Mycobacterial Spindle Cell Pseudotumor Presenting as a Pancreatic Head Mass: A Case Report
Abstract
1. Introduction
2. Case Report
2.1. Patient Background
2.2. Four Months Before Admission
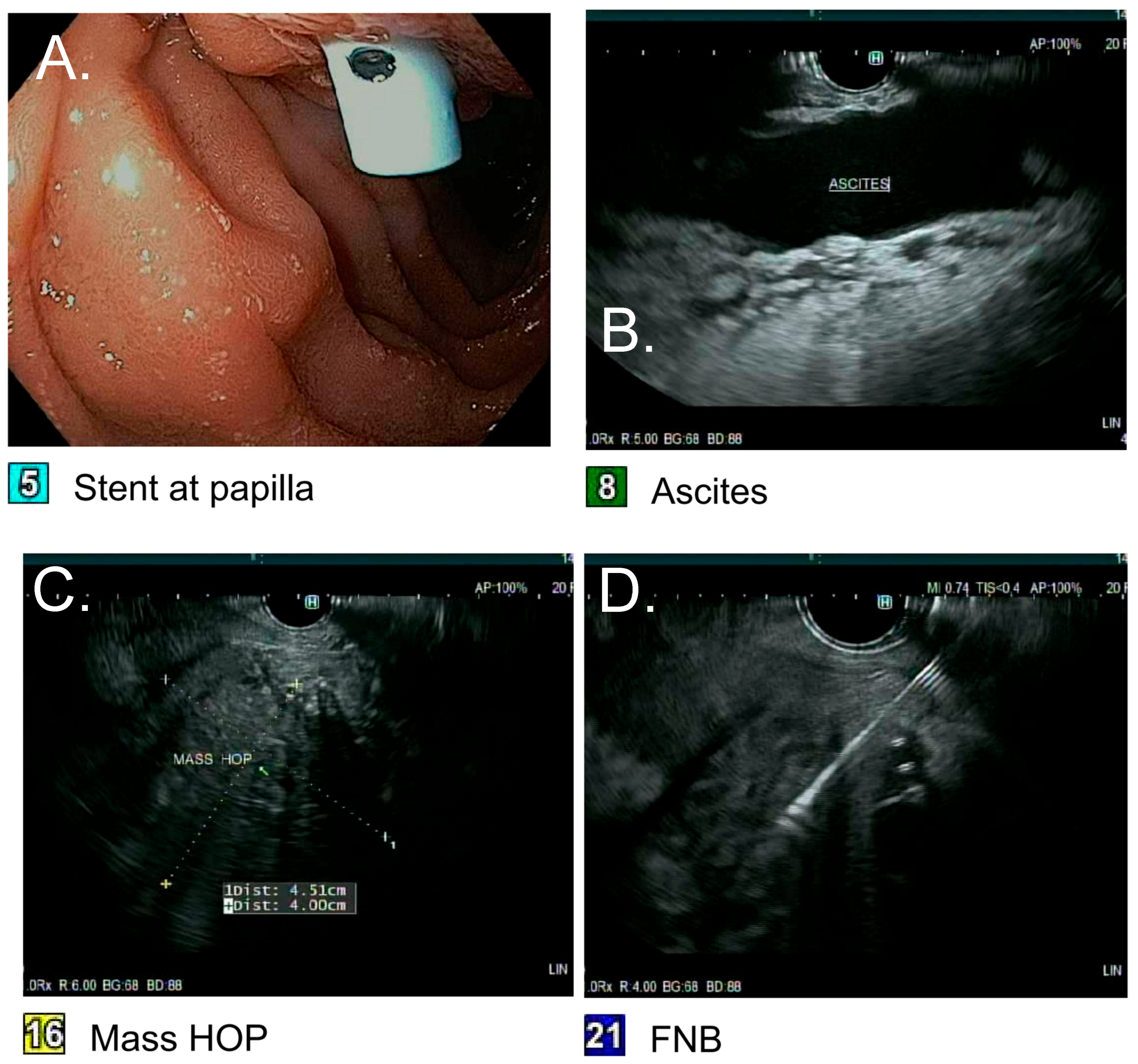
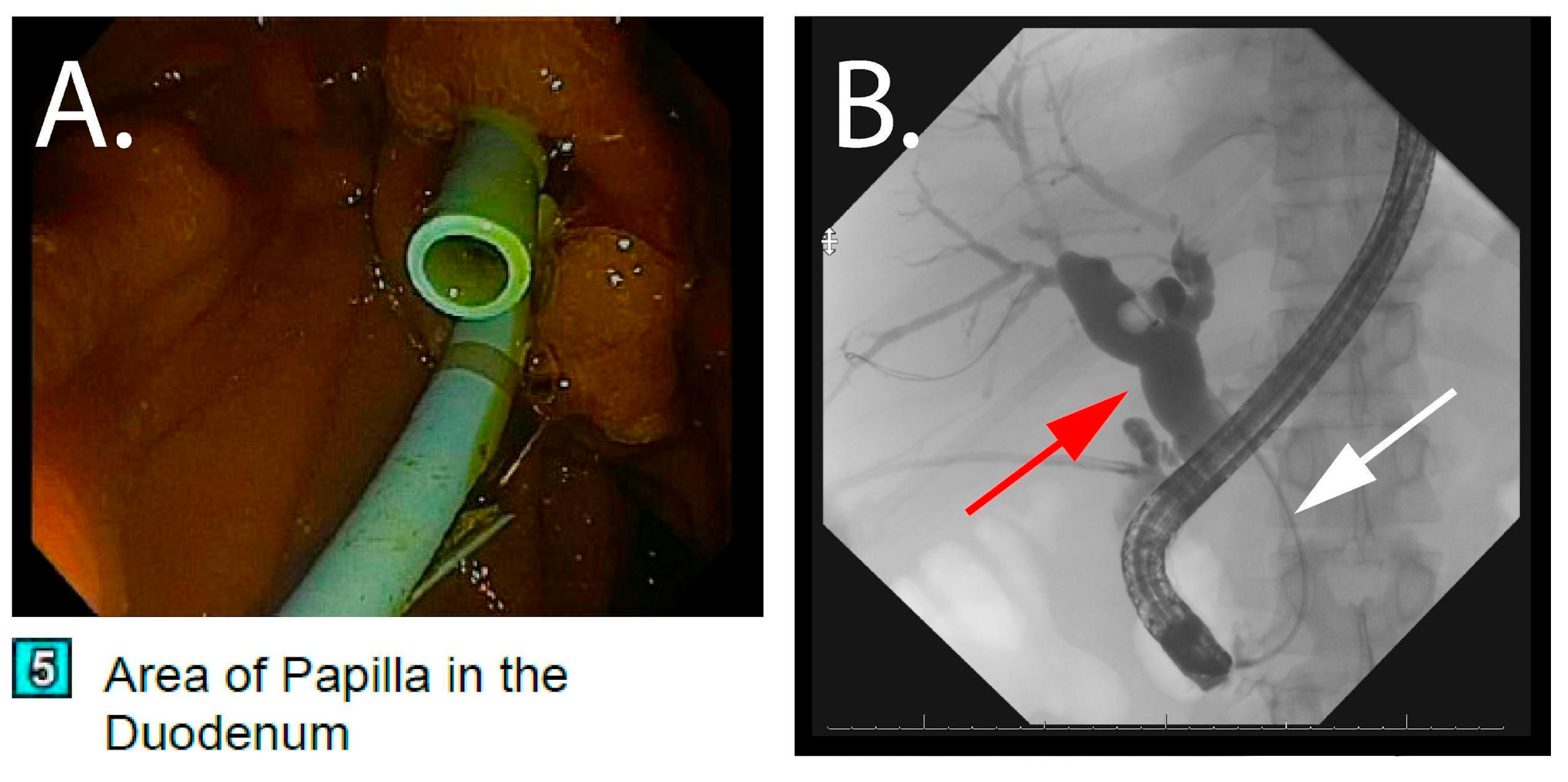
2.3. Index Hospitalization
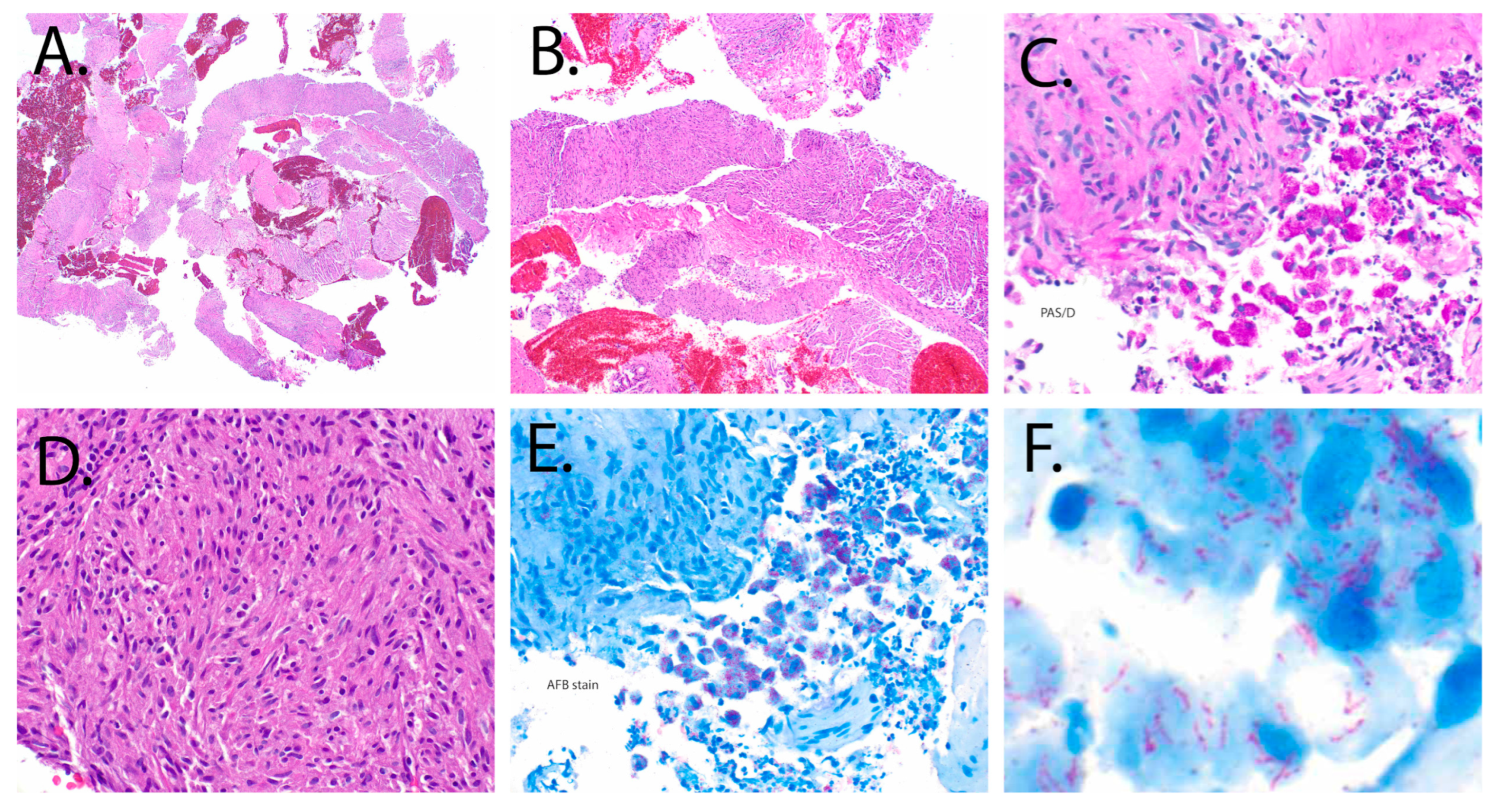
2.4. Pathology
2.5. Antimycobacterial Therapy and Outcomes
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MSCPs | mycobacterial spindle cell pseudotumors |
| HIV | human immunodeficiency virus |
| CD4 | cluster of differentiation 4 T-lymphocytes |
| MAC | Mycobacterium avium complex |
| IRIS | immune reconstitution inflammatory syndrome |
| CT | computed tomography |
| ERCP | endoscopic retrograde cholangiopancreatography |
| EUS | endoscopic ultrasound |
| IU | international units |
| L | liter |
| mg/dL | milligrams/deciliter |
| AST | aspartate transaminase |
| ALT | alanine transaminase |
| SAAG | serum ascites albumin gradient |
| EGD | esophagogastroduodenoscopy |
| IV | intravenously |
| mg | milligrams |
| g | grams |
| mm3 | cubic meters |
| NTM | non-tuberculous mycobacterial |
| RNA | ribonucleic acid |
| PCR | polymerase chain reaction |
| AIDS | acquired immunodeficiency syndrome |
References
- Sfeir, M.M.; Schuetz, A.; Van Besien, K.; Borczuk, A.C.; Soave, R.; Jenkins, S.G.; Walsh, T.J.; Small, C.B. Mycobacterial spindle cell pseudotumour: Epidemiology and clinical outcomes. J. Clin. Pathol. 2018, 71, 626–630. [Google Scholar] [CrossRef]
- Logani, S.; Lucas, D.R.; Cheng, J.D.; Ioachim, H.L.; Adsay, N.V. Spindle cell tumors associated with mycobacteria in lymph nodes of HIV-positive patients: ‘Kaposi sarcoma with mycobacteria’ and ‘mycobacterial pseudotumor. Am. J. Surg. Pathol. 1999, 23, 656–661. [Google Scholar] [CrossRef]
- Sekosan, M.; Cleto, M.; Senseng, C.; Farolan, M.; Sekosan, J. Spindle cell pseudotumors in the lungs due to Mycobacterium tuberculosis in a transplant patient. Am. J. Surg. Pathol. 1994, 18, 1065–1068. [Google Scholar] [CrossRef]
- Chesdachai, S.; Udompap, P.; Li, F.; Lake, J.R.; Kc, M. Pulmonary Mycobacterium Spindle Cell Pseudotumor in Patient With Liver Transplant. Am. J. Med. Sci. 2020, 359, 42–50. [Google Scholar] [CrossRef]
- Coelho, R.; Hanna, R.; Flagg, A.; Stempak, L.M.; Ondrejka, S.; Procop, G.W.; Harrington, S.; Zembillas, A.; Kusick, K.; Gonzalez, B.E. Mycobacterium genavense-induced spindle cell pseudotumor in a pediatric hematopoietic stem cell transplant recipient: Case report and review of the literature. Transpl. Infect. Dis. 2017, 19, e12656. [Google Scholar] [CrossRef]
- Franco, M.; Amoroso, A.; Burke, A.P.; Britt, E.J.; Reed, R.M. Pulmonary mycobacterial spindle cell pseudotumor in a lung transplant patient: Progression without therapy and response to therapy. Transpl. Infect. Dis. 2015, 17, 424–428. [Google Scholar] [CrossRef]
- Dhibar, D.P.; Sahu, K.K.; Singh, S.; Bal, A.; Chougale, A.; Dhir, V. Tubercular Mycobacterial Spindle Cell Pseudotumour: A Case Report. Iran J. Med. Sci. 2018, 43, 94–96. [Google Scholar] [PubMed]
- Phowthongkum, P.; Puengchitprapai, A.; Udomsantisook, N.; Tumwasorn, S.; Suankratay, C. Spindle cell pseudotumor of the brain associated with Mycobacterium haemophilum and Mycobacterium simiae mixed infection in a patient with AIDS: The first case report. Int. J. Infect. Dis. 2008, 12, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Philip, J.; Beasley, M.B.; Dua, S. Mycobacterial spindle cell pseudotumor of the lung. Chest 2012, 142, 783–784. [Google Scholar] [CrossRef]
- Poyuran, R.; Ponnambath, D.K.; Vilanilam, G.C.; Chandrasekharan, K.; Narasimhaiah, D. Mycobacterial spindle cell pseudotumour of the brain in an immunocompetent adult. Pathology 2021, 53, 782–785. [Google Scholar] [CrossRef]
- Rahmani, M.; Alroy, J.; Zoukhri, D.; Wein, R.O.; Tischler, A.S. Mycobacterial pseudotumor of the skin. Virchows Arch. Int. J. Pathol. 2013, 462, 843–846. [Google Scholar] [CrossRef]
- Shiomi, T.; Yamamoto, T.; Manabe, T. Mycobacterial spindle cell pseudotumor of the skin. J. Cutan. Pathol. 2007, 34, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.; Gyure, K.A.; Stone, J.; Wong, K.; McEvoy, P.; Koeller, K.; Mena, H. Mycobacterial spindle cell pseudotumor of the brain: A case report and review of the literature. Am. J. Surg. Pathol. 1999, 23, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Suster, S.; Moran, C.A.; Blanco, M. Mycobacterial spindle-cell pseudotumor of the spleen. Am. J. Clin. Pathol. 1994, 101, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Nieto, M.I.; Pérez-Robledo, J.P.; Díaz-San Andrés, B.; Nistal, M.; Rodríguez-Montes, J.A. Inflammatory pseudotumour of the spleen associated with splenic tuberculosis. World J. Gastrointest. Surg. 2014, 6, 248–252. [Google Scholar] [CrossRef]
- Pantiora, E.V.; Sakellaridis, E.P.; Kontis, E.A.; Fragulidis, G.P. Inflammatory Pseudotumor of the Liver Presented in a Patient with Cholelithiasis. Cureus 2018, 10, e3231. [Google Scholar] [CrossRef]
- Manitsas, S.; South, C.; Panchal, P.; Yearsley, M. Mycobacterial Spindle Cell Pseudotumor of the Colon: A Case Report. Am J Gastroenterol. 2008, 103, S235. [Google Scholar] [CrossRef]
- Basilio-de-Oliveira, C.; Eyer-Silva, W.A.; Valle, H.A.; Rodrigues, A.L.; Pinheiro Pimentel, A.L.; Morais-De-Sá, C.A. Mycobacterial spindle cell pseudotumor of the appendix vermiformis in a patient with aids. Braz. J. Infect. Dis. 2001, 5, 98–100. [Google Scholar] [CrossRef]
- Arthur, A.; Fortier, J.C.; Vincek, V.; Valdes Rodriguez, R.H. Mycobacterial Spindle Cell Pseudotumor (MSP): A case report. Cureus 2023, 15, e40432. [Google Scholar] [CrossRef]
- Daley, C.L.; Iaccarino, J.M.; Lange, C.; Cambau, E.; Wallace, R.J., Jr.; Andrejak, C.; Böttger, E.C.; Brozek, J.; Griffith, D.E.; Guglielmetti, L.; et al. Treatment of nontuberculous mycobacterial pulmonary disease: An official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur. Respir. J. 2020, 56, 2000535. [Google Scholar] [CrossRef]
- Duong, M.T.; Ungemach, A.; Malik, F.; Wasserman, N.; Cooper, K.; Pantel, A.R.; O’Neil, J.C.; Szep, Z. Mycobacterial spindle cell pseudotumor of the spinal cord: Case report and literature review. J. Neuroimmunol. 2024, 390, 578329. [Google Scholar] [CrossRef]
- Lim, H.-J.; Park, C.M.; Park, Y.S.; Lee, J.; Lee, S.-M.; Yang, S.-C.; Yoo, C.-G.; Kim, Y.W.; Han, S.K.; Yim, J.-J. Isolation of multiple nontuberculous mycobacteria species in the same patients. Int. J. Infect. Dis. 2011, 15, e795–e798. [Google Scholar] [CrossRef]

| Treatment Phase | Regimen | Approximate Duration | Reason for Change/Key Events |
|---|---|---|---|
| Initial | Amikacin, Ethambutol, Clarithromycin, Cefoxitin | First few weeks | Maculopapular rash attributed to cefoxitin |
| Phase 2 | Amikacin, Ethambutol, Clarithromycin, Imipenem | Subsequent 9 months | Ototoxicity from amikacin; amikacin discontinued |
| Phase 3 | Omadacycline, Imipenem, Azithromycin | Additional 9 months | Completed to total 18 months’ therapy; radiographic and clinical resolution |
| Reference (Year) | Organism Identified | Host (Status) | Site | Outcome |
|---|---|---|---|---|
| Current report | Mycobacterium avium complex (blood + supraclavicular lymph node culture), Mycobacterium grossiae (liver biopsy), Mycobacterium abscessus (liver biopsy) | Advanced HIV/AIDS; CD4 nadir < 50 | Pancreas | Mass effect resolved on prolonged multi-drug therapy; biliary/portal HTN complications improved |
| Suster et al., 1994 [14] | Nontuberculous mycobacteria (species not reported) | Advanced HIV/AIDS | Localized to spleen at autopsy | Died (Other cause-CNS toxoplasmosis; MSCP incidental at autopsy) |
| Sekosan et al., 1994 [3] | Mycobacterium tuberculosis | Solid-organ transplant recipient | Pulmonary | Died within 4 days of starting Tuberculosis treatment |
| Chesdachai et al., 2020 [4] | Mycobacterium avium–intracellulare complex | Liver transplant recipient | Pulmonary (masses), later also found on colonoscopy | Not reported |
| Morrison et al., 1999 [13] | Mycobacterium avium–intracellulare complex | HIV-negative; chronic steroids for sarcoidosis | Left frontal lobe, both parietal regions, and the right cerebellar hemisphere | Lesions resolved with surgical resection + anti-NTM therapy: Amikacin, clarithromycin, ethambutol + rifampin→ azithromycin, ethambutol + rifampine; amikacin was discontinued because of worsening ataxia. No comment on the duration of therapy. |
| Phowthongkum et al., 2008 [8] | Mycobacterium haemophilum + Mycobacterium simiae (mixed) | Advanced HIV/AIDS | Multiple lesions in the left cerebral peduncle and medulla | Partial surgical excision of the left medulla + anti-NTM therapy: Isoniazid, rifampin, pyrazinamide, ethambutol, + clarithromycin. No comment on the duration of therapy. Documented improvement with some residual neurological deficits. |
| Poyuran et al., 2021 [10] | Not reported | Immunocompetent adult | Intracranial lesion | Not reported |
| Basílio-de-Oliveira et al., 2001 [18] | NTM (species not determined) | Advanced HIV/AIDS (autopsy case) | liver, lymph nodes, spleen, skin, large and small intestine; also with disseminated histoplasmosis | Died (other cause-overwhelming sepsis) |
| Rahmani et al., 2013 [11] | Mycobacterium avium (PCR) | Liver transplant recipient | Cutaneous | Died (other cause- multiorgan failure) |
| Shiomi et al., 2007 [12] | Mycobacterium intracellulare | SLE (on prednisolone and azathioprine), insulin-dependent diabetes mellitus | Cutaneous | Not reported |
| Duong et al., 2024 [21] | Mycobacterium avium (blood + cerebrospinal fluid) | Advanced HIV/AIDS | Intradural spinal cord mass at L3–4 | Azithromycin, ethambutol, rifampin → azithromycin, ethambutol, rifabutin for >1 year with ART |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cusimano, F.A.; Herrera, T.; Brust, D.; Montgomery, E.; Amin, S.; Ayoade, F. Mycobacterial Spindle Cell Pseudotumor Presenting as a Pancreatic Head Mass: A Case Report. Pathogens 2025, 14, 889. https://doi.org/10.3390/pathogens14090889
Cusimano FA, Herrera T, Brust D, Montgomery E, Amin S, Ayoade F. Mycobacterial Spindle Cell Pseudotumor Presenting as a Pancreatic Head Mass: A Case Report. Pathogens. 2025; 14(9):889. https://doi.org/10.3390/pathogens14090889
Chicago/Turabian StyleCusimano, Frank A, Tara Herrera, Douglas Brust, Elizabeth Montgomery, Sunil Amin, and Folusakin Ayoade. 2025. "Mycobacterial Spindle Cell Pseudotumor Presenting as a Pancreatic Head Mass: A Case Report" Pathogens 14, no. 9: 889. https://doi.org/10.3390/pathogens14090889
APA StyleCusimano, F. A., Herrera, T., Brust, D., Montgomery, E., Amin, S., & Ayoade, F. (2025). Mycobacterial Spindle Cell Pseudotumor Presenting as a Pancreatic Head Mass: A Case Report. Pathogens, 14(9), 889. https://doi.org/10.3390/pathogens14090889






