Uncovering Hidden Transmission: Active Surveillance Reveals Cryptic Circulation of Yellow Fever Virus in Urban Marmosets in Belo Horizonte, Brazil, 2024
Abstract
1. Introduction
2. Materials and Methods
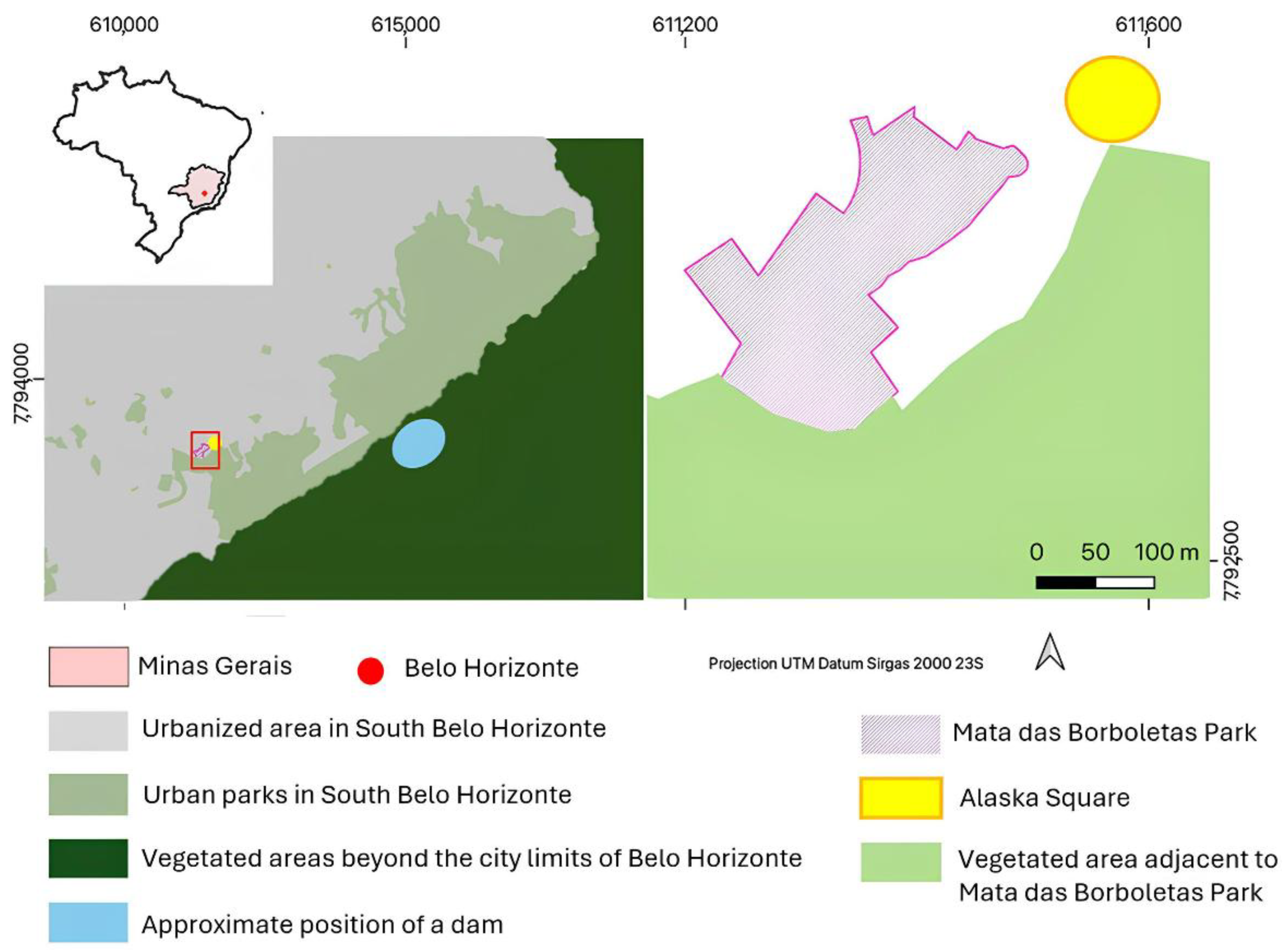
2.1. Study Area
2.2. Mosquito Sampling and Identification
2.3. YFV Molecular Screening
2.4. Serological Screening
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| µL | microliter |
| BH | Belo Horizonte |
| CEUA | Ethical Committee for Animal Experimentation |
| CHIKV | chikungunya virus |
| CMC | carboxymethylcellulose |
| COI | cytochrome c oxidase subunit I |
| DNA | deoxyribonucleic acid |
| E1 | envelope protein 1 |
| F | forward |
| HEPES | 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid |
| ID | identification |
| IgG | immunoglobulin G |
| IgM | immunoglobulin M |
| kg | kilogram |
| MEM | minimum essential medium |
| MG | Minas Gerais state |
| mg | milligram |
| mL | milliliter |
| n | number |
| na | not available |
| NCBI | National Center for Biotechnology Information |
| neg | negative |
| neut | neutralization |
| NHP | non-human primate |
| NS5 | nonstructural protein 5 |
| °C | Celsius degrees |
| OROV | Oropouche virus |
| P | probe |
| PCR | polymerase chain reaction |
| PFU | plaque-forming units |
| pos | positive |
| PRNT | plaque-reduction neutralization test |
| R | reverse |
| RNA | ribonucleic acid |
| RT-qPCR | reverse transcriptase polymerase chain reaction |
| SISBIO | Brazil’s Biodiversity Licensing and Information System |
| UTR | untranslated region |
| YF | yellow fever |
| YFV | yellow fever virus |
| ZIKV | Zika virus |
Appendix A
References
- Silva, N.I.O.; Sacchetto, L.; de Rezende, I.M.; Trindade, G.S.; LaBeaud, A.D.; de Thoisy, B.; Drumond, B.P. Recent Sylvatic Yellow Fever Virus Transmission in Brazil: The News from an Old Disease. Virol. J. 2020, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Secretaria de Estado de Saúde de Minas Gerais. Alerta-Cenário Epidemiológico Febre Amarela, 2021 (SES/SUBVS-SVE-DVAT-CEVARB 1877/2021). 2021. Available online: https://www.saude.mg.gov.br/febreamarela/ (accessed on 24 June 2025).
- Secretaria de Estado de Saúde de Minas Gerais. Boletim Epidemiológico Especial: Encerramento do Período Sazonal de Monitoramento da Febre Amarela (Julho/2022 a Junho/2023) e Fortalecimento das Ações de Imunização em Minas Gerais, 2023/2024 (Boletim Epidemiológico SES/SUBVS-SVE-DVAT-CEPI 4618/2023). 2023. Available online: https://www.saude.mg.gov.br/febreamarela/ (accessed on 24 June 2025).
- Secretaria de Estado de Planejamento e Gestão do Estado de Minas Gerais. Cobertura Vacinal Contra a Febre Amarela Quase Dobra em um Ano. MG.GOV.BR-Planejamento. 2018. Available online: https://www.mg.gov.br/planejamento/noticias/saude/01/2018/cobertura-vacinal-contra-febre-amarela-quase-dobra-em-um-ano (accessed on 24 June 2025).
- Andrade, M.S.; Campos, F.S.; de Oliveira, C.H.; Oliveira, R.S.; Campos, A.A.S.; de Almeida, M.A.B.; Fonseca, V.S.; Simonini-Teixeira, D.; Sevá, A.P.; Temponi, A.O.D.; et al. Fast Surveillance Response Reveals the Introduction of a New Yellow Fever Virus Sub-Lineage in 2021, in Minas Gerais, Brazil. Mem. Inst. Oswaldo Cru. 2022, 117, e220127. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Oliveira, G.F.; Guimarães, A.C.S.; Moreira, G.D.; Costa, T.A.; Arruda, M.S.; de Mello, É.M.; Silva, M.C.; de Almeida, M.G.; Hanley, K.A.; Vasilakis, N.; et al. Correction: Garcia-Oliveira et al. YELLOW ALERT: Persistent Yellow Fever Virus Circulation among Non-Human Primates in Urban Areas of Minas Gerais State, Brazil (2021–2023). Viruses 2024, 16, 1527. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, C.H.; Andrade, M.S.; Campos, F.S.; Cardoso, J.C.; Gonçalves-Dos-Santos, M.E.; Oliveira, R.S.; Aquino-Teixeira, S.M.; Campos, A.A.; Almeida, M.A.B.; Simonini-Teixeira, D.; et al. Yellow Fever Virus Maintained by Sabethes Mosquitoes during the Dry Season in Cerrado, a Semiarid Region of Brazil, in 2021. Viruses 2023, 15, 757. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.I.O.; Albery, G.F.; Arruda, M.S.; Oliveira, G.F.G.; Costa, T.A.; de Mello, É.M.; Moreira, G.D.; Reis, E.V.; da Silva, S.A.; Silva, M.C.; et al. Ecological Drivers of Sustained Enzootic Yellow Fever Virus Transmission in Brazil, 2017–2021. PLoS Negl. Trop. Dis. 2023, 17, e0011407. [Google Scholar] [CrossRef]
- Minas Gerais Department of Health. Distribuição das Epizootias Ocorridas em PNH e Classificação, Segundo Município e URS de Ocorrência, Minas Gerais, Período de Monitoramento 1 2024–2025. 2025. Available online: https://www.saude.mg.gov.br/wp-content/uploads/2025/05/Tabela-Epizootias05-05-2025-1.pdf (accessed on 17 July 2025).
- Brazilian Ministry of Health. Painel Febre Amarela. Ministério da Saúde. Available online: https://www.gov.br/saude/pt-br/composicao/svsa/cnie/painel-febre-amarela (accessed on 17 July 2025).
- Brazilian Ministry of Health. Guia de Vigilância de Epizootias em Primatas Não Humanos e Entomologia Aplicada à Vigilância da Febre Amarela, 2nd ed.; Brazilian Ministry of Health: Brasília, Brazil, 2014.
- Teixeira, B.; Hirsch, A.; Goulart, V.D.L.R.; Passos, L.; Teixeira, C.P.; James, P.; Young, R. Good Neighbours: Distribution of Black-Tufted Marmoset (Callithrix penicillata) in an Urban Environment. Wildl. Res. 2015, 42, 579–589. [Google Scholar] [CrossRef]
- de Abreu, F.V.S.; de Andreazzi, C.S.; Neves, M.S.A.S.; Meneguete, P.S.; Ribeiro, M.S.; Dias, C.M.G.; Motta, M.A.; Barcellos, C.; Romão, A.R.; Magalhães, M.A.F.M.; et al. Ecological and Environmental Factors Affecting Transmission of Sylvatic Yellow Fever in the 2017–2019 Outbreak in the Atlantic Forest, Brazil. Parasites Vectors 2022, 15, 23. [Google Scholar] [CrossRef]
- Fundação João Pinheiro. Panorama de Belo Horizonte–Atlas Histórico; Fundação João Pinheiro: Belo Horizonte, Brazil, 1997. [Google Scholar]
- Centro de Informações Estratégicas em Saúde do Estado de Minas Gerais. Orientação aos Profissionais de Saúde acerca da Detecção de Febre Amarela em Primata Não Humano no Município de Belo Horizonte, Minas Gerais (Alerta Epidemiológico 5). 2024. Available online: https://www.saude.mg.gov.br/aedes/orientacoes/ (accessed on 24 June 2025).
- Fundação Municipal de Parques e Zoobotânica da Prefeitura de Belo Horizonte. Parque Mata das Borboletas. Available online: https://prefeitura.pbh.gov.br/fundacao-de-parques-e-zoobotanica/informacoes/parques/parque-mata-das-borboletas (accessed on 24 June 2025).
- Forattini, O.P. Culicidologia Médica: Identificação, Biologia e Epidemiologia, 2nd ed.; EDUSP: São Paulo, Brazil, 2002; Volume 2. [Google Scholar]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; Dewaard, J.R. Biological Identifications through DNA Barcodes. Proc. R. Soc. Lond. B. 2003, 270, 313–321. [Google Scholar] [CrossRef]
- de Rezende, I.M.; Alves, P.A.; Arruda, M.S.; Gonçalves, A.P.; Oliveira, G.F.G.; Pereira, L.S.; Dutra, M.R.T.; Campi-Azevedo, A.C.; Valim, V.; Tourinho, R.; et al. Yellow Fever Virus Genotyping Tool and Investigation of Suspected Adverse Events Following Yellow Fever Vaccination. Vaccines 2019, 7, 206. [Google Scholar] [CrossRef]
- Domingo, C.; Patel, P.; Yillah, J.; Weidmann, M.; Méndez, J.A.; Nakouné, E.R.; Niedrig, M. Advanced Yellow Fever Virus Genome Detection in Point-of-Care Facilities and Reference Laboratories. J. Clin. Microbiol. 2012, 50, 4054–4060. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and Serologic Properties of Zika Virus Associated with an Epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008, 14, 1232–1239. [Google Scholar] [CrossRef]
- Naveca, F.G.; do Nascimento, V.A.; de Souza, V.C.; Nunes, B.T.D.; Rodrigues, D.S.G.; Vasconcelos, P.F.C. Multiplexed Reverse Transcription Real-Time Polymerase Chain Reaction for Simultaneous Detection of Mayaro, Oropouche, and Oropouche-like Viruses. Mem. Inst. Oswaldo Cruz. 2017, 112, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.J.; Welch, S.R.; Chamberlain, J.; Hewson, R.; Tolley, H.; Cane, P.A.; Lloyd, G. Molecular Diagnosis and Analysis of Chikungunya Virus. J. Clin. Virol. 2007, 39, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Landt, O.; Kaiser, M.; Faye, O.; Koppe, T.; Lass, U.; Sall, A.A.; Niedrig, M. Development of One-Step Quantitative Reverse Transcription PCR for the Rapid Detection of Flaviviruses. Virol. J. 2013, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA Sequencing with Chain-Terminating Inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef]
- Moreira, G.D. Samanthex, Version 2.0; GitHub: San Francisco, CA, USA, 2025; Available online: https://github.com/Dias0202/SAMANTHEX_2.0 (accessed on 10 June 2025).
- Moreira, G.D. Samanthex, version 1.0.0; Zenodo: Geneva, Switzerland, 2025. [Google Scholar] [CrossRef]
- BOLD Systems. BOLD-The Barcode of Life Data Systems; BOLD Systems: Toronto, ON, Canada. Available online: https://boldsystems.org/ (accessed on 24 June 2025).
- Yamamoto, M.E. From Dependence to Sexual Maturity: The Behavioural Ontogeny of Callitrichidae. In Marmosets and Tamarins: Systematics, Behaviour, and Ecology; Rylands, A.B., Ed.; Oxford University Press: Oxford, UK, 1993; pp. 235–254. [Google Scholar]
- Couto-Lima, D.; Madec, Y.; Bersot, M.I.; Campos, S.S.; Motta, M.A.; dos Santos, F.B.; Vazeille, M.; Vasconcelos, P.F.C.; Lourenço-De-Oliveira, R.; Failloux, A.-B. Potential Risk of Re-Emergence of Urban Transmission of Yellow Fever Virus in Brazil Facilitated by Competent Aedes Populations. Sci. Rep. 2017, 7, 4848. [Google Scholar] [CrossRef]
- Damasceno-Caldeira, R.; Nunes-Neto, J.P.; Aragão, C.F.; Freitas, M.N.O.; Ferreira, M.S.; de Castro, P.H.G.; Dias, D.D.; Araújo, P.A.S.; Brandão, R.C.F.; Nunes, B.T.D.; et al. Vector Competence of Aedes albopictus for Yellow Fever Virus: Risk of Reemergence of Urban Yellow Fever in Brazil. Viruses 2023, 15, 1019. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.S.; Faria, N.R.; Caleiro, G.S.; Candido, D.S.; Hill, S.C.; Claro, I.M.; da Costa, A.C.; Nogueira, J.S.; Maeda, A.Y.; da Silva, F.G.; et al. Genomic Evidence of Yellow Fever Virus in Aedes scapularis, Southeastern Brazil, 2016. Acta Trop. 2020, 205, 105390. [Google Scholar] [CrossRef]
- Shinde, D.P.; Plante, J.A.; Scharton, D.; Mitchell, B.; Walker, J.; Azar, S.R.; Campos, R.K.; Sacchetto, L.; Drumond, B.P.; Vasilakis, N.; et al. Potential Role of Heterologous Flavivirus Immunity in Preventing Urban Transmission of Yellow Fever Virus. Nat. Commun. 2024, 15, 9728. [Google Scholar] [CrossRef]
- Hill, S.C.; Dellicour, S.; Claro, I.M.; Sequeira, P.C.; Adelino, T.; Thézé, J.; Wu, C.-H.; Moreira, F.R.R.; Giovanetti, M.; Li, S.L.; et al. Climate and Land-Use Shape the Spread of Zoonotic Yellow Fever Virus. medRxiv 2022. [Google Scholar] [CrossRef]
- Sun, J.; Tang, X.; Bai, R.; Liang, C.; Zeng, L.; Lin, H.; Yuan, R.; Zhou, P.; Huang, X.; Xiong, Q.; et al. The Kinetics of Viral Load and Antibodies to SARS-CoV-2. Clin. Microbiol. Infect. 2020, 26, 1690.e1–1690.e4. [Google Scholar] [CrossRef]
- Mason, R.A.; Tauraso, N.M.; Spertzel, R.O.; Ginn, R.K. Yellow Fever Vaccine: Direct Challenge of Monkeys Given Graded Doses of 17D Vaccine. Appl. Microbiol. 1973, 25, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Hanley, K.A.; Azar, S.R.; Campos, R.K.; Vasilakis, N.; Rossi, S.L. Support for the Transmission-Clearance Trade-Off Hypothesis from a Study of Zika Virus Delivered by Mosquito Bite to Mice. Viruses. 2019, 11, 1072. [Google Scholar] [CrossRef] [PubMed]
- Hanley, K.A.; Cecilia, H.; Azar, S.R.; Moehn, B.A.; Gass, J.T.; da Silva, N.I.O.; Yu, W.; Yun, R.; Althouse, B.M.; Vasilakis, N.; et al. Trade-Offs Shaping Transmission of Sylvatic Dengue and Zika Viruses in Monkey Hosts. Nat. Commun. 2024, 15, 2682. [Google Scholar] [CrossRef] [PubMed]
- Hayes, E.B.; Fischer, M. Flaviviruses. In Principles and Practice of Pediatric Infectious Diseases, 4th ed.; Long, S.S., Ed.; Elsevier: Edinburgh, UK, 2012; pp. 1099–1102.e2. [Google Scholar]
- Rezende, I.M.; Pereira, L.S.; Fradico, J.R.B.; Xavier, M.A.P.; Alves, P.A.; Campi-Azevedo, A.C.; Speziali, E.; dos Santos, L.Z.M.; Albuquerque, N.S.; Penido, I.; et al. Late-Relapsing Hepatitis after Yellow Fever. Viruses 2020, 12, 222. [Google Scholar] [CrossRef]
- Monath, T.P. Yellow Fever: An Update. Lancet Infect. Dis. 2001, 1, 11–20. [Google Scholar] [CrossRef]
- Medeiros-Sousa, A.R.; Ceretti-Júnior, W.; de Carvalho, G.C.; Nardi, M.S.; Araujo, A.B.; Vendrami, D.P.; Marrelli, M.T. Diversity and Abundance of Mosquitoes (Diptera: Culicidae) in an Urban Park: Larval Habitats and Temporal Variation. Acta Trop. 2015, 150, 200–209. [Google Scholar] [CrossRef]
- Tátila-Ferreira, A.; Maia, D.D.A.; De Abreu, F.V.S.; Rodrigues, W.C.; Alencar, J. Oviposition Behavior of Haemagogus leucocelaenus (Diptera: Culicidae), a Vector of Wild Yellow Fever in Brazil. Rev. Inst. Med. Trop. Sao Paulo 2017, 59, e60. [Google Scholar] [CrossRef][Green Version]
| Target | Sequence | Reference |
|---|---|---|
| COI | 5′-GGTCAACAAATCATAAAGATATTGG-3′ (F) 5′-TAAACTTCAGGGTGACCAAAAAATCA-3′ (R) | Hebert et al., 2003 [18] |
| β-actin | 5′-CCAACCGCGAGAAGATGA-3′ (F) 5′-CCAGAGGCGTACAGGGATAG-3′ (R) | Rezende et al., 2019 [19] |
| YFV | 5′-GCTAATTGAGGTGYATTGGTCTGC-3′ (F) 5′-ATCGAATGCACCGCACACT-3′ (R) 5′-ATCGAGTTGCTAGGCAATAAACAC-3′ (P) | Domingo et al., 2012 [20] |
| ZIKV | 5′-CCGCTGCCCAACACAAG-3′ (F) 5′-CCACTAACGTTCTTTTGCAGACAT-3′ (R) 5′-AGCCTACCTTGACAAGCAGTCAGACACTCAA-3′ (P) | Lanciotti et al., 2008 [21] |
| OROV | 5′ TCCGGAGGCAGCATATGTG-3′ (F) 5′-ACAACACCAGCATTGAGCACTT-3′ (R) 5′-CATTTGAAGCTAGATACGG-3′ (P) | Naveca et al., 2017 [22] |
| CHIKV | 5′-TCGACGCGCCCTCTTTAA-3′ (F) 5′-CTGCTAATCGCTCAAMGAACG-3′ (R) 5′-ACCAGCCTGCACCCATTCCTCAGAC-3′ (P) | Edwards et al., 2007 [23] |
| Pan-orthoflavivirus | 5′-TACAACATGATGGGGAARAGAGARAA-3′ (F) 5′-GTGTCCCAGCCNGCKGTGTCATCWGC-3′ (R) | Patel et al., 2013 [24] |
| Genus/species | Ground | Canopy | Total |
|---|---|---|---|
| Aedes aegypti | 5 | 1 | 6 |
| Aedes albopictus | 13 | 1 | 14 |
| Aedes fluviatilis | 112 | 11 | 123 |
| Aedes scapularis | 66 | 8 | 74 |
| Aedes spp. | 3 | 0 | 3 |
| Culex spp. | 9 | 13 | 22 |
| Wyeomyia spp. | 1 | 0 | 1 |
| Wyeomyia melanocephala | 3 | 2 | 5 |
| Total | 212 | 36 | 248 * |
| NHP ID | Month | Age | RT-qPCR | PRNT50 | Lateral Flow Test | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| YFV | CHIKV | ZIKV | OROV | Pan Flavi | YFV IgM | DENV IgM/ IgG | ZIKV IgM/ IgG | ||||
| CT24-214 | July 2024 | adult | neg | neg | neg | neg | neg | neg | pos | neg/ neg | neg/ neg |
| CT24-215 | July 2024 | adult | neg | neg | neg | neg | neg | neg | neg | na | na |
| CT24-216 | July 2024 | adult | neg | neg | neg | neg | neg | neg | neg | na | na |
| CT24-217 | July 2024 | juvenile | neg | neg | neg | neg | neg | neg | pos | neg/ neg | neg/ neg |
| CT24-218 | July 2024 | juvenile | neg | neg | neg | neg | neg | neg | pos | neg/ neg | neg/ neg |
| CT24-219 | July 2024 | adult | neg | neg | neg | neg | neg | neg | pos | neg/ neg | neg/ neg |
| CT24-220 | July 2024 | old adult | neg | neg | neg | neg | neg | neg | neg | na | na |
| CT24-216 * | September 2024 | adult | neg | neg | neg | neg | neg | neg | pos | neg/ neg | neg/ neg |
| CT24-234 | April 2025 | old adult | neg | neg | neg | neg | neg | neg | neg | na | na |
| CT24-214 * | April 2025 | adult | neg | neg | neg | neg | neg | neg | neg | na | na |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arruda, M.S.; Costa, T.A.; Moreira, G.D.; Jacob, D.; de Oliveira, M.A.; Biccas, M.F.; de Oliveira Paschoal, A.M.; Guimarães, A.C.D.S.; Viegas, S.S.F.M.; Garcia-Oliveira, G.F.; et al. Uncovering Hidden Transmission: Active Surveillance Reveals Cryptic Circulation of Yellow Fever Virus in Urban Marmosets in Belo Horizonte, Brazil, 2024. Pathogens 2025, 14, 866. https://doi.org/10.3390/pathogens14090866
Arruda MS, Costa TA, Moreira GD, Jacob D, de Oliveira MA, Biccas MF, de Oliveira Paschoal AM, Guimarães ACDS, Viegas SSFM, Garcia-Oliveira GF, et al. Uncovering Hidden Transmission: Active Surveillance Reveals Cryptic Circulation of Yellow Fever Virus in Urban Marmosets in Belo Horizonte, Brazil, 2024. Pathogens. 2025; 14(9):866. https://doi.org/10.3390/pathogens14090866
Chicago/Turabian StyleArruda, Matheus Soares, Thaís Alkifeles Costa, Gabriel Dias Moreira, Daniel Jacob, Marcelle Alves de Oliveira, Mikaelly Frasson Biccas, Ana Maria de Oliveira Paschoal, Anna Catarina Dias Soares Guimarães, Samantha Stephany Fiuza Meneses Viegas, Gabriela Fernanda Garcia-Oliveira, and et al. 2025. "Uncovering Hidden Transmission: Active Surveillance Reveals Cryptic Circulation of Yellow Fever Virus in Urban Marmosets in Belo Horizonte, Brazil, 2024" Pathogens 14, no. 9: 866. https://doi.org/10.3390/pathogens14090866
APA StyleArruda, M. S., Costa, T. A., Moreira, G. D., Jacob, D., de Oliveira, M. A., Biccas, M. F., de Oliveira Paschoal, A. M., Guimarães, A. C. D. S., Viegas, S. S. F. M., Garcia-Oliveira, G. F., Cruz, A. L. C., Almeida, L. T., Souza e Silva, M. F. A., da Rocha Vilela, D. A., Mendes, T. M., Alves, P. A., Hanley, K. A., Vasilakis, N., do Vale Beirão, M., & Drumond, B. P. (2025). Uncovering Hidden Transmission: Active Surveillance Reveals Cryptic Circulation of Yellow Fever Virus in Urban Marmosets in Belo Horizonte, Brazil, 2024. Pathogens, 14(9), 866. https://doi.org/10.3390/pathogens14090866









