β-Giardin as an Immunomagnetic Enrichment Target for Multi-Host Detection of Giardia duodenalis Cysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. G. duodenalis Isolation, Cultivation, Encystation and Cysts Preparation
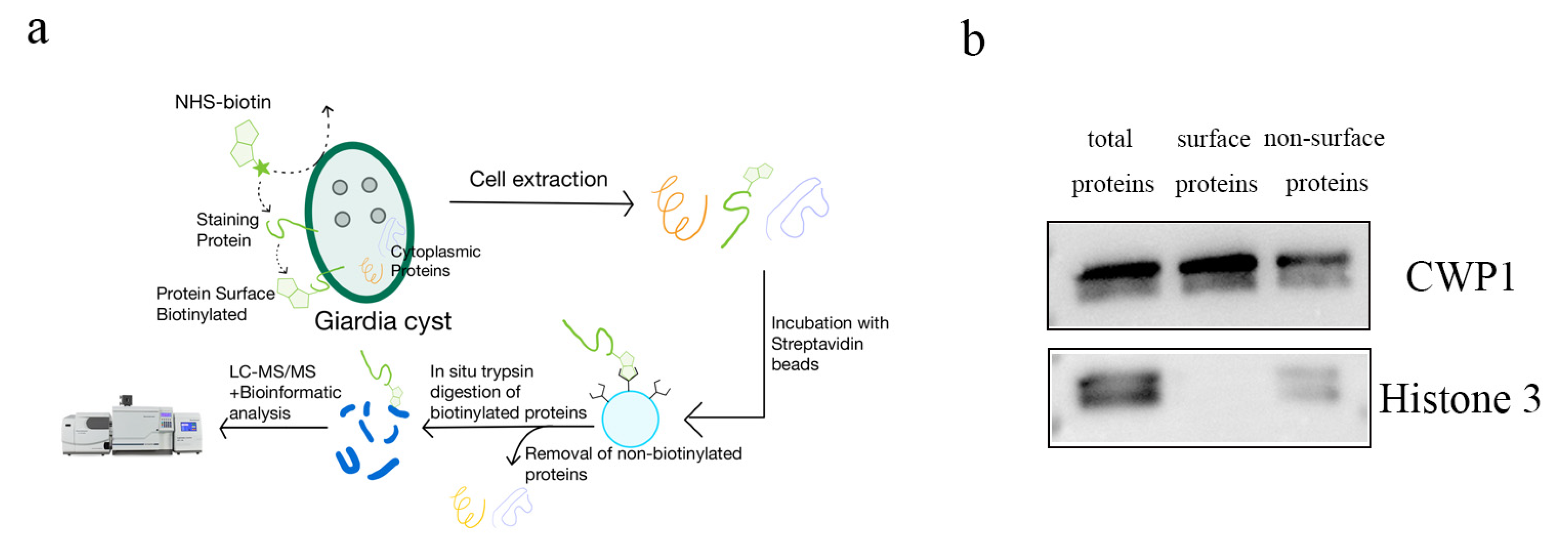
2.3. Biotinylation of G. duodenalis Cyst Outer Wall Proteins and Purification of Biotinylated Proteins
2.4. Western Blot
2.5. LC-MS/MS Analysis of the G. duodenalis Cyst Outer Wall Proteins
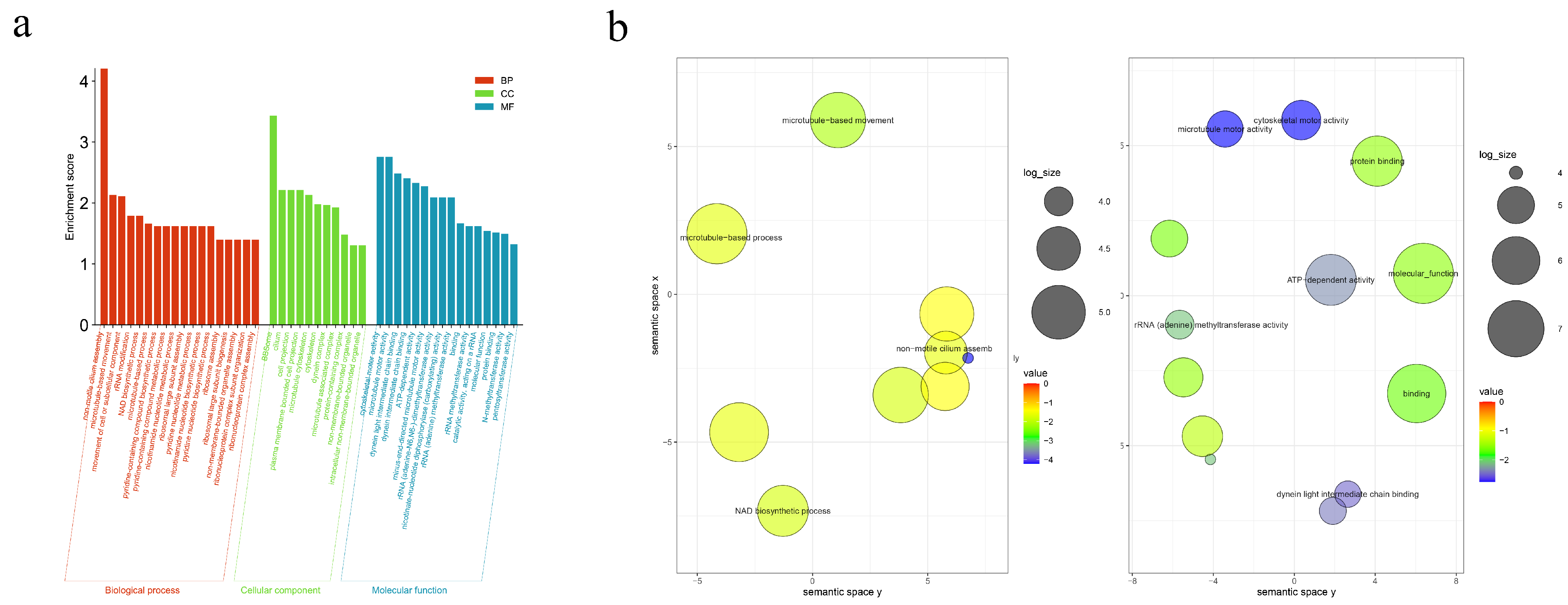
2.6. Bioinformatic Analysis
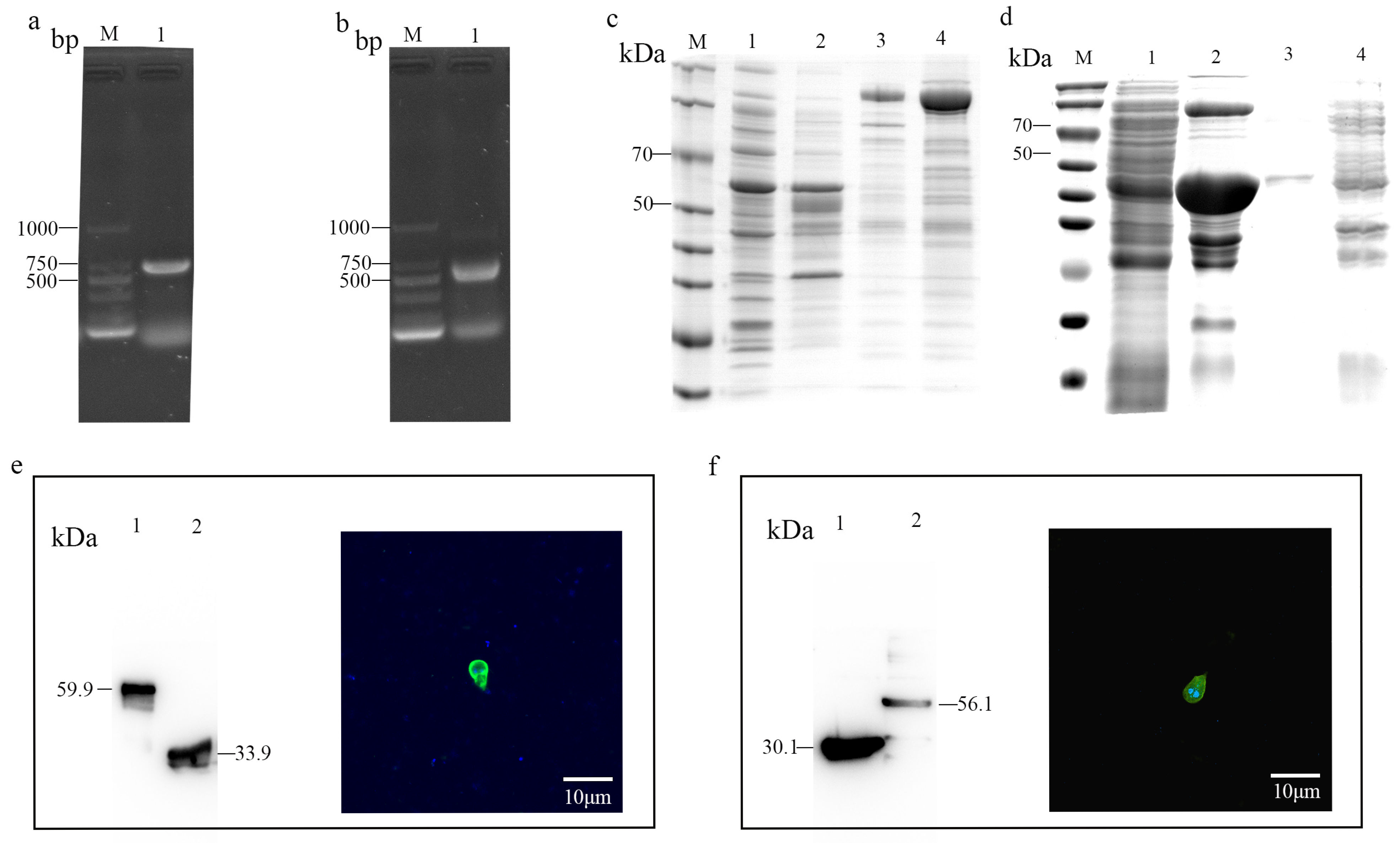
2.7. Production of Recombinant Proteins and Preparation of Polyclonal Antibodies
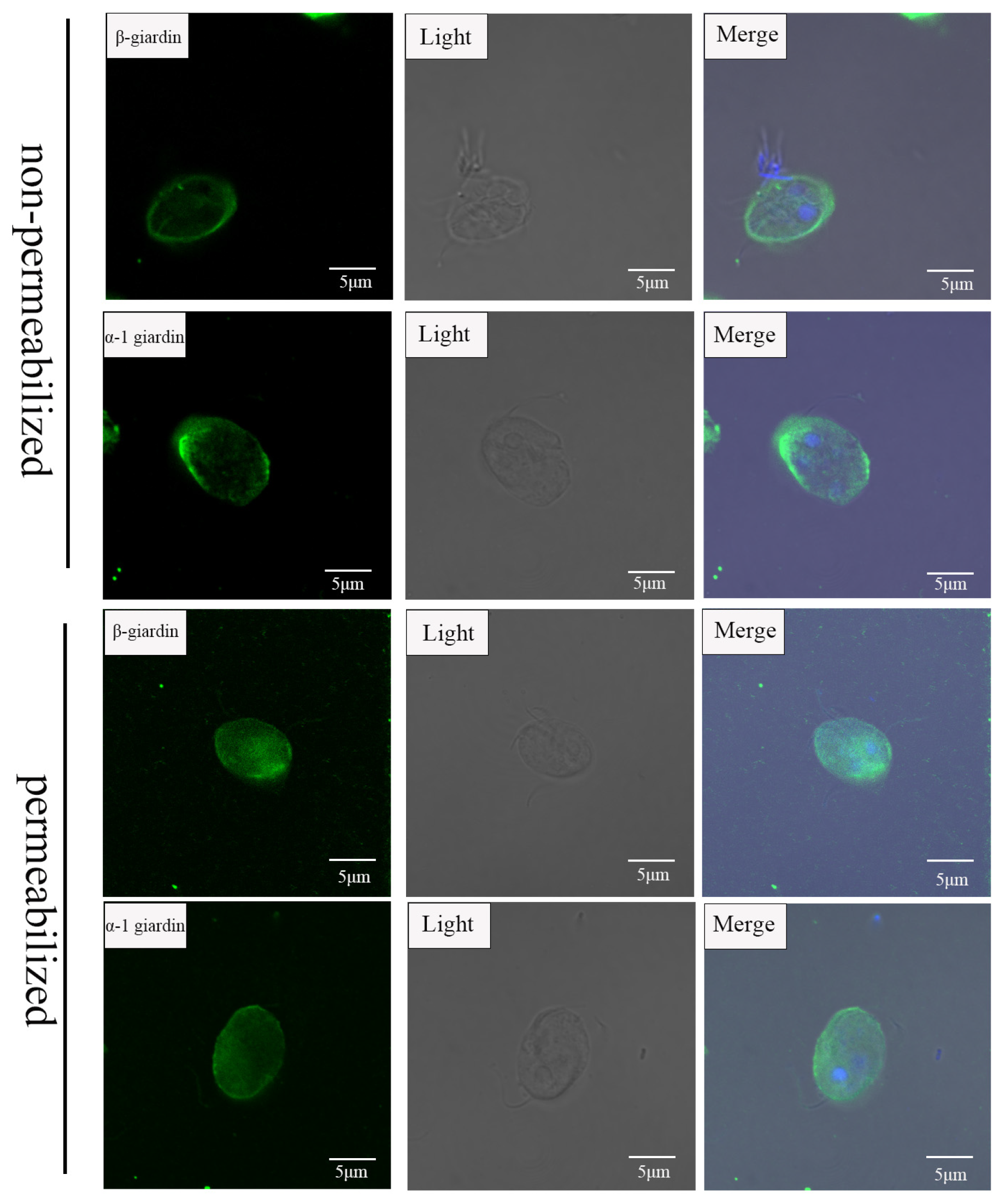
2.8. Indirect Immunofluorescence Localization
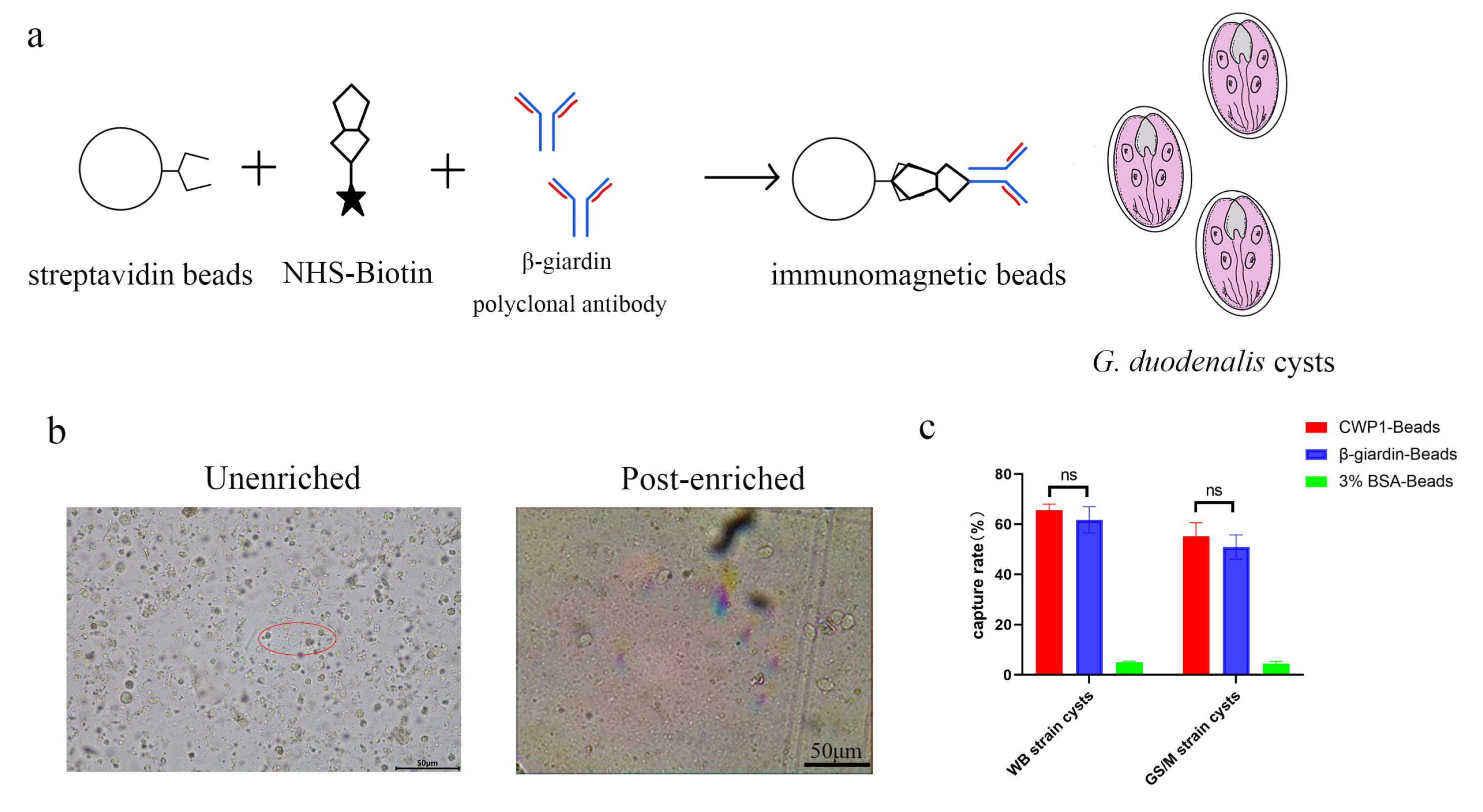
2.9. Preparation of Immunomagnetic Beads
2.10. Assessment of Immunomagnetic Beads Enrichment Efficiency
2.11. Clinical Sample Collection
2.12. Nested PCR
2.13. Statistical Analysis
3. Results
3.1. Proteomic Analysis of G. duodenalis Cyst Surface Protein
| Gene ID | Name | Sequences (5′–3′) | Size (bp) | Application |
|---|---|---|---|---|
| GL50803_005638 | CWP1-F | GGATCCATGATGCTCGCTCTCCTTGC | 726 | protein expression |
| CWP1-R | GAATTCTCAAGGCGGGGTGAGGC | |||
| GL50803_0011654 | α-1 giardin-F | GGATCCATGCCGAAGGTCACCGAC | 888 | protein expression |
| α-1 giardin-R | GAATTCCTACTTCACGCGCCAGAGG | |||
| GL50803_004812 | β-giardin-F | GGATCCATGTCTATGTTCACCTCCACCCG | 819 | protein expression |
| β-giardin-R | GAATTCTTAGTGCTTTGTGACCATCGAGAGG |
| Gene Name | Description | Previously Identified (Yes/No) | Prot Score |
|---|---|---|---|
| GL50581_1724 | CWP2 | Yes | 128 |
| GL50581_403 | CWP1 | Yes | 101 |
| GL50581_2806 | CWP3 | Yes | 36 |
| GL50803_00101168 | Ankyrin repeat protein 1 | No | 124 |
| GL50803_101291 | Tubulin beta chain | No | 114 |
| GL50581_2741 | Beta-giardin | No | 77 |
| GL50803_9515 | Coiled-coil protein | No | 75 |
| GL50803_88765 | Cytosolic HSP70 | No | 68 |
| GL50803_15276 | Glucosamine-6-phosphate isomerase | No | 66 |
| GL50803_0017043 | Glyceraldehyde-3-phosphate dehydrogenase | No | 59 |
| GL50803 0011684 | Leucine-rich repeat protein | No | 49 |
| GL50803_0017230 | Gamma giardin | No | 47 |
| GL50803_0011654 | Alpha-1 giardin | No | 37 |
| GL50803_13561 | Protein Translation Elongation Factor 1B | No | 47 |
| GL50803_0013220 | ATP-dependent RNA helicase | No | 49 |
3.2. Functional Annotation of Proteins
3.3. Expression of Several Recombinant Surface Proteins
3.4. β-Giardin and α-1 Giardin Are Located in G. duodenalis Cyst Surface
3.5. Detectability of Selected Conserved Surface Proteins
3.6. Clinical Performance Evaluation of Giardia Cysts via Immunomagnetic Beads Detection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rojas-López, L.; Marques, R.C.; Svärd, S.G. Giardia duodenalis. Trends Parasitol. 2022, 38, 605–606. [Google Scholar] [CrossRef]
- Li, L.; Li, X.; Li, G.; Gong, P.; Zhang, X.; Yang, Z.; Yang, J.; Li, J. Mouse macrophages capture and kill Giardia lamblia by means of releasing extracellular trap. Dev. Comp. Immunol. 2018, 88, 206–212. [Google Scholar] [CrossRef]
- Kalavani, S.; Matin, S.; Rahmanian, V.; Meshkin, A.; Bahadori Mazidi, B.; Taghipour, A.; Abdoli, A. Prevalence of Giardia duodenalis among African children: A systematic review and meta-analysis. Parasite Epidemiol. Control 2024, 26, e00365. [Google Scholar] [CrossRef]
- Bourque, D.L.; Neumayr, A.; Libman, M.; Chen, L.H. Treatment strategies for nitroimidazole-refractory giardiasis: A systematic review. J. Travel Med. 2022, 29, taab120. [Google Scholar] [CrossRef]
- Rojas, L.; Grüttner, J.; Ma’ayeh, S.; Xu, F.; Svärd, S.G. Dual RNA Sequencing Reveals Key Events When Different Giardia Life Cycle Stages Interact with Human Intestinal Epithelial Cells In Vitro. Front. Cell. Infect. Microbiol. 2022, 12, 862211. [Google Scholar] [CrossRef]
- Thomas, E.B.; Sutanto, R.; Johnson, R.S.; Shih, H.W.; Alas, G.C.M.; Krtková, J.; MacCoss, M.J.; Paredez, A.R. Staging Encystation Progression in Giardia lamblia Using Encystation-Specific Vesicle Morphology and Associating Molecular Markers. Front. Cell. Dev. Biol. 2021, 9, 662945. [Google Scholar] [CrossRef] [PubMed]
- Luján, H.D.; Mowatt, M.R.; Conrad, J.T.; Bowers, B.; Nash, T.E. Identification of a novel Giardia lamblia cyst wall protein with leucine-rich repeats. Implications for secretory granule formation and protein assembly into the cyst wall. J. Biol. Chem. 1995, 270, 29307–29313. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.H.; McCaffery, J.M.; Reiner, D.S.; Gillin, F.D. Mining the Giardia lamblia genome for new cyst wall proteins. J. Biol. Chem. 2003, 278, 21701–21708. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.W.; Lin, Z.Q.; Tung, S.Y.; Su, L.H.; Ho, C.C.; Lee, G.A.; Sun, C.H. A Novel Multiprotein Bridging Factor 1-Like Protein Induces Cyst Wall Protein Gene Expression and Cyst Differentiation in Giardia lamblia. Int. J. Mol. Sci. 2021, 22, 1370. [Google Scholar] [CrossRef]
- Chatterjee, A.; Carpentieri, A.; Ratner, D.M.; Bullitt, E.; Costello, C.E.; Robbins, P.W.; Samuelson, J.; Johnson, P.J. Giardia cyst wall protein 1 is a lectin that binds to curled fibrils of the GalNAc homopolymer. PLoS Pathog. 2010, 6, e1001059. [Google Scholar] [CrossRef]
- Vicente, B.; Freitas, A.; Freitas, M.; Midlej, V. Systematic Review of Diagnostic Approaches for Human Giardiasis: Unveiling Optimal Strategies. Diagnostics 2024, 14, 364. [Google Scholar] [CrossRef]
- Weitzel, T.; Dittrich, S.; Möhl, I.; Adusu, E.; Jelinek, T. Evaluation of seven commercial antigen detection tests for Giardia and Cryptosporidium in stool samples. Clin. Microbiol. Infect. 2006, 12, 656–659. [Google Scholar] [CrossRef]
- Bahwaireth, E.O.; Wakid, M.H. Molecular, Microscopic, and Immunochromatographic Detection of Enteroparasitic Infections in Hemodialysis Patients and Related Risk Factors. Foodborne Pathog. Dis. 2022, 19, 830–838. [Google Scholar] [CrossRef]
- Pacheco, F.T.F.; de Carvalho, S.S.; Santos, S.A.; das Chagas, G.M.T.; Santos, M.C.; Santos, J.G.S.; da Costa-Ribeiro, H.J.; Ribeiro, T.C.M.; de Mattos, Â.P.; Gomes, M.A.; et al. Specific IgG and IgA Antibody Reactivities in Sera of Children by Enzyme-Linked Immunoassay and Comparison with Giardia duodenalis Diagnosis in Feces. Ann. Lab. Med. 2020, 40, 382–389. [Google Scholar] [CrossRef]
- Peeling, R.W.; Mabey, D. Point-of-care tests for diagnosing infections in the developing world. Clin. Microbiol. Infect. 2010, 16, 1062–1069. [Google Scholar] [CrossRef]
- Tao, T.; Li, Z.; Xu, S.; Rehman, S.U.; Chen, R.; Xu, H.; Xia, H.; Zhang, J.; Zhao, H.; Wang, J.; et al. Boosting SARS-CoV-2 Enrichment with Ultrasmall Immunomagnetic Beads Featuring Superior Magnetic Moment. Anal. Chem. 2023, 95, 11542–11549. [Google Scholar] [CrossRef]
- Luo, S.; Nguyen, K.T.; Nguyen, B.T.T.; Feng, S.; Shi, Y.; Elsayed, A.; Zhang, Y.; Zhou, X.; Wen, B.; Chierchia, G.; et al. Deep learning-enabled imaging flow cytometry for high-speed Cryptosporidium and Giardia detection. Cytom. Part A 2021, 99, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.V.; Ward, H.D.; Keusch, G.T.; Pereira, M.E. In vitro encystation of Giardia lamblia: Large-scale production of in vitro cysts and strain and clone differences in encystation efficiency. J. Parasitol. 1991, 6, 974–981. [Google Scholar] [CrossRef]
- Kurnosova, O.P.; Panova, O.A.; Arisov, M.V. Prevalence of Giardia duodenalis in dogs and cats: Age-related predisposition, symptomatic, and asymptomatic cyst shedding. Vet. World 2024, 17, 379–383. [Google Scholar] [CrossRef]
- Davids, B.J.; Liu, C.M.; Hanson, E.M.; Le, C.H.Y.; Ang, J.; Hanevik, K.; Fischer, M.; Radunovic, M.; Langeland, N.; Ferella, M.; et al. Identification of Conserved Candidate Vaccine Antigens in the Surface Proteome of Giardia lamblia. Infect. Immun. 2019, 87, e00219-19. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, X.; Gong, P.; Xia, F.; Li, L.; Yang, Z.; Li, J. TLR2(−/−) Mice Display Decreased Severity of Giardiasis via Enhanced Proinflammatory Cytokines Production Dependent on AKT Signal Pathway. Front. Immunol. 2017, 8, 1186. [Google Scholar] [CrossRef]
- Dumètre, A.; Aubert, D.; Puech, P.H.; Hohweyer, J.; Azas, N.; Villena, I. Interaction forces drive the environmental transmission of pathogenic protozoa. Appl. Environ. Microbiol. 2012, 78, 905–912. [Google Scholar] [CrossRef]
- Ankarklev, J.; Jerlström-Hultqvist, J.; Ringqvist, E.; Troell, K.; Svärd, S.G. Behind the smile: Cell biology and disease mechanisms of Giardia species. Nat. Rev. Microbiol. 2010, 8, 413–422. [Google Scholar] [CrossRef]
- Davids, B.J.; Reiner, D.S.; Birkeland, S.R.; Preheim, S.P.; Cipriano, M.J.; McArthur, A.G.; Gillin, F.D. A new family of giardial cysteine-rich non-VSP protein genes and a novel cyst protein. PLoS ONE 2006, 1, e44. [Google Scholar] [CrossRef]
- Chiu, P.W.; Huang, Y.C.; Pan, Y.J.; Wang, C.H.; Sun, C.H. A novel family of cyst proteins with epidermal growth factor repeats in Giardia lamblia. PLoS Neglected Trop. Dis. 2010, 4, e677. [Google Scholar] [CrossRef]
- Fradette, M.S.; Charette, S.J. Working toward improved monitoring of Cryptosporidium and Giardia (oo)cysts in water samples: Testing alternatives to elution and immunomagnetic separation from USEPA Method 1623.1. BMC Res. Notes 2022, 15, 254. [Google Scholar] [CrossRef]
- Moss, J.A.; Gordy, J.; Snyder, R.A. Effective concentration and detection of cryptosporidium, giardia, and the microsporidia from environmental matrices. J. Pathog. 2014, 2014, 408204. [Google Scholar] [CrossRef]
- Tayachi, I.; Galai, Y.; Ben-Abid, M.; Saidi, N.; Ben-Sghaier, I.; Aoun, K.; Bouratbine, A. Use of Immunomagnetic Separation tool in Leishmania promastigotes capture. Acta Trop. 2021, 215, 105804. [Google Scholar] [CrossRef] [PubMed]
- Yeğenoğlu Akçinar, H.; Aslim, B.; Torul, H.; Güven, B.; Zengin, A.; Suludere, Z.; Boyaci, I.H.; Tamer, U. Immunomagnetic separation and Listeriamonocytogenes detection with surface-enhanced Raman scattering. Turk. J. Med. Sci. 2020, 50, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Wang, Y.; Zhang, C.; Wang, Y.; Wei, J.; Liao, X.; Wang, J.; Anfossi, L.; Wang, Y. Nanobody-based immunomagnetic separation platform for rapid isolation and detection of Salmonella enteritidis in food samples. Food Chem. 2023, 424, 136416. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.; Zhou, Y.; Fan, Y.; Bi, Y.; Yang, R.; Zhou, Y.; Wang, X. Yersinia pestis detection by loop-mediated isothermal amplification combined with magnetic bead capture of DNA. Braz. J. Microbiol. 2018, 49, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Qiu, Y.; Chen, S.; Chen, X.; Wu, Y.; He, S.; Li, X.; Chen, H. A rapid immunomagnetic beads-based sELISA method for the detection of bovine αs1-casein based on specific epitopes. Food Chem. 2024, 444, 138565. [Google Scholar] [CrossRef]
- Chuang, Y.S.; Wang, C.K.; Li, C.Y.; Li, C.; Wu, C.C. Rapid label-free impedimetric detection of progesterone enhanced by immunomagnetic bead-based competitive immunoreaction. Talanta 2024, 276, 126204. [Google Scholar] [CrossRef]
- Yatsomboon, A.; Sermswan, R.W.; Wongratanacheewin, S. Development of an immunomagnetic separation-ELISA for the detection of Burkholderia pseudomallei in blood samples. Asian Pac. J. Allergy Immunol. 2021, 39, 35–43. [Google Scholar] [CrossRef]
- Zeng, J.; Wei, H.; Zhang, L.; Liu, X.; Zhang, H.; Cheng, J.; Ma, D.; Zhang, X.; Fu, P.; Liu, L. Rapid detection of Vibrio parahaemolyticus in raw oysters using immunomagnetic separation combined with loop-mediated isothermal amplification. Int. J. Food Microbiol. 2014, 174, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Cao, S.; Sun, M.; Yang, Q.; Huang, T.; Yang, X.; Li, J.; Zhang, X.; Li, X.; Wang, X.; et al. Rapid visual detection of Giardia duodenalis in faecal samples using an RPA-CRISPR/Cas12a system. Parasitol. Res. 2024, 123, 176. [Google Scholar] [CrossRef] [PubMed]

| Sample | Antibody Dilution Radio | Estimated Number of Cysts Captured by IMB | IMB Efficiency | Optical Microscopy Observation |
|---|---|---|---|---|
| S1 | 1:100 | 1.0 × 104 | 41% | + |
| S2 | 1:200 | 1.25 × 104 | 52% | + |
| S3 | 1:500 | 5 × 103 | 20% | + |
| S4 | 1:1000 | 5.0 × 103 | 20% | + |
| S5 | 1:2000 | 2.5 × 103 | 10% | + |
| Sample | Incubation Time Between IMB and Cysts | Estimated Number of Cysts Captured by IMB | IMB Efficiency | Optical Microscopy Observation |
|---|---|---|---|---|
| S1 | 10 min | + | ||
| S2 | 30 min | 7.5 × 103 | 31% | + |
| S3 | 60 min | 1.25 × 104 | 52% | + |
| S4 | 120 min | 1.0 × 104 | 41% | + |
| S5 | 180 min | 7.5 × 103 | 31% | + |
| Sample | Estimated Number of Cysts in 500 μL | Estimated Number of Cysts Captured by IMB | IMB Efficiency | Optical Microscopy Observation |
|---|---|---|---|---|
| S1 | 1 × 104 | + | ||
| S2 | 2 × 104 | 1.75 × 103 | 8.5% | + |
| S3 | 5 × 104 | 1.25 × 104 | 25% | + |
| S4 | 1 × 105 | 6.5 × 104 | 65% | + |
| S5 | 2 × 105 | 1.05 × 105 | 52.5% | + |
| Sample | Unriched Cysts in 1 mL | Post-Enriched Cysts Captured by IMB | IMB Efficiency |
|---|---|---|---|
| Ferret | 1.25 × 104 | 6.25 × 103 | 50% |
| Red-crowned crane | 8 × 103 | 3.5 × 103 | 43.75% |
| Siberian tiger | 1.5 × 104 | 8.25 × 104 | 55% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Yang, H.; Li, C.; Chen, M.; Wang, X.; Zhang, X.; Gong, P.; Zhang, N.; Zhang, X.; Li, J.; et al. β-Giardin as an Immunomagnetic Enrichment Target for Multi-Host Detection of Giardia duodenalis Cysts. Pathogens 2025, 14, 918. https://doi.org/10.3390/pathogens14090918
Wang H, Yang H, Li C, Chen M, Wang X, Zhang X, Gong P, Zhang N, Zhang X, Li J, et al. β-Giardin as an Immunomagnetic Enrichment Target for Multi-Host Detection of Giardia duodenalis Cysts. Pathogens. 2025; 14(9):918. https://doi.org/10.3390/pathogens14090918
Chicago/Turabian StyleWang, Hongyu, Heng Yang, Chaofan Li, Mengge Chen, Xiaocen Wang, Xu Zhang, Pengtao Gong, Nan Zhang, Xichen Zhang, Jianhua Li, and et al. 2025. "β-Giardin as an Immunomagnetic Enrichment Target for Multi-Host Detection of Giardia duodenalis Cysts" Pathogens 14, no. 9: 918. https://doi.org/10.3390/pathogens14090918
APA StyleWang, H., Yang, H., Li, C., Chen, M., Wang, X., Zhang, X., Gong, P., Zhang, N., Zhang, X., Li, J., & Li, X. (2025). β-Giardin as an Immunomagnetic Enrichment Target for Multi-Host Detection of Giardia duodenalis Cysts. Pathogens, 14(9), 918. https://doi.org/10.3390/pathogens14090918








