.F.; Laurent, V.; Santolini, J.; Melki, R.; Didry, D.; Xia, G.X.; Hong, Y.; Chua, N.H.; Pantaloni, D. Actin depolymerizing factor (ADF/cofilin) enhances the rate of filament turnover: Implication in actin-based motility. J. Cell Biol. 1997, 136, 1307–1322. [Google Scholar] [CrossRef]
- Haggstrom, M. Medical gallery of mikael haggstrom 2014. WikiJournal Med. 2014, 1, 1–53. [Google Scholar] [CrossRef]
- Gupta, C.M.; Ambaru, B.; Bajaj, R. Emerging functions of actin and actin-binding proteins in trypanosomatids. Front. Cell Dev. Biol. 2020, 8, 587685. [Google Scholar] [CrossRef]
- Kabsch, W.; Mannherz, H.G.; Suck, D.; Pai, E.F.; Holmes, K.C. Atomic structure of the actin: DNase I complex. Nature 1990, 347, 37–44. [Google Scholar] [CrossRef]
- Dominguez, R.; Holmes, K.C. Actin structure and function. Annu. Rev. Biophys. 2011, 40, 169–186. [Google Scholar] [CrossRef]
- Drouin, G.; Moniz de Sá, M.; Zuker, M. The Giardia lamblia actin gene and the phylogeny of eukaryotes. J. Mol. Evol. 1995, 41, 841–849. [Google Scholar] [CrossRef]
- Paredez, A.R.; Assaf, Z.J.; Sept, D.; Timofejeva, L.; Dawson, S.C.; Wang, C.-J.R.; Cande, W.Z. An actin cytoskeleton with evolutionarily conserved functions in the absence of canonical actin-binding proteins. Proc. Natl. Acad. Sci. USA 2011, 108, 6151–6156. [Google Scholar] [CrossRef]
- Gupta, C.M.; Thiyagarajan, S.; Sahasrabuddhe, A.A. Unconventional actins and actin-binding proteins in human protozoan parasites. Int. J. Parasitol. 2015, 45, 435–447. [Google Scholar] [CrossRef]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; den Boer, M.; WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- Gossage, S.M.; Rogers, M.E.; Bates, P.A. Two separate growth phases during the development of Leishmania in sand flies: Implications for understanding the life cycle. Int. J. Parasitol. 2003, 33, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Mortara, R.A. Studies on trypanosomatid actin. I. Immunochemical and biochemical identification. J. Protozool. 1989, 36, 8–13. [Google Scholar] [CrossRef]
- Gull, K. The cytoskeleton of trypanosomatid parasites. Ann. Rev. Microbiol. 1999, 53, 629–655. [Google Scholar] [CrossRef]
- Ivens, A.C.; Peacock, C.S.; Worthey, E.A.; Murphy, L.; Aggarwal, G.; Berriman, M.; Sisk, E.; Rajandream, M.-A.; Adlem, E.; Aert, R.; et al. The genome of the kinetoplastid parasite Leishmania major. Science 2005, 309, 436–442. [Google Scholar] [CrossRef]
- Nomura, K.; Hayakawa, K.; Tatsumi, H.; Ono, S.J. Actin-interacting protein 1 promotes disassembly of actin-depolymerizing factor/cofilin-bound actin filaments in a pH-dependent manner. J. Biol. Chem. 2016, 291, 5146–5156. [Google Scholar] [CrossRef]
- Xu, J.; Casella, J.F.; Pollard, T.D. Effect of capping protein (CapZ) on the length of actin filaments and mechanical properties of actin filament networks. Cell Motil. Cytoskelet. 1999, 42, 73–81. [Google Scholar] [CrossRef]
- Sahasrabuddhe, A.A.; Bajpai, V.K.; Gupta, C.M. A novel form of actin in Leishmania: Molecular characterisation, subcellular localisation and association with subpellicular microtubules. Mol. Biochem. Parasitol. 2004, 134, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, P.; Sahasrabuddhe, A.A.; Kumar, A.; Mitra, K.; Siddiqi, M.I.; Gupta, C.M. An unconventional form of actin in protozoan hemoflagellate, Leishmania. J. Biol. Chem. 2008, 283, 22760–22773. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, P.; Kumar, A.; Naik, R.; Ganguli, M.; Siddiqi, M.I.; Sahasrabuddhe, A.A.; Gupta, C.M. Leishmania actin binds and nicks kDNA as well as inhibits decatenation activity of type II topoisomerase. Nucleic Acids Res. 2010, 38, 3308–3317. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Y.; Motyka, S.A.; Agbo, E.E.C.; Englund, P.T. Fellowship of the rings: The replication of kinetoplast DNA. Trends Parasitol. 2005, 21, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Kotila, T.; Wioland, H.; Selvaraj, M.; Kogan, K.; Antenucci, L.; Jégou, A.; Huiskonen, J.T.; Romet-Lemonne, G.; Lappalainen, P. Structural basis of rapid actin dynamics in the evolutionarily divergent Leishmania parasite. Nat. Commun. 2022, 13, 3442. [Google Scholar] [CrossRef]
- Pollard, T.D.; Beltzner, C.C. Structure and function of the Arp2/3 complex. Curr. Opin. Struct. Biol. 2002, 12, 768–774. [Google Scholar] [CrossRef]
- Pizarro-Cerdá, J.; Chorev, D.S.; Geiger, B.; Cossar, P. The diverse family of Arp2/3 complexes. Trends Cell Biol. 2017, 27, 93–100. [Google Scholar] [CrossRef]
- Diniz, M.C.; Costa, M.P.; Pacheco, A.C.N.; Kamimura, M.T.; Silva, S.C.; Carneiro, L.D.; Sousa, A.P.; Soares, C.E.; Souza, C.S.; de Oliveira, D.M. Actin-interacting and flagellar proteins in Leishmania spp.: Bioinformatics predictions to functional assignments in phagosome formation. Genet. Mol. Biol. 2009, 32, 652–665. [Google Scholar] [CrossRef]
- Vizcaíno-Castillo, A.; Kotila, T.; Kogan, K.; Yanase, R.; Michelot, A.; Sunter, J.D.; Lappalainen, P. Leishmania profilin interacts with actin through an unusual structural mechanism to control cytoskeletal dynamics in parasites. J. Biol. Chem. 2024, 300, 10574. [Google Scholar] [CrossRef]
- Kurisu, S.; Takenawa, T. The WASP and WAVE family proteins. Genome Biol. 2009, 10, 226. [Google Scholar] [CrossRef]
- Liu, S.L.; Needham, K.M.; May, J.R.; Nolen, B.J. Mechanism of a concentration-dependent switch between activation and inhibition of Arp2/3 complex by coronin. J. Biol. Chem. 2011, 286, 17039–17046. [Google Scholar] [CrossRef] [PubMed]
- Humphries, C.L.; Balcer, H.I.; D’Agostino, J.L.; Winsor, B.; Drubin, D.G.; Barnes, G.; Andrews, B.J.; Goode, B.L. Direct regulation of Arp2/3 complex activity and function by the actin binding protein coronin. J. Cell Biol. 2002, 159, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Goode, B.L.; Eck, M.J. Mechanism and function of formins in the control of actin assembly. Annu. Rev. Biochem. 2007, 76, 593–627. [Google Scholar] [CrossRef] [PubMed]
- Otomo, T.; Tomchick, D.R.; Otomo, C.; Panchal, S.C.; Machius, M.; Rosen, M.K. Structural basis of actin filament nucleation and processive capping by a formin homology 2 domain. Nature 2005, 433, 488–494. [Google Scholar] [CrossRef]
- Schutt, C.E.; Karlen, M.; Karlsson, R. A structural model of the profilin-formin pacemaker system for actin filament elongation. Sci. Rep. 2022, 12, 20515. [Google Scholar] [CrossRef]
- Mahanta, B.; Courtemanche, N. The mode of subunit addition regulates the processive elongation of actin filaments by formin. J. Biol. Chem. 2025, 301, 108071. [Google Scholar] [CrossRef]
- Kushwaha, R.; Seth, A.; Jijumon, A.S.; Reshmi, P.B.; Dileep, B.; Datta, R.; Maiti, S. Formins play important role in Leishmania physiology by acting as cytosolic actin bundlers. bioRxiv 2021. [Google Scholar] [CrossRef]
- Krishnan, K.; Moens, P.D.J. Structure and functions of profilins. Biophys. Rev. 2009, 1, 71–81. [Google Scholar] [CrossRef]
- Lappalainen, P.; Kotila, T.; Jégou, A.; Romet-Lemonne, G. Biochemical and mechanical regulation of actin dynamics. Nature Rev. Mol. Cell Biol. 2022, 23, 836–852. [Google Scholar] [CrossRef]
- Eads, J.C.; Mahoney, N.M.; Vorobiev, S.; Bresnick, A.R.; Wen, K.K.; Rubenstein, P.A.; Haarer, B.K.; Almo, S.C. Structure determination and characterization of Saccharomyces cerevisiae profilin. Biochemistry 1998, 37, 11171–11181. [Google Scholar] [CrossRef]
- Ambaru, B.; Gopalsamy, A.; Tammana, T.V.S.; Subramanya, H.S.; Gupta, C.M. Actin Sequestering Protein, Profilin, Regulates Intracellular Vesicle Transport in Leishmania. Mol. Biochem. Parasitol. 2020, 238, 111280. [Google Scholar] [CrossRef]
- Paunola, E.; Mattila, P.K.; Lappalainen, P. WH2 Domain: A Small, Versatile Adapter for Actin Monomers. FEBS Lett. 2002, 513, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Costa, R.; Sousa, M.M. Profilin as a dual regulator of actin and microtubule dynamics. Cytoskeleton 2020, 77, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Ambaru, B.; Gangadharan, G.M.; Subramanya, H.S.; Gupta, C.M. Profilin Is Involved in G1 to S Phase Progression and Mitotic Spindle Orientation during Leishmania donovani Cell Division Cycle. PLoS ONE 2022, 17, e0265692. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.; Rai, P.K.; Kumar, S.H.; Thiyagarajan, S.; Gupta, C.M. Loss of Direct Binding of Leishmania Profilin with Actin Adversely Affected Its Functions and Interactions with Other Cellular Proteins. bioRxiv 2025. [Google Scholar] [CrossRef]
- Pusnik, M.; Charrie’re, F.; Ma, P.; Waller, R.F.; Dagley, M.J.; Lithgow, T.; Schneider, A. The single mitochondrial porin of Trypanosoma brucei is the main metabolite transporter in the outer mitochondrial membrane. Mol. Biol. Evol. 2009, 26, 671–680. [Google Scholar] [CrossRef]
- Dhalia, R.; Reis, C.R.S.; Freire, E.R.; Rocha, P.O.; Katz, R.; Muniz, J.R.C.; Standart, N.; Neto, O.P.d.M. Translation initiation in Leish mania major: Characterization of multiple eIF4F subunit homologues. Mol. Biochem. Parasit. 2005, 140, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Silva-Almeida, M.; Pereira, B.A.S.; Ribeiro-Guimarães, M.P.; Alves, C.R. Proteinases as Virulence Factors in Leishmania spp. Infection in Mammals. Parasites Vectors 2012, 5, 160. [Google Scholar] [CrossRef]
- Siqueira-Neto, J.L.; Debnath, A.; McCall, L.-I.; Bernatchez, J.A.; Ndao, M.; Reed, S.L.; Rosenthal, P.J.; Sinnis, P. Cysteine proteases in protozoan parasites. PLoS Negl. Trop. Dis. 2018, 12, e0006512. [Google Scholar] [CrossRef]
- Dubessay, P.; Blaineau, C.; Bastien, P.; Tasse, L.; Van Dijk, J.; Crobu, L.; Pagès, M. Cell cycle-dependent expression regulation by the proteasome pathway and characterization of the nuclear targeting signal of a Leishmania major Kin-13 kinesin. Mol. Microbiol. 2006, 59, 1162–1174. [Google Scholar] [CrossRef]
- Carlier, M.-F.; Ressad, F.; Pantaloni, D. Control of actin dynamics in cell motility—Role of ADF/cofilin. J. Biol. Chem. 1999, 274, 33827–33830. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, B.W.; Bamburg, J.R. ADF/Cofilin: A functional node in cell biology. Trends Cell Biol. 2010, 20, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Kanellos, G.; Frame, M.C. Cellular functions of the ADF/cofilin family at a glance. J. Cell Sci. 2016, 129, 3211–3218. [Google Scholar] [CrossRef]
- Wioland, H.; Guichard, B.; Senju, Y.; Myram, S.; Lappalainen, P.; Jégou, A.; Romet-Lemonne, G. ADF/Cofilin accelerates actin dynamics by severing filaments and promoting their depolymerization at both ends. Curr. Biol. 2017, 27, 1956–1967.e7. [Google Scholar] [CrossRef]
- Tanaka, K.; Takeda, S.; Mitsuoka, K.; Oda, T.; Kimura-Sakiyama, C.; Maéda, Y.; Narita, A. Structural basis for cofilin binding and actin filament disassembly. Nat. Commun. 2018, 9, 1860. [Google Scholar] [CrossRef]
- Moon, A.; Drubin, D.G. The ADF/cofilin proteins: Stimulus-responsive modulators of actin dynamics. Mol. Biol. Cell 1995, 6, 1423–1431. [Google Scholar] [CrossRef]
- Mizuno, K. Signaling mechanisms and functional roles of cofilin phosphorylation and dephosphorylation. Cell Signal. 2013, 25, 457–469. [Google Scholar] [CrossRef]
- Zhao, H.; Hakala, M.; Lappalainen, P. ADF/cofilin binds phosphoinositides in a multivalent manner to act as a PIP2-density sensor. Biophys. J. 2010, 98, 2327–2336. [Google Scholar] [CrossRef]
- Didry, D.; Carlier, M.-F.; Pantaloni, D. Synergy between actin depolymerizing factor/cofilin and profilin in increasing actin filament turnover. J. Biol. Chem. 1998, 273, 25602–25611. [Google Scholar] [CrossRef]
- Mohri, K.; Ono, K.; Yu, R.; Yamashiro, S.; Ono, S. Enhancement of actin-depolymerizing factor/cofilin-dependent actin disassembly by actin-interacting protein 1 is required for organized actin filament assembly in the Caenorhabditis elegans body wall muscle. Mol. Biol. Cell 2006, 17, 2190–2199. [Google Scholar] [CrossRef]
- Ono, S.; Ono, K. Tropomyosin inhibits ADF/cofilin-dependent actin filament dynamics. J. Cell Biol. 2002, 156, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Tammana, T.V.S.; Sahasrabuddhe, A.A.; Mitra, K.; Bajpai, V.K.; Gupta, C.M. Actin-depolymerizing factor, ADF/cofilin, is essentially required in assembly of Leishmania flagellum. Mol. Microbiol. 2008, 70, 837–852. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Srivastava, R.; Mitra, K.; Sahasrabuddhe, A.A.; Gupta, C.M. Overexpression of S4D mutant of Leishmania donovani ADF/cofilin impairs flagellum assembly by affecting actin dynamics. Eukaryot. Cell 2012, 11, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Morgan, G.V.; Hall, B.S.; Denny, P.W.; Carrington, M.; Field, M.C. The kinetoplastida endocytic apparatus. Part I: A dynamic system for nutrition and evasion of host defences. Trends Parasitol. 2002, 18, 491–496. [Google Scholar] [CrossRef]
- Morgan, G.W.; Hall, B.S.; Denny, P.W.; Field, M.C.; Carrington, M. The endocytic apparatus of the kinetoplastida. Part II: Machinery and components of the system. Trends Parasitol. 2002, 18, 540–546. [Google Scholar] [CrossRef]
- Pathak, P.P.; Pulavarti, S.V.S.R.K.; Jain, A.; Sahasrabuddhe, A.A.; Gupta, C.M.; Arora, A. Solution structure and dynamics of ADF/cofilin from Leishmania donovani. J. Struct. Biol. 2010, 172, 219–224. [Google Scholar] [CrossRef]
- Tammana, T.V.S.; Sahasrabuddhe, A.A.; Bajpai, V.K.; Gupta, C.M. ADF/cofilin-driven actin dynamics in early events of Leishmania cell division. J. Cell Sci. 2010, 123, 1894–1901. [Google Scholar] [CrossRef]
- de Hostos, E.L. The coronin family of actin-associated proteins. Trends Cell Biol. 1999, 9, 345–350. [Google Scholar] [CrossRef]
- Uetrecht, A.C.; Bear, J.E. Coronins: The return of the crown. Trends Cell Biol. 2006, 16, 421–426. [Google Scholar] [CrossRef]
- Keefe, T.; Chan, K.; Creed, S.J.; Bear, J.E. Unraveling the enigma: Progress towards understanding the coronin family of actin regulators. Trends Cell Biol. 2011, 21, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Marshall, T.W.; Uetrecht, A.C.; Schafer, D.A.; Bear, J.E. Coronin 1B coordinates Arp2/3 complex and cofilin activities at the leading edge. Cell 2007, 128, 915–929. [Google Scholar] [CrossRef] [PubMed]
- Xavier, C.P.; Rastetter, R.; Blömacher, M.; Stumpf, M.; Himmel, M.; Morgan, R.O.; Fernandez, M.-P.; Wang, C.; Osman, A.; Miyata, Y.; et al. Phosphorylation of CRN2 by CK2 regulates F-actin and Arp2/3 interaction and inhibits cell migration. Sci. Rep. 2012, 2, 241. [Google Scholar] [CrossRef] [PubMed]
- Goode, B.L.; Wong, J.J.; Butty, A.-C.; Peter, M.; McCormack, A.L.; Yates, J.R.; Drubin, D.G.; Barnes, G. Coronin promotes the rapid assembly and cross-linking of actin filaments and may link the actin and microtubule cytoskeletons in yeast. J. Cell Biol. 1999, 144, 83–98. [Google Scholar] [CrossRef]
- Cai, L.; Holoweckyj, N.; Schaller, M.D.; Bear, J.E. Phosphorylation of coronin 1B by protein kinase C regulates interaction with Arp2/3 and cell motility. J. Biol. Chem. 2005, 280, 31913–31923. [Google Scholar] [CrossRef]
- Nayak, R.C.; Sahasrabuddhe, A.A.; Bajpai, V.K.; Gupta, C.M. A novel homologue of coronin colocalizes with actin in filament-like structures in Leishmania. Mol. Biochem. Parasitol. 2005, 143, 152–164. [Google Scholar] [CrossRef]
- Srivastava, V.K.; Rana, A.K.; Sahasrabuddhe, A.A.; Gupta, C.M.; Pratap, J.V. Cloning, overexpression, purification and crystallization of the CRN12 coiled-coil domain from Leishmania donovani. Acta Crystallogr. F Struct. Biol. Commun. 2013, 69, 535–539. [Google Scholar] [CrossRef]
- Nayak, A.R.; Karade, S.S.; Srivastava, V.K.; Rana, A.K.; Gupta, C.M.; Sahasrabuddhe, A.A.; Pratap, J. Structure of Leishmania donovani coronin coiled coil domain reveals an antiparallel 4 helix bundle with inherent asymmetry. J. Struct. Biol. 2016, 195, 129–138. [Google Scholar] [CrossRef]
- Srivastava, R.; Kajuluri, L.P.; Pathak, N.; Gupta, C.M.; Sahasrabuddhe, A.A. Oligomerization of coronin: Implication on actin filament length in Leishmania. Cytoskeleton 2015, 72, 621–632. [Google Scholar] [CrossRef]
- Sahasrabuddhe, A.A.; Nayak, R.C.; Gupta, C.M. Ancient Leishmania coronin (CRN12) is involved in microtubule remodeling during cytokinesis. J. Cell Sci. 2009, 122, 1691–1699. [Google Scholar] [CrossRef]
- Ojala, P.J.; Paavilainen, V.O.; Vartiainen, M.K.; Tuma, R.; Weeds, A.G.; Lappalainen, P. The two ADF-H domains of twinfilin play functionally distinct roles in interactions with actin monomers. Mol. Biol. Cell 2002, 13, 3811–3821. [Google Scholar] [CrossRef] [PubMed]
- Helfer, E.; Nevalainen, E.M.; Naumanen, P.; Romero, S.; Didry, D.; Pantaloni, D.; Lappalainen, P.; Carlier, M.-F. Mammalian twinfilin sequesters ADP-G-actin and caps filament barbed ends: Implications in motility. EMBO J. 2006, 25, 1184–1195. [Google Scholar] [CrossRef] [PubMed]
- Goode, B.L.; Drubin, D.G.; Lappalainen, P. Regulation of the cortical actin cytoskeleton in budding yeast by twinfilin, a ubiquitous actin monomer-sequestering protein. J. Cell Biol. 1998, 142, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, S.; Hoeprich, G.J.; Gelles, J.; Goode, B.L. Twinfilin bypasses assembly conditions and actin filament aging to drive barbed end depolymerization. J. Cell Biol. 2021, 220, e202006022. [Google Scholar] [CrossRef]
- Hakala, M.; Wioland, H.; Tolonen, M.; Kotila, T.; Jégou, A.; Romet-Lemonne, G.; Lappalainen, P. Twinfilin uncaps filament barbed ends to promote turnover of lamellipodial actin networks. Nat. Cell Biol. 2021, 23, 147–159. [Google Scholar] [CrossRef]
- Reddy, V.; Arya, A.; Shekhar, S. Twinfilin is a nonprocessive depolymerase which synergizes with formin to dramatically accelerate actin filament uncapping by 300-fold. Proc. Natl. Acad. Sci. USA 2025, 122, e2501078122. [Google Scholar] [CrossRef]
- Kumar, G.; Kajuluri, L.P.; Gupta, C.M.; Sahasrabuddhe, A.A. A twinfilin-like protein coordinates karyokinesis by influencing mitotic spindle elongation and DNA replication in Leishmania. Mol. Microbiol. 2016, 100, 173–187. [Google Scholar] [CrossRef]
- Rust, M.B.; Khudayberdiev, S.; Pelucchi, S.; Marcello, E. CAPt’n of actin dynamics: Recent advances in the molecular, developmental and physiological functions of cyclase-associated protein (CAP). Front. Cell Dev. Biol. 2020, 8, 586631. [Google Scholar] [CrossRef]
- Dodatko, T.; Fedorov, A.A.; Grynberg, M.; Patskovsky, Y.; Rozwarski, D.A.; Jaroszewski, L.; Aronoff-Spencer, E.; Kondraskina, E.; Irving, T.; Godzik, A.; et al. Crystal structure of the actin binding domain of the cyclase-associated protein. Biochemistry 2004, 43, 10628–10641. [Google Scholar] [CrossRef]
- Shekhar, S.; Chung, J.; Kondev, J.; Gelles, J.; Goode, B.L. Synergy between cyclase-associated protein and cofilin accelerates actin filament depolymerization by two orders of magnitude. Nat. Commun. 2019, 10, 53. [Google Scholar] [CrossRef]
- Alimov, N.; Hoeprich, G.J.; Padrick, S.B.; Goode, B.L. Cyclase-associated protein interacts with actin filament barbed ends to promote depolymerization and formin displacement. J. Biol. Chem. 2023, 299, 105367. [Google Scholar] [CrossRef] [PubMed]
- Sellers, J.R. Myosins: A diverse superfamily. Biochim. Biophys. Acta 2000, 1496, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, D.V.; Nag, S.; Spudich, A.; Ruppel, K.M.; Spudich, J.A. The myosin family of mechanoenzymes: From mechanisms to therapeutic approaches. Annu. Rev. Biochem. 2020, 89, 667–693. [Google Scholar] [CrossRef] [PubMed]
- Karcher, R.L.; Deacon, S.W.; Gelfand, V.I. Motor-cargo interactions: The key to transport specificity. Trends Cell Biol. 2002, 12, 21–27. [Google Scholar] [CrossRef]
- Krendel, M.; Mooseker, M.S. Myosins: Tails (and heads) of functional diversity. Physiology 2005, 20, 239–251. [Google Scholar] [CrossRef]
- Foth, B.J.; Goedecke, M.C.; Soldati, D. New insights into myosin evolution and classification. Proc. Natl. Acad. Sci. USA 2006, 103, 3681–3686. [Google Scholar] [CrossRef]
- Sebé-Pedrós, A.; Grau-Bové, X.; Richards, T.A.; Ruiz-Trillo, I. Evolution and classification of myosins, a paneukaryotic whole-genome approach. Genome Biol. Evol. 2014, 6, 290–305. [Google Scholar] [CrossRef]
- de Souza, D.A.S.; Pavoni, D.P.; Krieger, M.A.; Ludwig, A. Evolutionary analyses of myosin genes in trypanosomatids show a history of expansion, secondary losses and neofunctionalization. Sci. Rep. 2018, 8, 1376. [Google Scholar] [CrossRef]
- Katta, S.S.; Sahasrabuddhe, A.A.; Gupta, C.M. Flagellar localization of a novel isoform of myosin, myosin XXI, in Leishmania. Mol. Biochem. Parasitol. 2009, 164, 105–110. [Google Scholar] [CrossRef]
- Batters, C.; Woodall, K.A.; Toseland, C.P.; Hundschell, C.; Veigel, C. Cloning, expression, and characterization of a novel molecular motor, Leishmania myosin-XXI. J. Biol. Chem. 2012, 287, 27556–27566. [Google Scholar] [CrossRef]
- Batters, C.; Ellrich, H.; Helbig, C.; Woodall, K.A.; Hundschell, C.; Brack, D.; Veigel, C. Calmodulin regulates dimerization, motility, and lipid binding of Leishmania myosin XXI. Proc. Natl. Acad. Sci. USA 2014, 111, E227–E236. [Google Scholar] [CrossRef]
- Bajaj, R.; Ambaru, B.; Gupta, C.M. Deciphering the role of UBA like domains in intraflagellar distribution and functions of myosin XXI in Leishmania. PLoS ONE 2020, 15, e0232116. [Google Scholar] [CrossRef]
- Katta, S.S.; Tammana, T.V.S.; Sahasrabuddhe, A.A.; Bajpai, V.K.; Gupta, C.M. Trafficking activity of myosin XXI is required in assembly of Leishmania flagellum. J. Cell Sci. 2010, 123, 2035–2044. [Google Scholar] [CrossRef]
- Machesky, L.M.; Insall, R.H. Signaling to actin dynamics. J. Cell Biol. 1999, 146, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Dominguez, R. Regulation of actin cytoskeleton dynamics in cells. Mol. Cells 2010, 29, 311–325. [Google Scholar] [CrossRef]
- Homma, Y.; Hiragi, S.; Fukuda, M. Rab family of small GTPases: An updated view on their regulation and functions. FEBS J. 2020, 288, 36–55. [Google Scholar] [CrossRef] [PubMed]
- Marwaha, R.; Dwivedi, D.; Sharma, M. Emerging roles of Arf-like GTP-binding proteins: From membrane trafficking to cytoskeleton dynamics and beyond. Proc. Indian Natl. Sci. Acad. 2019, 85, 189–212. [Google Scholar]
- Bhuin, T.; Roy, J.K. Rab proteins: The key regulators of intracellular vesicle transport. Exp. Cell Res. 2014, 328, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Izadi, M.; Hou, W.; Qualmann, B.; Kessels, M.M. Direct effects of Ca2+/calmodulin on actin filament formation. Biochem. Biophys. Res. Commun. 2018, 506, 355–360. [Google Scholar] [CrossRef]
- Lehne, F.; Bogdan, S. Getting cells into shape by calcium-dependent actin cross-linking proteins. Front. Cell Dev. Biol. 2023, 11, 1171930. [Google Scholar] [CrossRef]
- Greka, A.; Mundel, P. Calcium regulates podocyte actin dynamics. Semin. Nephrol. 2012, 32, 319–326. [Google Scholar] [CrossRef]
- Figueroa, N.E.; Franz, P.; Luzarowski, M.; Martinez-Seidel, F.; Moreno, J.C.; Childs, D.; Ziemblicka, A.; Sampathkumar, A.; Andersen, T.G.; Tsiavaliaris, G.; et al. Protein interactome of 3′, 5′-cAMP reveals its role in regulating the actin cytoskeleton. Plant J. 2023, 115, 1214–1230. [Google Scholar] [CrossRef] [PubMed]
- Howe, A.K. Regulation of actin-based cell migration by cAMP/PKA. Biochim. Biophys. Acta 2004, 1692, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Senju, Y.; Lappalainen, P. Regulation of actin dynamics by PI(4,5)P2 in cell migration and endocytosis. Curr. Opin. Cell Biol. 2019, 56, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Caroni, P. Actin cytoskeleton regulation through modulation of PI(4,5)P2 rafts. EMBO J. 2001, 20, 4332–4336. [Google Scholar] [CrossRef]
- Posor, Y.; Eichhorn-Gruenig, M.; Puchkov, D.; Schöneberg, J.; Ullrich, A.; Lampe, A.; Müller, R.; Zarbakhsh, S.; Gulluni, F.; Hirsch, E.; et al. Spatiotemporal control of endocytosis by phosphatidylinositol-3,4-bisphosphate. Nature 2013, 499, 233–237. [Google Scholar] [CrossRef]
- De Pablos, L.M.; Ferreira, T.R.; Walrad, P.B. Developmental differentiation in Leishmania lifecycle progression: Post-transcriptional control conducts the orchestra. Curr. Opin. Microbiol. 2016, 34, 82–89. [Google Scholar] [CrossRef]
- Dandugudumula, R.; Fischer-Weinberger, R.; Zilberstein, D. Morphogenesis dynamics in Leishmania differentiation. Pathogens 2022, 11, 952. [Google Scholar] [CrossRef]
- Besteiro, S.B.; Williams, R.A.M.; Coombs, G.H.; Mottram, J.C. Protein turnover and differentiation in Leishmania. Int. J. Parasitol. 2007, 37, 1063–1075. [Google Scholar] [CrossRef]
- Dostálová, A.; Volf, P. Leishmania development in sand flies: Parasite-vector interactions overview. Parasites Vectors 2012, 5, 276. [Google Scholar] [CrossRef]
- Ralston, K.S.; Hill, K.L. The flagellum of Trypanosoma brucei: New tricks from an old dog. Int. J. Parasitol. 2008, 38, 869–884. [Google Scholar] [CrossRef]
- Maga, J.A.; LeBowitz, J.H. Unravelling the kinetoplastid paraflagellar rod. Trends Cell Biol. 1999, 9, 409–413. [Google Scholar] [CrossRef]
- Wheeler, R.J.; Gluenz, E.; Gull, K. The cell cycle of Leishmania: Morphogenetic events and their implications for parasite biology. Mol. Microbiol. 2011, 79, 647–662. [Google Scholar] [CrossRef] [PubMed]
- Ambit, A.; Woods, K.L.; Cull, B.; Coombs, G.H.; Mottram, J.C. Morphological events during the cell cycle of Leishmania major. Eukaryot. Cell 2011, 10, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Veluru, N.K.; Trivedi, V.; Gupta, C.M.; Sahasrabuddhe, A.A. An actin-like protein is involved in regulation of mitochondrial and flagellar functions as well as in intramacrophage survival of Leishmania donovani. Mol. Microbiol. 2014, 91, 562–578. [Google Scholar] [CrossRef] [PubMed]
- Kajuluri, L.P.; Singh, A.; Bajpai, R.; Veluru, N.K.; Mitra, K.; Sahasrabuddhe, A.A. Actin-related protein 4: An unconventional negative regulator of mitochondrial calcium in protozoan parasite Leishmania. Mitochondrion 2022, 62, 31–40. [Google Scholar] [CrossRef]
- Pradhan, S.; Schwartz, R.A.; Patil, A.; Grabbe, S.; Goldust, M. Treatment options for Leishmaniasis. Clin. Exp. Dermatol. 2022, 47, 516–521. [Google Scholar] [CrossRef]
- Sundar, S.; Singh, J.; Singh, V.K.; Agrawal, N.; Kumar, R. Current and emerging therapies for the treatment of Leishmaniasis. Expert Opin. Orphan Drugs. 2024, 12, 19–32. [Google Scholar] [CrossRef]
- Izdebska, M.; ZieliNska, W.; Grzanka, D.; Gagat, M. The role of actin dynamics and actin-binding proteins expression in epithelial-to-mesenchymal transition and its association with cancer progression and evaluation of possible therapeutic targets. Biomed Res. Int. 2018, 2018, 4578373. [Google Scholar] [CrossRef]
- Wang, A.; Wang, Y.; Wan, R.; Hu, L. Profilin 1 Protein and Its Implications for Cancers. Oncology 2021, 35, 402–409. [Google Scholar] [CrossRef]
- Day, S.M.; Tardiff, J.C.; Ostap, E.M. Myosin modulators: Emerging approaches for the treatment of cardiomyopathies and heart failure. J. Clin. Investig. 2022, 132, e148557. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Dash, R.; Jeong, K.; Lee, W. Role of actin-binding proteins in skeletal myogenesis. Cells 2023, 12, 2523. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Nakamura, F. Actin-associated proteins and small molecules targeting the actin cytoskeleton. Int. J. Mol. Sci. 2022, 23, 2118. [Google Scholar] [CrossRef]
- Moussaoui, D.; Robblee, J.P.; Paganin, J.R.; Auguin, D.; Fisher, F.; Fagnant, P.M.; Macfarlane, J.E.; Schaletzky, J.; Wehri, E.; Mueller-Dieckmann, C.; et al. Mechanism of small molecule inhibition of Plasmodium falciparum myosin A informs antimalarial drug design. Nat. Commun. 2023, 14, 3463. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, D.V.; Karabina, A.; Bergnes, G.; Racca, A.; Wander, H.; Jung, S.; Mittal, N.; Huijs, T.; Ouchida, S.; Ruijgrok, P.V.; et al. A small-molecule myosin inhibitor as a targeted multi-stage antimalarial. bioRxiv 2022. [Google Scholar] [CrossRef]
- Kelsen, A.; Kent, R.S.; Snyder, A.S.; Wehri, E.; Bishop, S.J.; Stadler, R.V.; Powell, C.; di Genova, B.M.; Rompikuntal, P.K.; Boulanger, M.J.; et al. MyosinA is a druggable target in the widespread protozoan parasite Toxoplasma gondii. PLoS Biol. 2023, 21, e3002110. [Google Scholar] [CrossRef]
- Raza, S.; Sahasrabuddhe, A.A.; Gupta, C.M. Nuclear localization of an actin-related protein (ORF LmjF21.0230) in Leishmania. Mol. Biochem. Parasitol. 2007, 153, 216–219. [Google Scholar] [CrossRef]
- García-Salcedo, J.A.; Pérez-Morga, D.; Gijón, P.; Dilbeck, V.; Pays, E.; Nolan, D.P. A differential role for actin during the life cycle of Trypanosoma brucei. EMBO J. 2004, 23, 780–789. [Google Scholar] [CrossRef]
| Species | % Identity | % Similarity |
|---|---|---|
| L. major | 69.5 | 86.5 |
| L. donovani | 69.8 | 86.5 |
| L. infantum | 70.0 | 86.5 |
| L. chagasi | 70.0 | 86.5 |
| L. braziliensis | 70.0 | 85.9 |
| L. mexicana | 70.0 | 86.5 |
| Category | Protein(s) | Function |
|---|---|---|
| 1. Actin Nucleating Proteins | ARP2/3 Complex | Promotes actin nucleation and initiates formation of branched filaments by binding to sides of existing filaments. Activated by WASP/WAVE proteins. |
| Formins (mDia1, mDia2, DAAM1) | Promote nucleation and elongation of linear actin filaments by binding to the barbed end. | |
| 2. Polymerization/Depolymerization Promoters | Profilin | Sequesters ADP-actin to prevent uncontrolled polymerization; promotes ATP exchange and delivers ATP-actin to barbed ends. |
| Thymosin-β4 | Sequesters G-actin, preventing polymerization. | |
| Cofilin/ADF | Binds ADP-G-actin and filaments; promotes severing and depolymerization. | |
| Aip1 | Enhances cofilin-mediated actin filament severing. | |
| Ena/VASP | Promotes elongation by protecting barbed ends from capping. | |
| Twinfilin | Prevents filament assembly at barbed end while allowing disassembly. | |
| Cyclase-Associated Protein (CAP/Srv2) | Promotes pointed-end depolymerization of cofilin-decorated filaments; regulates monomer pool. | |
| Gelsolin | Caps, severs, and nucleates filaments in a calcium-dependent manner. | |
| 3. Actin Capping Proteins | Cap Z | Caps barbed ends, preventing further polymerization. |
| Tropomodulin | Caps pointed ends of actin filaments. | |
| 4.Crosslinking and Bundling Proteins | α-Actinin | Crosslinks actin filaments into bundles or networks. |
| Fimbrin/Plastin | Bundles actin filaments into tight parallel arrays. | |
| Filamin | Crosslinks actin filaments into orthogonal networks. | |
| Spectrin | Links actin filaments to the plasma membrane; forms part of the cortical cytoskeleton. | |
| 5. Actin-Membrane Linkers | Ezrin/Radixin/Moesin (ERM) | Link actin filaments to the plasma membrane. |
| Talin | Links actin filaments to integrins at focal adhesions. | |
| Vinculin | Stabilizes actin–membrane interactions at focal adhesions. | |
| 6. Other Actin-Associated Proteins | Myosin Motors | Interact with actin filaments to generate contractile forces. |
| Tropomyosin | Stabilizes actin filaments and regulates their interaction with other proteins. | |
| Coronin | Binds to actin filaments and stabilizes them. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, C.M.; Thiyagarajan, S. Structure and Functions of Actin and Actin-Binding Proteins in Leishmania. Pathogens 2025, 14, 948. https://doi.org/10.3390/pathogens14090948
Gupta CM, Thiyagarajan S. Structure and Functions of Actin and Actin-Binding Proteins in Leishmania. Pathogens. 2025; 14(9):948. https://doi.org/10.3390/pathogens14090948
Chicago/Turabian StyleGupta, Chhitar M., and Saravanamuthu Thiyagarajan. 2025. "Structure and Functions of Actin and Actin-Binding Proteins in Leishmania" Pathogens 14, no. 9: 948. https://doi.org/10.3390/pathogens14090948
APA StyleGupta, C. M., & Thiyagarajan, S. (2025). Structure and Functions of Actin and Actin-Binding Proteins in Leishmania. Pathogens, 14(9), 948. https://doi.org/10.3390/pathogens14090948






