Rabies Surveillance in Mainland Tanzania: A Scoping Review of Animal Rabies Occurrences (1993–2023)
Abstract
1. Introduction
2. Methods
2.1. Data Source and Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Synthesis
3. Results
3.1. Study Selection
3.2. Critical Appraisal of Sources
3.3. Characteristics of the Studies Included in the Review
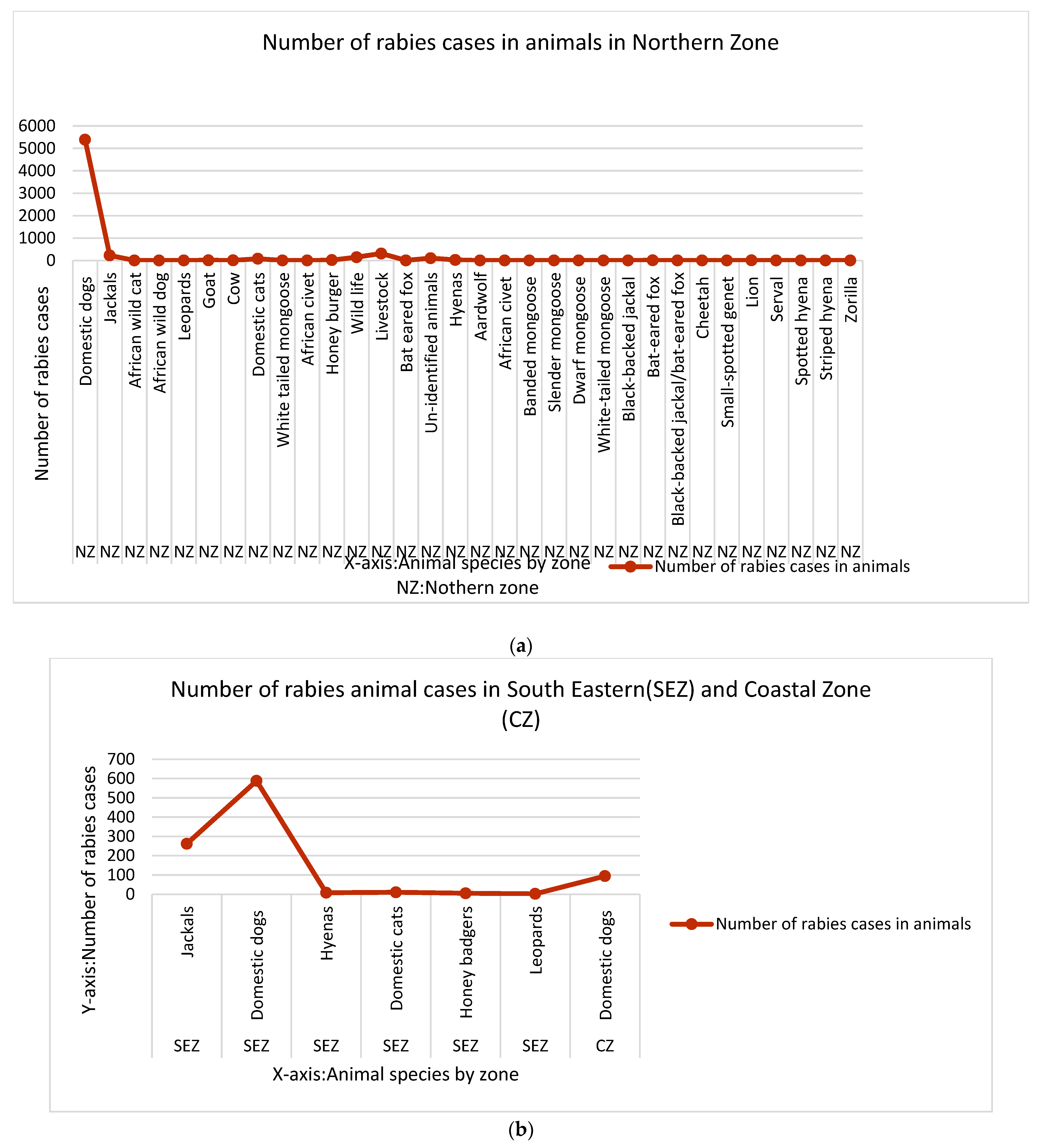
3.4. Reservoir Hosts and Their Role in the Circulation of Lyssavirus in Tanzania
3.5. Rabies Hotspots in Tanzania Mainland (1993–2023)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, R. Rabies—Epidemiology, pathogenesis, public health concerns and advances in diagnosis and control: A comprehensive review. Vet. Q. 2017, 37, 212–251. [Google Scholar] [CrossRef]
- Hikufe, E.H.; Freuling, C.M.; Athingo, R.; Shilongo, A.; Ndevaetela, E.-E.; Helao, M.; Shiindi, M.; Hassel, R.; Bishi, A.; Khaiseb, S.; et al. Ecology and epidemiology of rabies in humans, domestic animals and wildlife in Namibia, 2011–2017. PLoS Negl. Trop. Dis. 2019, 13, e0007355. [Google Scholar] [CrossRef]
- Muleya, W.; Chambaro, H.M.; Sasaki, M.; Gwenhure, L.F.; Mwenechanya, R.; Kajihara, M.; Saasa, N.; Mupila, Z.; Mori-Kajihara, A.; Qiu, Y.; et al. Genetic diversity of rabies virus in different host species and geographic regions of Zambia and Zimbabwe. Virus Genes 2019, 55, 713–719. [Google Scholar] [CrossRef]
- World Health Organization. Rabies vaccines: WHO position paper, April 2018–Recommendations. Vaccine 2018, 36, 5500–5503. [Google Scholar] [CrossRef]
- Cleaveland, S.; Fèvre, E.M.; Kaare, M.; Coleman, P.G. Estimating human rabies mortality in the United Republic of Tanzania from dog bite injuries. Bull. World Health Organ. 2002, 80, 304–310. [Google Scholar]
- Taylor, L.H.; Hampson, K.; Fahrion, A.; Abela-Ridder, B.; Nel, L.H. Difficulties in estimating the human burden of canine rabies. Acta Trop. 2017, 165, 133–140. [Google Scholar] [CrossRef]
- Kipanyula, M.J. Why has canine rabies remained endemic in the Kilosa district of Tanzania? Lessons learnt and the way forward. Infect. Dis. Poverty 2015, 4, 52. [Google Scholar] [CrossRef][Green Version]
- Hampson, K.; Coudeville, L.; Lembo, T.; Sambo, M.; Kieffer, A.; Attlan, M.; Barrat, J.; Blanton, J.D.; Briggs, D.J.; Cleaveland, S.; et al. Estimating the global burden of endemic canine rabies. PLoS Negl. Trop. Dis. 2015, 9, e0003709. [Google Scholar] [CrossRef]
- Hampson, K.; Dobson, A.; Kaare, M.; Dushoff, J.; Magoto, M.; Sindoya, E.; Cleaveland, S. Rabies exposures, post-exposure prophylaxis and deaths in a region of endemic canine rabies. PLoS Negl. Trop. Dis. 2008, 2, e339. [Google Scholar] [CrossRef]
- Sambo, M.; Hampson, K.; Changalucha, J.; Cleaveland, S.; Lembo, T.; Lushasi, K.; Mbunda, E.; Mtema, Z.; Sikana, L.; Johnson, P.C.D. Estimating the size of dog populations in Tanzania to inform rabies control. Vet. Sci. 2018, 5, 77. [Google Scholar] [CrossRef]
- Gsell, A.S.; Knobel, D.L.; Kazwala, R.R.; Vounatsou, P.; Zinsstag, J. Domestic dog demographic structure and dynamics relevant to rabies control planning in urban areas in Africa: The case of Iringa, Tanzania. BMC Vet. Res. 2012, 8, 236. [Google Scholar] [CrossRef]
- Nel, L. Discrepancies in Data Reporting for Rabies, Africa. Emerg. Infect. Dis. J. 2013, 19, 529. [Google Scholar] [CrossRef] [PubMed]
- Lushasi, K.; Hayes, S.; Ferguson, E.A.; Changalucha, J.; Cleaveland, S.; Govella, N.J.; Haydon, D.T.; Sambo, M.; Mchau, G.J.; Mpolya, E.A.; et al. Reservoir dynamics of rabies in south-east Tanzania and the roles of cross-species transmission and domestic dog vaccination. J. Appl. Ecol. 2021, 58, 2673–2685. [Google Scholar] [CrossRef]
- Gsell, A.; Knobel, D.L.; Cleaveland, S.; Kazwala, R.R.; Vounatsou, P.; Zinsstag, J. Features of domestic dog demography relevant to rabies control planning in tanzania. J. Vet. Behav. 2009, 4, 63. [Google Scholar] [CrossRef]
- Confiance, A.; Vaz, M.; Chilengue, I.; Ali, S.; Baltazar, C.; Inlamea, O.F.; Development, R.; Epidemiology, F.; Program, T. Article Program evaluation Evaluation of the rabies surveillance system using the One Health approach in Mozambique, 2020–2021. PAMJ-One Health 2024, 13, 4. [Google Scholar]
- Knobel, D.L.; Cleaveland, S.; Coleman, P.G.; Fèvre, E.M.; Meltzer, M.I.; Miranda, M.E.G.; Shaw, A.; Zinsstag, J.; Meslin, F.-X. Re-evaluating the burden of rabies in Africa and Asia. Bull. World Health Organ. 2005, 83, 360–368. [Google Scholar] [PubMed]
- Lembo, T.; Hampson, K.; Kaare, M.T.; Ernest, E.; Knobel, D.; Kazwala, R.R.; Haydon, D.T.; Cleaveland, S. The feasibility of canine rabies elimination in Africa: Dispelling doubts with data. PLoS Negl. Trop. Dis. 2010, 4, e626. Available online: https://pubmed.ncbi.nlm.nih.gov/20186330 (accessed on 24 July 2025). [CrossRef]
- Knobel, D.L.; Laurenson, M.K.; Kazwala, R.R.; Boden, L.A.; Cleaveland, S. A cross-sectional study of factors associated with dog ownership in Tanzania. BMC Vet Res. 2008, 4, 5. [Google Scholar] [CrossRef]
- Lawson, E.T.; Ayivor, J.S.; Ohemeng, F.; Ntiamoa, Y. Avoiding bites and scratches ? Understanding the public health implication of human—Bat interactions in Ghana. Zoonoses Public Health 2018, 66, 108–116. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. 25 PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2016, 169, 467–473. [Google Scholar] [CrossRef]
- Khalil, H.; Peters, M.D.J.; Tricco, A.C.; Pollock, D.; Alexander, L.; McInerney, P.; Godfrey, C.M.; Munn, Z. Conducting high quality scoping reviews-challenges and solutions. J. Clin. Epidemiol. 2021, 130, 156–160. [Google Scholar] [CrossRef]
- Olofsson, H.; Brolund, A.; Hellberg, C.; Silverstein, R.; Stenström, K.; Österberg, M.; Dagerhamn, J. Can abstract screening workload be reduced using text mining? User experiences of the tool Rayyan. Res. Synth. Methods 2017, 8, 275–280. [Google Scholar] [CrossRef]
- Gascoyne, S.C.; Laurenson, M.K.; Lelo, S.; Borner, M. Rabies in African wild dogs (Lycaon pictus) in the Serengeti region, Tanzania. J. Wildl. Dis. 1993, 29, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Cleaveland, S.; Dye, C. Maintenance of a microparasite infecting several host species: Rabies in the Serengeti. Parasitology 1995, 111, S33–S47. [Google Scholar] [CrossRef] [PubMed]
- Cleaveland, S.; Barrat, J.; Barrat, M.J.; Selve, M.; Kaare, M.; Esterhuysen, J. A rabies serosurvey of domestic dogs in rural Tanzania: Results of a rapid fluorescent focus inhibition test (RFFIT) and a liquid-phase blocking ELISA used in parallel. Epidemiol. Infect. 1999, 123, 157–164. [Google Scholar] [CrossRef] [PubMed]
- East, M.L.; Hofer, H.; Cox, J.H.; Wulle, U.; Wiik, H.; Pitra, C. Regular exposure to rabies virus and lack of symptomatic disease in Serengeti spotted hyenas. Proc. Natl. Acad. Sci. USA 2001, 98, 15026–15031. [Google Scholar] [CrossRef]
- Lushasi, K.; Steenson, R.; Bernard, J.; Changalucha, J.J.; Govella, N.J.; Haydon, D.T.; Hoffu, H.; Lankester, F.; Magoti, F.; Mpolya, E.A.; et al. One Health in Practice: Using Integrated Bite Case Management to Increase Detection of Rabid Animals in Tanzania. Front. Public Heal. 2020, 8, 13. [Google Scholar] [CrossRef]
- Lembo, T.; Niezgoda, M.; Velasco-Villa, A.; Cleaveland, S.; Ernest, E.; Rupprecht, C.E. Evaluation of a direct, rapid immunohistochemical test for rabies diagnosis. Emerg. Infect. Dis. 2006, 12, 310–313. [Google Scholar] [CrossRef]
- Lembo, T.; Haydon, D.T.; Velasco-Villa, A.; Rupprecht, C.E.; Packer, C.; Brandão, P.E.; Kuzmin, I.V.; Fooks, A.R.; Barrat, J.; Cleaveland, S. Molecular epidemiology identifies only a single rabies virus variant circulating in complex carnivore communities of the Serengeti. Proc. Biol. Sci. 2007, 274, 2123–2130. [Google Scholar] [CrossRef] [PubMed]
- Lembo, T.; Hampson, K.; Haydon, D.T.; Craft, M.; Dobson, A.; Dushoff, J.; Ernest, E.; Hoare, R.; Kaare, M.; Mlengeya, T.; et al. Exploring reservoir dynamics: A case study of rabies in the Serengeti ecosystem. J. Appl. Ecol. 2008, 45, 1246–1257. [Google Scholar] [CrossRef]
- Swai, E.S.; Moshy, W.E.; Kaaya, J.E.; Mtui, P.F. Spatial and temporal distribution of rabies in northern Tanzania in the period of 1993-2002. Tanzan J. Health Res. 2010, 12, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Marston, D.A.; Horton, D.L.; Ngeleja, C.; Hampson, K.; McElhinney, L.M.; Banyard, A.C.; Haydon, D.; Cleaveland, S.; Rupprecht, C.E.; Bigambo, M.; et al. Ikoma lyssavirus, highly divergent novel lyssavirus in an African civet. Emerg. Infect. Dis. 2012, 18, 664–667. [Google Scholar] [CrossRef] [PubMed]
- Horton, D.L.; Banyard, A.C.; Marston, D.A.; Wise, E.; Selden, D.; Nunez, A.; Hicks, D.; Lembo, T.; Cleaveland, S.; Peel, A.J.; et al. Antigenic and genetic characterization of a divergent African virus, Ikoma lyssavirus. J. Gen. Virol. 2014, 95 Pt 5, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Brunker, K.; Marston, D.A.; Horton, D.L.; Cleaveland, S.; Fooks, A.R.; Kazwala, R.; Ngeleja, C.; Lembo, T.; Sambo, M.; Mtema, Z.J.; et al. Elucidating the phylodynamics of endemic rabies virus in eastern Africa using whole-genome sequencing. Virus Evol. 2015, 1, vev011. [Google Scholar] [CrossRef]
- Mpolya, E.A.; Lembo, T.; Lushasi, K.; Mancy, R.; Mbunda, E.M.; Makungu, S.; Maziku, M.; Sikana, L.; Jaswant, G.; Townsend, S.; et al. Toward Elimination of Dog-Mediated Human Rabies: Experiences from Implementing a Large-scale Demonstration Project in Southern Tanzania. Front. Vet. Sci. 2017, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Brunker, K.; Lemey, P.; Marston, D.A.; Fooks, A.R.; Lugelo, A.; Ngeleja, C.; Hampson, K.; Biek, R. Landscape attributes governing local transmission of an endemic zoonosis: Rabies virus in domestic dogs. Mol. Ecol. 2018, 27, 773–788. [Google Scholar] [CrossRef]
- Mtui-Malamsha, N.; Sallu, R.; Mahiti, G.R.; Mohamed, H.; OleNeselle, M.; Rubegwa, B.; Swai, E.S.; Makungu, S.; Otieno, E.G.; Lupindu, A.M.; et al. Ecological and Epidemiological Findings Associated with Zoonotic Rabies Outbreaks and Control in Moshi, Tanzania, 2017–2018. Int. J. Environ. Res. Public Health 2019, 16, 2816. [Google Scholar] [CrossRef]
- Brunker, K.; Jaswant, G.; Thumbi, S.M.; Lushasi, K.; Lugelo, A.; Czupryna, A.M.; Ade, F.; Wambura, G.; Chuchu, V.; Steenson, R.; et al. Rapid in-country sequencing of whole virus genomes to inform rabies elimination programmes [version 1; peer review: 3 approved]. Wellcome Open Res. 2020, 5, 3. [Google Scholar] [CrossRef]
- Hayes, S.; Lushasi, K.; Sambo, M.; Changalucha, J.; Ferguson, E.A.; Sikana, L.; Hampson, K.; Nouvellet, P.; Donnelly, C.A. Understanding the incidence and timing of rabies cases in domestic animals and wildlife in south-east Tanzania in the presence of widespread domestic dog vaccination campaigns. Vet. Res. 2022, 53, 106. [Google Scholar] [CrossRef]
- Mancy, R.; Rajeev, M.; Lugelo, A.; Brunker, K.; Cleaveland, S.; Ferguson, E.A.; Hotopp, K.; Kazwala, R.; Magoto, M.; Rysava, K.; et al. Rabies shows how scale of transmission can enable acute infections to persist at low prevalence. Science 2022, 376, 512–516. [Google Scholar] [CrossRef]
- Bautista, C.; Jaswant, G.; French, H.; Campbell, K.; Durrant, R.; Gifford, R.; Kia, G.S.N.; Ogoti, B.; Hampson, K.; Brunker, K. Whole Genome Sequencing for Rapid Characterization of Rabies Virus Using Nanopore Technology. J. Vis. Exp. 2023, 191, e65414. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.; Colquhoun, H.; Kastner, M.; Levac, D.; Ng, C.; Sharpe, J.P.; Wilson, K.; et al. A scoping review on the conduct and reporting of scoping reviews. BMC Med. Res. Methodol. 2016, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Nyasulu, P.S.; Weyer, J.; Tschopp, R.; Mihret, A.; Aseffa, A.; Nuvor, S.V.; Tamuzi, J.L.; Nyakarahuka, L.; Helegbe, G.K.; Ntinginya, N.E.; et al. Rabies mortality and morbidity associated with animal bites in Africa: A case for integrated rabies disease surveillance, prevention and control: A scoping review. BMJ Open 2021, 11, e048551. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, R. AgriFoSe2030; SLU Global, Swedish University of Agricultural Sciences: Uppsala, Sweden, 2018; pp. 1–40. [Google Scholar]
- Broban, A.; Tejiokem, M.C.; Tiembré, I.; Druelles, S.; L’Azou, M. Bolstering human rabies surveillance in Africa is crucial to eliminating canine-mediated rabies. PLoS Negl. Trop. Dis. 2018, 12, e0006367. [Google Scholar] [CrossRef]
- Sambo, M.; Cleaveland, S.; Ferguson, H.; Lembo, T.; Simon, C.; Urassa, H.; Hampson, K. The Burden of Rabies in Tanzania and Its Impact on Local Communities. PLoS Negl. Trop. Dis. 2013, 7, e2510. [Google Scholar] [CrossRef] [PubMed]
- Sambo, M.; Mahiti, G.R.; Mohamed, H.; Bernard, J.; Coetzer, A.; Felix, A.K.; Kakoko, D.; Kimboka, J.E.; Mtui-Malamsha, N.; Mbunde, M.V. Rabies intervention in Tanzania: An innovative animal vaccination model to train One Health focused student workforces to control and eliminate rabies. 2022. preprint. [Google Scholar] [CrossRef]
- Borse, R.H.; Atkins, C.Y.; Gambhir, M.; Undurraga, E.A.; Blanton, J.D.; Kahn, E.B.; Dyer, J.L.; Rupprecht, C.E.; Meltzer, M.I. Cost-effectiveness of dog rabies vaccination programs in East Africa. PLoS Negl. Trop. Dis. 2018, 12, e0006490. [Google Scholar] [CrossRef]
- Ma, X.; Boutelle, C.; Bonaparte, S.; Orciari, L.A.; Condori, R.E.; Kirby, J.D.; Chipman, R.B.; Fehlner-Gardiner, C.; Thang, C.; Cedillo, V.G.; et al. Rabies surveillance in the United States during 2022. J. Am. Vet. Med. Assoc. 2024, 262, 1518–1525. Available online: https://avmajournals.avma.org/view/journals/javma/262/11/javma.24.05.0354.xml (accessed on 24 July 2025).
- Duamor, C.T.; Lankester, F.; Mpolya, E.; Ferguson, E.A.; Johnson, P.C.D.; Wyke, S.; Cleaveland, S.; Hampson, K.; Kreppel, K. Participation in mass dog vaccination campaigns in Tanzania: Benefits of community engagement. Front. Public Health 2022, 10, 971967. [Google Scholar] [CrossRef]
- Kuzmin, I.V.; Niezgoda, M.; Franka, R.; Agwanda, B.; Markotter, W.; Beagley, J.C.; Urazova, O.Y.; Breiman, R.F.; Rupprecht, C.E. Lagos Bat Virus in Kenya. J. Clin. Microbiol. 2008, 46, 1451–1461. [Google Scholar] [CrossRef]
- Warrell, M. Rabies and African bat lyssavirus encephalitis and its prevention. Int. J. Antimicrob. Agents 2010, 36 (Suppl. 1), S47–S52. [Google Scholar] [CrossRef] [PubMed]
- Ameh, V.O.; Chirima, G.J.; Quan, M.; Sabeta, C. Public Health Awareness on Bat Rabies among Bat Handlers and Persons Residing near Bat Roosts in Makurdi, Nigeria. Pathogen 2022, 11, 975. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Villa, A.; Escobar, L.E.; Sanchez, A.; Shi, M.; Streicker, D.G.; Gallardo-Romero, N.F.; Vargas-Pino, F.; Gutierrez-Cedillo, V.; Damon, I.; Emerson, G. Successful strategies implemented towards the elimination of canine rabies in the Western Hemisphere. Antiviral Res. 2017, 143, 1–12. [Google Scholar] [CrossRef] [PubMed]

| Study Authors | Tanzania Administrative Zones (s) | Type of Rabies Diagnosis Performed | Type of Surveillance | Host Animals, Sample Types, and Related Clinical/Epidemiological Data | Assumed Lyssavirus Variant in Circulation |
|---|---|---|---|---|---|
| Gascoyn et al., 1993 [23] | Northern Zone | Sero-surveillance | Passive surveillance | African wild dogs serum samples | Canine RABV variant |
| Cleaveland and Dye 1995 [24] | Northern Zone | Fluorescent antibody test, Sero-surveillance and Clinical signs observation | Passive surveillance | Rabies case records (1977–1994) and diagnostic brain and saliva samples were collected from domestic dogs, cows, bat-eared foxes (Otocyon megalotis), and African wild dogs (Lycaon pictus). | Canine RABV variant |
| Cleveland., 1999 [25] | Northern Zone | Sero-surveillance | Passive surveillance | Domestic dogs serum | Canine RABV variant |
| East et al., 2001 [26] and Lushashi et al, 2020 [27] | Northern Zone | Sero-surveillance, Clinical signs observation and Molecular screening | Passive surveillance | Samples (brain, tissue, serum, saliva) collected in 1999 from spotted hyena, bat-eared fox, black-backed jackal, white-tailed mongoose, banded mongoose, slender mongoose, and dwarf mongoose. | Canine RABV variant |
| Lembo et al., 2006 [28] | Northern Zone | Direct rapid immunohistochemical test (dRIT) | Passive surveillance | Brainstem samples from domestic dogs, cats, cows, goats, white-tailed mongooses, black-backed jackals/bat-eared foxes, small-spotted genets, and spotted hyenas were collected between 2002 and 2004. | Canine RABV variant |
| Lembo et al., 2007 [29] | Northern Zone | Gene sequencing | Passive surveillance | Archived brain samples from domestic dogs collected between 1994 and 2001. | Canine RABV variant |
| Lembo et al., 2008 [30] | Northern Zone | Clinical and epidemiological history, Fluorescent antibody test, Inoculation of murine neuroblastoma cells and mouse inoculation and nucleoprotein gene sequencing | Passive and active surveillance | Archived brain samples from domestic cats, livestock (cows, goats), spotted hyenas, honey badgers, African wildcats, white-tailed mongooses, bat-eared foxes, small-spotted genets, jackals, and leopards tested by 2001, with further tests until 2006. | Canine RABV variant |
| Swai et al., 2010 [31] | Northern Zone | Fluorescent Antibody Technique test | Passive surveillance | Archived samples from domestic dogs, jackals, and hyenas collected between 1993 and 2002 | Canine RABV variant |
| Marston et al., 2012 [32] | Northern Zone | Gene sequencing | Passive surveillance | Archived brain samples collected from an African civet. | Assumed to be the Bat rabies variant |
| Horton et al., 2014 [33] | Northern Zone | Sero-surveillance | Passive surveillance | Serum bats samples | N/A |
| Brunker et al., 2015 [34] | Northern Zone | Gene sequencing | Passive surveillance | Archived brain samples from domestic dogs collected between 2003–2012 | Canine RABV variant |
| Mpolya. et al., 2017 [35] | Southern Eastern Zone | Clinical signs and Fluorescent antibody test | Passive surveillance | Rabid host data: clinical, historical, and epidemiological | Canine RABV variant |
| Brunker et al.,2018 [36] | Northern Zone | Genomic sequencing | Passive surveillance | Archived brain samples from domestic dogs collected between 2004 and 2013. | Canine RABV variant |
| Mtui-Malamsha et al., 2019 [37] | Coastal Zone | Sero-surveillance | Passive surveillance | Saliva and serum from randomly selected domestic dogs | Canine RABV variant |
| Kirstyn et al., 2020 [38] | Northern Zone | Nucleoprotein gene sequencing | Passive surveillance | Archived brain samples from domestic dogs, livestock, and wildlife collected between 2017 and 2019. | Canine RABV variant |
| Lushasi et al., 2020 [27] | Southern Eastern Zone | Clinical signs and epidemiological history | Passive surveillance | Rabid host data from domestic dogs: clinical signs, biting history, and epidemiology. | Canine RABV variant |
| Lushashi et al., 2021 [13] | Southern Eastern Zone | Clinical signs, epidemiological history and Fluorescent antibody test | Active surveillance | Rabid host data: Cats, domestic dogs, jackals, honey badger (Mellivora capensis), hyena, and leopard (Panthera pardus) based on clinical, historical, and epidemiological records. | Canine RABV variant |
| Hayes et al., 2022 [39] | Southern Eastern Zone | Clinical signs and Fluorescent antibody test | Passive surveillance | From 2011 to 2018, suspected rabies cases in domestic dogs and jackals were documented. | Canine RABV variant |
| Mancy et al., 2022 [40] | Northern Zone | Fluorescent antibody test, gene sequencing and Clinical signs | Passive surveillance | Rabid domestic dog data: diagnosis, clinical signs, history, and epidemiology. | Canine RABV variant |
| Bautista et al., 2023 [41] | Southern Eastern Zone | Genomic sequencing | Passive surveillance | Domestic dogs Samples archived from 2021 | Canine RABV variant |
| Host Type | Examples of Species | Role in Rabies Ecology | Relationship to Transmission Dynamics | Hotspot Zones |
|---|---|---|---|---|
| Reservoir (Primary host) | Domestic dogs | Main reservoir sustaining rabies transmission | Key source of transmission to incidental hosts and wildlife | Serengeti, Southeast Zone |
| Potential Reservoirs | Jackals, Hyenas, African wild dogs, Bats | Possible secondary reservoirs (uncertain role) | Facilitate spillover and occasional spillback to domestic dogs | Serengeti, Southeast Zone |
| Incidental Hosts | Domestic cats, Livestock (Cows, Goats), Wild carnivores (Honey badgers, Mongooses), Humans | Do not sustain the virus; acquire rabies through contact with reservoirs or potential reservoirs | Dead-end hosts in the rabies transmission chain | Serengeti, Southeast Zone, Coast Zone |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bunuma, E.K.; Keyyu, J.; Maziku, J.; Bitanyi, S.; Fyumagwa, R.; Changula, K.; Mubemba, B.; Simulundu, E.; Chitanga, S.; Horton, D.L.; et al. Rabies Surveillance in Mainland Tanzania: A Scoping Review of Animal Rabies Occurrences (1993–2023). Pathogens 2025, 14, 919. https://doi.org/10.3390/pathogens14090919
Bunuma EK, Keyyu J, Maziku J, Bitanyi S, Fyumagwa R, Changula K, Mubemba B, Simulundu E, Chitanga S, Horton DL, et al. Rabies Surveillance in Mainland Tanzania: A Scoping Review of Animal Rabies Occurrences (1993–2023). Pathogens. 2025; 14(9):919. https://doi.org/10.3390/pathogens14090919
Chicago/Turabian StyleBunuma, Emmanuel Kulwa, Julius Keyyu, Joseph Maziku, Stella Bitanyi, Robert Fyumagwa, Katendi Changula, Benjamin Mubemba, Edgar Simulundu, Simbarashe Chitanga, Daniel L. Horton, and et al. 2025. "Rabies Surveillance in Mainland Tanzania: A Scoping Review of Animal Rabies Occurrences (1993–2023)" Pathogens 14, no. 9: 919. https://doi.org/10.3390/pathogens14090919
APA StyleBunuma, E. K., Keyyu, J., Maziku, J., Bitanyi, S., Fyumagwa, R., Changula, K., Mubemba, B., Simulundu, E., Chitanga, S., Horton, D. L., Ekiri, A. B., Sawa, H., & Muleya, W. (2025). Rabies Surveillance in Mainland Tanzania: A Scoping Review of Animal Rabies Occurrences (1993–2023). Pathogens, 14(9), 919. https://doi.org/10.3390/pathogens14090919






