Viral Infection Correlates with a Better Clinical Outcome than Pulmonary Exacerbation of Bacterial Origin in Paediatric Patients with Cystic Fibrosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Clinical Analysis
2.3. PCR Testing for Respiratory Viruses and Bacteria Detection
2.4. Statistical Analysis
3. Results
3.1. Clinical Description of the Studied Group
3.2. Results of Epidemiological Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CF | Cystic fibrosis |
| CFTR | Cystic fibrosis transmembrane regulator |
| SK | Shwachman–Kulczycki (score) |
| PE | Pulmonary exacerbation |
| BMI | Body mass index |
| SD | Standard deviation |
| FEV1 | Forced expiratory volume in the first second |
| FRES | Resonant Frequency |
| AX | Area of reactance |
| TLC | Total lung capacity |
References
- Sanders, D.B.; Bittner, R.C.L.; Rosenfeld, M.; Hoffman, L.R.; Redding, G.J.; Goss, C.H. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am. J. Respir. Crit. Care Med. 2010, 182, 627–632. [Google Scholar] [CrossRef]
- Sanders, D.B.; Hoffman, L.R.; Emerson, J.; Gibson, R.L.; Rosenfeld, M.; Redding, G.J.; Goss, C.H. Return of FEV1 after pulmonary exacerbation in children with cystic fibrosis. Pediatr. Pulmonol. 2010, 45, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Goss, C.H. Acute Pulmonary Exacerbations in Cystic Fibrosis. Crit. Care Med. 2019, 40, 792–803. [Google Scholar] [CrossRef]
- Emerson, J.; Rosenfeld, M.; McNamara, S.; Ramsey, B.; Gibson, R.L. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr. Pulmonol. 2002, 34, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Konstan, M.W.; Morgan, W.J.; Butler, S.M.; Pasta, D.J.; Craib, M.L.; Silva, S.J.; Stokes, D.C.; Wohl, M.E.B.; Wagener, J.S.; Regelmann, W.E.; et al. Risk Factors For Rate of Decline in Forced Expiratory Volume in One Second in Children and Adolescents with Cystic Fibrosis. J. Pediatr. 2007, 151, 134–139.e1. [Google Scholar] [CrossRef]
- Waters, V.; Ratjen, F. Pulmonary exacerbations in children with cystic fibrosis. Ann. Am. Thorac. Soc. 2015, 12, S200–S206. [Google Scholar] [CrossRef]
- Aaron, S.D.; Ramotar, K.; Ferris, W.; Vandemheen, K.; Saginur, R.; Tullis, E.; Haase, D.; Kottachchi, D.; Denis, M.S.; Chan, F. Adult cystic fibrosis exacerbations and new strains of Pseudomonas aeruginosa. Am. J. Respir. Crit. Care Med. 2004, 169, 811–815. [Google Scholar] [CrossRef]
- Blanchard, A.C.; Waters, V.J. Microbiology of Cystic Fibrosis Airway Disease. Semin. Respir. Crit. Care Med. 2019, 40, 727–736. [Google Scholar] [CrossRef]
- Caverly, L.J.; Vandevanter, D.R. The Elusive Role of Airway Infection in Cystic Fibrosis Exacerbation. J. Pediatr. Infect. Dis. Soc. 2022, 11 (Suppl. S2), S40–S45. [Google Scholar] [CrossRef]
- Van Ewijk, B.E.; Wolfs, T.F.; Aerts, P.C.; Van Kessel, K.P.; Fleer, A.; Kimpen, J.L.; Van der Ent, C.K. RSV mediates Pseudomonas aeruginosa binding to cystic fibrosis and normal epithelial cells. Pediatr. Res. 2007, 61, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Chattoraj, S.S.; Ganesan, S.; Jones, A.M.; Helm, J.M.; Comstock, A.T.; Bright-Thomas, R.; LiPuma, J.J.; Hershenson, M.B.; Sajjan, U.S. Rhinovirus infection liberates planktonic bacteria from biofilm and increases chemokine responses in cystic fibrosis airway epithelial cells. Thorax 2011, 66, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Kiedrowski, M.R.; Bomberger, J.M. Viral-Bacterial Co-infections in the Cystic Fibrosis Respiratory Tract. Front. Immunol. 2018, 9, 3067. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.R.; Neuzil, K.M.; Victor, J.C.; Wald, A.; Aitken, M.L.; Goss, C.H. Influenza-associated cystic fibrosis pulmonary exacerbations. Chest 2010, 137, 852–860. [Google Scholar] [CrossRef]
- Somayaji, R.; Goss, C.H.; Khan, U.; Neradilek, M.; Neuzil, K.M.; Ortiz, J.R. Cystic fibrosis pulmonary exacerbations attributable to respiratory syncytial virus and influenza: A population-based study. Clin. Infect. Dis. 2017, 64, 1760–1767. [Google Scholar] [CrossRef]
- Kerem, E.; Viviani, L.; Zolin, A.; MacNeill, S.; Hatziagorou, E.; Ellemunter, H.; Drevinek, P.; Gulmans, V.; Krivec, U.; Olesen, H. Factors associated with FEV1 decline in cystic fibrosis: Analysis of the ECFS patient registry. Eur. Respir. J. 2014, 43, 125–133. [Google Scholar] [CrossRef]
- Dalbøge, C.S.; Hansen, C.R.; Pressler, T.; Høiby, N.; Johansen, H.K. Chronic pulmonary infection with Stenotrophomonas maltophilia and lung function in patients with cystic fibrosis. J. Cyst. Fibros. 2011, 10, 318–325. [Google Scholar] [CrossRef]
- 2023 Annual Data Report. Available online: http://www.ecfs.eu/ecfspr (accessed on 12 August 2025).
- Burgel, P.-R.; Southern, K.W.; Addy, C.; Battezzati, A.; Berry, C.; Bouchara, J.-P.; Brokaar, E.; Brown, W.; Azevedo, P.; Durieu, I.; et al. Standards for the care of people with cystic fibrosis (CF); recognising and addressing CF health issues. J. Cyst. Fibros. 2024, 23, 187–202. [Google Scholar] [CrossRef]
- Cleveland, R.H.; Stamoulis, C.; Sawicki, G.; Kelliher, E.; Zucker, E.J.; Wood, C.; Zurakowski, D.; Lee, E. Brasfield and Wisconsin scoring systems have equal value as outcome assessment tools of cystic fibrosis lung disease. Pediatr. Radiol. 2014, 44, 529–534. [Google Scholar] [CrossRef]
- Hafen, G.M.; Ranganathan, S.C.; Robertson, C.F.; Robinson, P.J. Clinical scoring systems in cystic fibrosis. Pediatr. Pulmonol. 2006, 41, 602–617. [Google Scholar] [CrossRef] [PubMed]
- van Ewijk, B.E.; van der Zalm, M.M.; Wolfs, T.F.W.; van der Ent, C.K. Viral respiratory infections in cystic fibrosis. J. Cyst. Fibros. 2005, 4 (Suppl. S2), 31–36. [Google Scholar] [CrossRef]
- Hizal, M.; Yalcin, E.; Alp, A.; Ozden, M.; Karakaya, J.; Polat, S.E.; Tugcu, G.; Dogru, D.; Ozcelik, U.; Kiper, N. Respiratory viruses: What is their role in acute exacerbations in children with cystic fibrosis? Pediatr. Pulmonol. 2020, 55, 1646–1652. [Google Scholar] [CrossRef]
- Wat, D.; Doull, I. Respiratory virus infections in cystic fibrosis. Paediatr. Respir. Rev. 2003, 4, 172–177. [Google Scholar] [CrossRef]
- Armstrong, D.; Grimwood, K.; Carlin, J.B.; Carzino, R.; Hull, J.; Olinsky, A.; Phelan, P.D. Severe viral respiratory infections in infants with cystic fibrosis. Pediatr. Pulmonol. 1998, 26, 371–379. [Google Scholar] [CrossRef]
- Hiatt, P.W.; Grace, S.C.; Kozinetz, C.A.; Raboudi, S.H.; Treece, D.G.; Taber, L.H.; Piedra, P.A. Effects of viral lower respiratory tract infection on lung function in infants with cystic fibrosis. Pediatrics 1999, 103, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Smyth, R.L.; Smyth, A.R.; Tong, C.Y.W.; Hart, C.A.; Heaf, D.P. Effect of respiratory virus infections including rhinovirus on clinical status in cystic fibrosis. Arch. Dis. Child. 1995, 73, 117–120. [Google Scholar] [CrossRef]
- Bilton, D.; Canny, G.; Conway, S.; Dumcius, S.; Hjelte, L.; Proesmans, M.; Tümmler, B.; Vavrova, V.; De Boeck, K. Pulmonary exacerbation: Towards a definition for use in clinical trials. Report from the EuroCareCF Working Group on outcome parameters in clinical trials. J. Cyst. Fibros. 2011, 10, 79–81. [Google Scholar] [CrossRef]
- Castellani, C.; Simmonds, N.J.; Barben, J.; Addy, C.; Bevan, A.; Burgel, P.-R.; Drevinek, P.; Gartner, S.; Gramegna, A.; Lammertyn, E.; et al. Standards for the care of people with cystic fibrosis (CF): A timely and accurate diagnosis. J. Cyst. Fibros. 2023, 22, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, B.W.; Gore, E.J.; Smith, A.L.; Cooney, M.K.; Redding, G.J.; Foy, H. The Effect of Respiratory Viral Infections on Patients With Cystic Fibrosis. Arch. Pediatr. Adolesc. Med. 1989, 143, 662–668. [Google Scholar] [CrossRef]
- Esther, C.R.; Lin, F.C.; Kerr, A.; Miller, M.B.; Gilligan, P.H. Respiratory viruses are associated with common respiratory pathogens in cystic fibrosis. Pediatr. Pulmonol. 2014, 49, 926–931. [Google Scholar] [CrossRef]
- Jones, A.M. Infection control in cystic fibrosis: Evolving perspectives and challenges. Curr. Opin. Pulm. Med. 2022, 28, 571–576. [Google Scholar] [CrossRef]
- VMeyer, V.M.C.; Siqueira, M.M.; Costa, P.F.B.M.; Caetano, B.C.; Lopes, J.C.O.; Folescu, T.W.; Motta, F.D.C.; Omri, A. Clinical impact of respiratory virus in pulmonary exacerbations of children with Cystic Fibrosis. PLoS ONE 2020, 15, e0240452. [Google Scholar] [CrossRef]
- Gulla, K.M.; Balaji, A.; Mukherjee, A.; Jat, K.R.; Sankar, J.; Lodha, R.; Kabra, S.K. Course of illness after viral infection in Indian children with cystic fibrosis. J. Trop. Pediatr. 2019, 65, 176–182. [Google Scholar] [CrossRef]
- Scott, J.E.; O’Toole, G.A. The yin and yang of Streptococcus lung infections in cystic fibrosis: A model for studying polymicrobial interactions. J. Bacteriol. 2019, 201, e00115-19. [Google Scholar] [CrossRef] [PubMed]
- Scherz, V.; Caruana, G.; Taffé, P.; Brouillet, R.; Bertelli, C.; Jaton, K.; Fougère, Y.; Posfay-Barbe, K.M.; Mornand, A.; Rochat-Guignard, I.; et al. Unexpected associations between respiratory viruses and bacteria with Pulmonary Function Testing in children suffering from Cystic Fibrosis (MUCOVIB study). J. Cyst. Fibros. 2022, 21, e158–e164. [Google Scholar] [CrossRef]
- Hamed, D.H.; Soliman, M.S.; Emam, O.S.; El Attar, M.M. Is there a role of viral infection in cystic fibrosis exacerbation in children? Turk. J. Pediatr. 2022, 64, 549–557. [Google Scholar] [CrossRef]
- Wat, D.; Gelder, C.; Hibbitts, S.; Cafferty, F.; Bowler, I.; Pierrepoint, M.; Evans, R.; Doull, I. The role of respiratory viruses in cystic fibrosis. J. Cyst. Fibros. 2008, 7, 320–328. [Google Scholar] [CrossRef]
- Gonzalez-Rosales, N.; Kasi, A.S.; McCracken, C.E.; Silva, G.L.; Starks, M.; Stecenko, A.; Guglani, L. Impact of viral respiratory infections on pulmonary exacerbations in children with cystic fibrosis. Pediatr. Pulmonol. 2023, 58, 871–877. [Google Scholar] [CrossRef]
- Carter, S.C.; Franciosi, A.N.; O’sHea, K.M.; O’cArroll, O.M.; Sharma, A.; Bell, A.; Keogan, B.; O’rEilly, P.; Coughlan, S.; Law, S.M.; et al. Acute Pulmonary Exacerbation Phenotypes in Patients with Cystic Fibrosis. Ann. Am. Thorac. Soc. 2022, 19, 1818–1826. [Google Scholar] [CrossRef]
- Hilliam, Y.; Armbruster, C.R.; Atteih, S.E.; Rapsinski, G.J.; Moore, J.; Koirala, J.; Krainz, L.; Gaston, J.; Williams, J.; Cooper, V.S.; et al. Respiratory viral infection is associated with increased Pseudomonas abundance in cystic fibrosis airways. bioRxiv 2025. [Google Scholar] [CrossRef] [PubMed]
- Kartsiouni, E.; Chatzipanagiotou, S.; Tamvakeras, P.; Douros, K. The role of viral infections in pulmonary exacerbations of patients with non-cystic fibrosis bronchiectasis: A systematic review. Respir. Investig. 2022, 60, 625–632. [Google Scholar] [CrossRef]
- Sinha, M.; Zabini, D.; Guntur, D.; Nagaraj, C.; Enyedi, P.; Olschewski, H.; Kuebler, W.M.; Olschewski, A. Chloride channels in the lung: Challenges and perspectives for viral infections, pulmonary arterial hypertension, and cystic fibrosis. Pharmacol. Ther. 2022, 237, 108249. [Google Scholar] [CrossRef] [PubMed]
- Londino, J.D.; Lazrak, A.; Jurkuvenaite, A.; Collawn, J.F.; Noah, J.W.; Matalon, S. Influenza matrix protein 2 alters CFTR expression and function through its ion channel activity. Am. J. Physiol. Cell. Mol. Physiol. 2013, 304, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Londino, J.D.; Lazrak, A.; Noah, J.W.; Aggarwal, S.; Bali, V.; Woodworth, B.A.; Bebok, Z.; Matalon, S. Influenza virus M2 targets cystic fibrosis transmembrane conductance regulator for lysosomal degradation during viral infection. ASEB J. 2015, 29, 2712–2725. [Google Scholar] [CrossRef]
- Cao, K.; Chen, M.; Jie, X.; Wang, Y.; Li, Q.; Xu, J. H5N1 virus hemagglutinin inhibition of cAMP-dependent CFTR via TLR4-mediated Janus tyrosine kinase 3 activation exacerbates lung inflammation. Mol. Med. 2015, 21, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kwon, H.J.; Jang, Y.J. Effects of rhinovirus infection on the expression and function of cystic fibrosis transmembrane conductance regulator and epithelial sodium channel in human nasal mucosa. Ann. Allergy Asthma Immunol. 2012, 108, 182–187. [Google Scholar] [CrossRef]
- Annual Reports|European Cystic Fibrosis Society (ECFS). Available online: https://www.ecfs.eu/projects/ecfs-patient-registry/annual-reports (accessed on 4 June 2025).
- Saluzzo, F.; Riberi, L.; Messore, B.; Loré, N.I.; Esposito, I.; Bignamini, E.; De Rose, V. CFTR Modulator Therapies: Potential Impact on Airway Infections in Cystic Fibrosis. Cells 2022, 11, 1243. [Google Scholar] [CrossRef]
- De Jong, E.; Garratt, L.W.; Looi, K.; Lee, A.H.; Ling, K.-M.; Smith, M.L.; Falsafi, R.; Sutanto, E.N.; Hillas, J.; Iosifidis, T.; et al. Ivacaftor or lumacaftor/ivacaftor treatment does not alter the core CF airway epithelial gene response to rhinovirus. J. Cyst. Fibros. 2021, 20, 97–105. [Google Scholar] [CrossRef]
- Thornton, C.S.; Caverly, L.J.; Kalikin, L.M.; Carmody, L.A.; McClellan, S.; LeBar, W.; Sanders, D.B.; West, N.E.; Goss, C.H.; Flume, P.A.; et al. Prevalence and Clinical Impact of Respiratory Viral Infections from the STOP2 Study of Cystic Fibrosis Pulmonary Exacerbations. Ann. Am. Thorac. Soc. 2024, 21, 595–603. [Google Scholar] [CrossRef]
- Fireizen, Y.; Ahmed, M.; Vigers, T.; Akong, K.; Ryu, J.; Hahn, A.; Fanous, H.; Koumbourlis, A.; Tirakitsoontorn, P.; Arrieta, A.; et al. Changing Epidemiology of Pediatric Pulmonary Exacerbations in Cystic Fibrosis. Pediatr. Pulmonol. 2025, 60, e71019. [Google Scholar] [CrossRef]
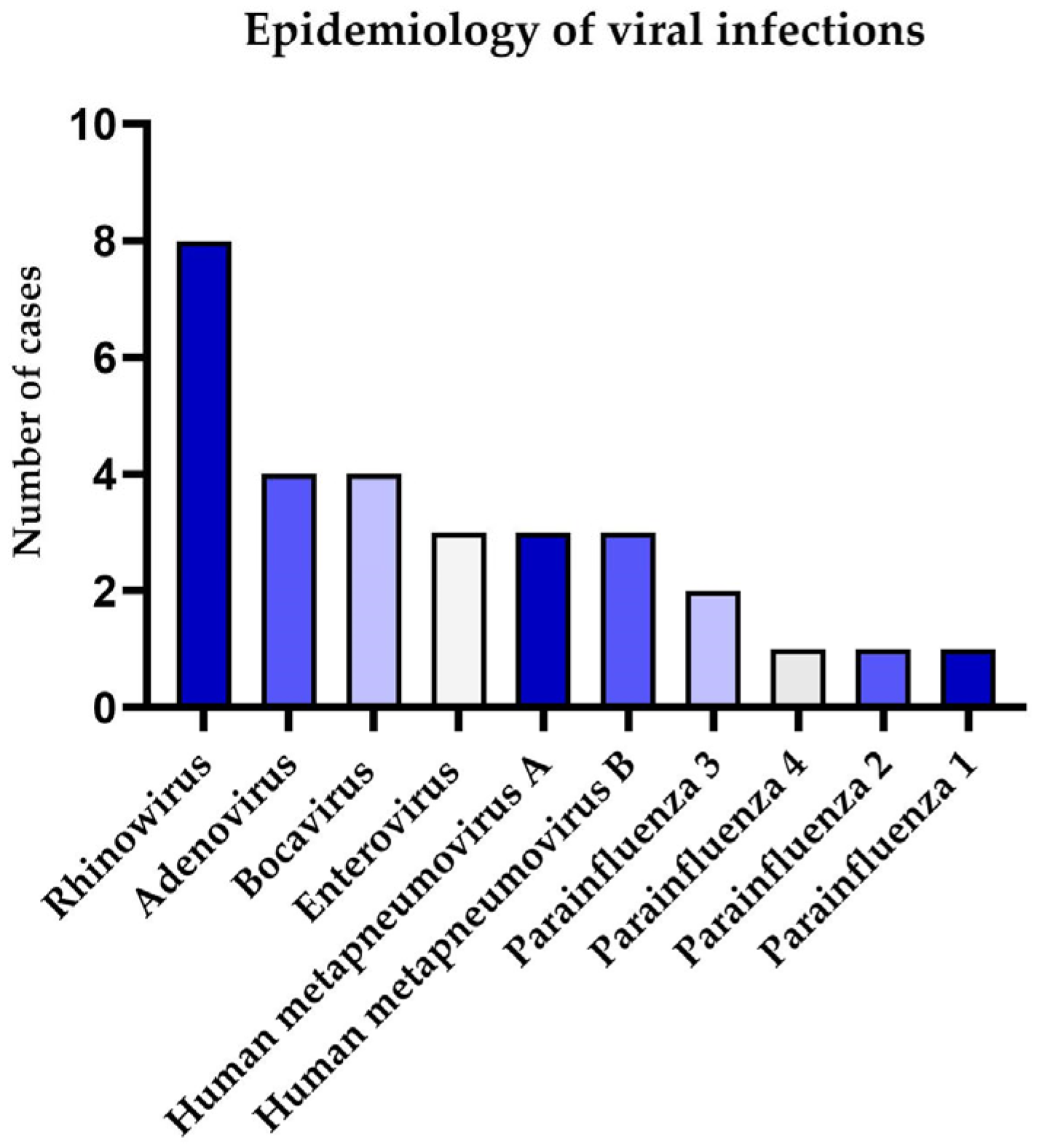

| Variable | PE | Viral-Negative | Viral-Positive | p-Value |
|---|---|---|---|---|
| Number of samples | 49 | 30 | 19 | - |
| Sex, female | 22 (44.9%) | 12 (40.0%) | 10 (52.6%) | - |
| Median age, years (range) | 10 (0.5–17) | 11 (2–17) | 6 (0.5–17) | 0.002 |
| F508del homozygous | 20 (40.8%) | 10 (33.3%) | 10 (52.6%) | 1.000 |
| F508del heterozygous | 18 (36.7%) | 9 (30.0%) | 9 (47.4%) | 1.000 |
| Other variants | 11 (22.4%) | 11 (36.7%) | 0 | 0.003 |
| Median body mass index, BMI, kg/m2 (range) | 14.81 (11.2–21.53) | 14.93 (13.33–20.9) | 14.79 (11.2–21.53) | 0.700 |
| Pancreatic insufficiency | 43 (87.8%) | 27 (90.0%) | 16 (84.0%) | 0.547 |
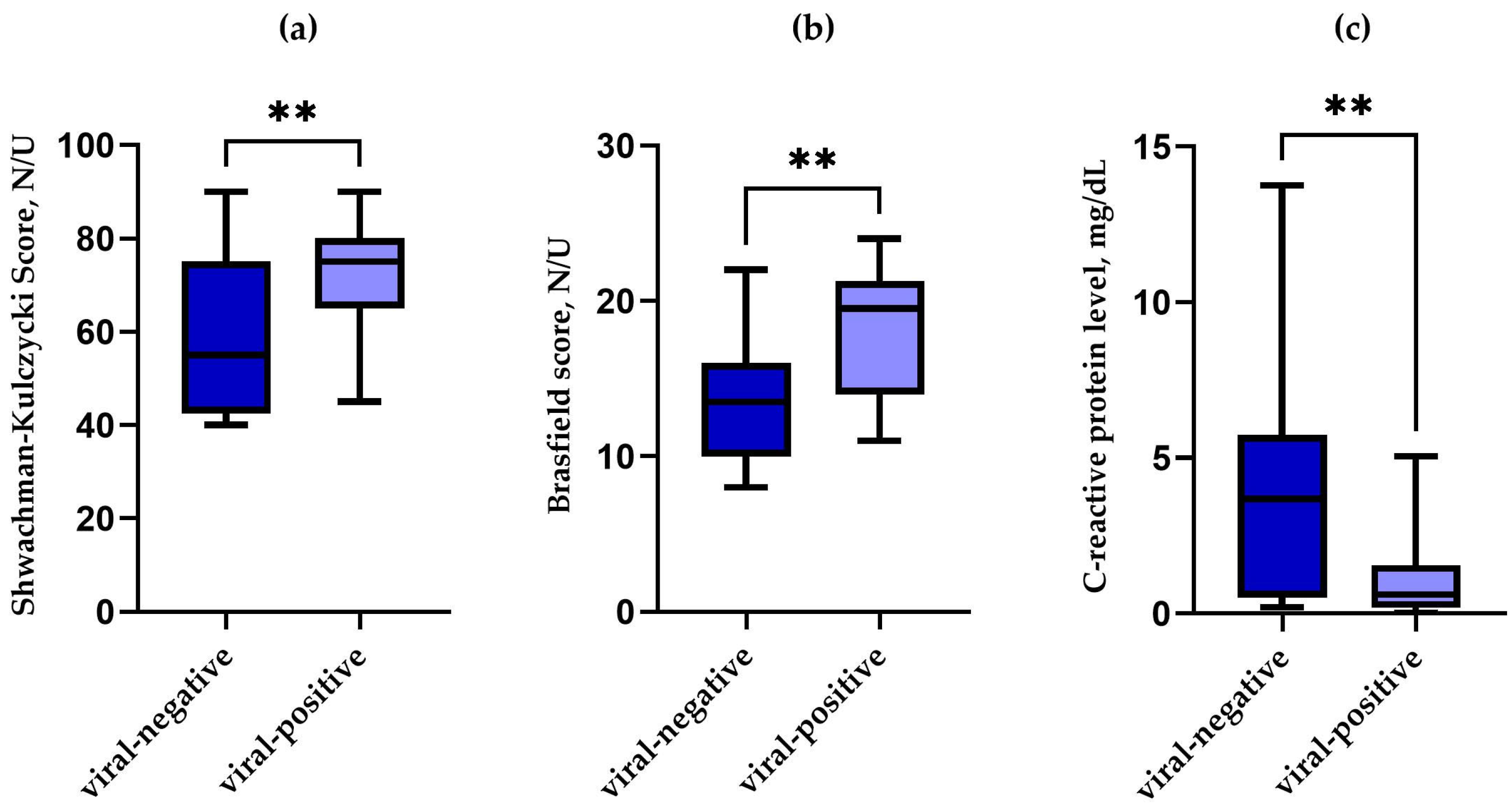
| Median SK score (range) | 65 (40–90) | 57.5 (40–90) | 75 (45–90) | 0.006 |
| Median Brasfield score (range) | 15 (8–24) | 13.5 (8–22) | 19 (11–24) | 0.002 |
| Median oxygen saturation, % | 92.0 (50.1–99.5) | 90.7 (56.6–99.0) | 94.3 (50.1–99.5) | 0.209 |
| Median C-reactive protein level, mg/dL, (range) | 1.50 (0.02–13.75) | 3.68 (0.20–13.75) | 0.61 (0.02–5.05) | 0.002 |
| Median absolute neutrophil count, 103/mm3 (range) | 6.68 (1.33–24.28) | 6.70 (1.33–17.39) | 5.08 (1.58–24.28) | 0.312 |
| Mean forced expiratory volume in the first second, FEV1 z-score (SD) | −2.02 (±1.82) | −1.99 (±1.82) | −2.12 (±1.88) | 0.840 |
| Median Resonant Frequency, Fres (range) | 19.74 (11.45–31.37) | 19.71 (11.45–31.37) | 21.54 (12.37–29.47) | 0.639 |
| Median reactance at 10 Hz % due value, 10x due value (range) | 212 (−2748–3493) | 240 (−2748–3493) | 185.5 (−66–427) | 0.577 |
| Median area of reactance, AX (range) | 1.60 (0.22–5.14) | 1.43 (0.22–5.14) | 1.79 (0.32–4.45) | 0.566 |
| Mean total lung capacity, TLC (SD) | 4.29 (±1.14) | 4.52 (±1.10) | 3.94 (±1.17) | 0.077 |
| Variable | Stable | Viral-Negative | Viral-Positive | p-Value |
|---|---|---|---|---|
| Number of samples | 16 | 10 | 6 | - |
| Sex, female | 10 (62.5%) | 8 (80.0%) | 2 (33.3%) | - |
| Mean age, years (SD) | 8.43 (±4.20) | 8.65 (±4.40) | 8.08 (±4.21) | 0.804 |
| F508del homozygous | 7 (43.8%) | 4 (40.0%) | 3 (50.0%) | 0.696 |
| F508del heterozygous | 6 (37.5%) | 4 (40.0%) | 2 (33.3%) | 0.790 |
| Other variants | 3 (18.7%) | 2 (20.0%) | 1 (16.7%) | 0.869 |
| Mean body mass index, BMI, kg/m2 (SD) | 15.16 (±2.27) | 15.41 (±2.46) | 14.17 (±2.06) | 0.591 |
| Pancreatic insufficiency | 15 (93.8%) | 9 (90.0%) | 6 (100%) | 0.790 |
| Median SK score (range) | 70 (40–85) | 60 (40–80) | 77.5 (55–85) | 0.106 |
| Median Brasfield score (range) | 18 (11–24) | 17 (11–24) | 20 (11–24) | 0.999 |
| Median oxygen saturation, % | 90.65 (47.7–96.6) | 92.1 (47.7–96.6) | 87.9 (73–96.6) | 0.476 |
| Median C-reactive protein level, mg/dL, (range) | 0.2 (0.02–4.57) | 0.2 (0.02–1.57) | 0.71 (0.03–4.57) | 0.196 |
| Mean absolute neutrophil count, 103/mm3 (SD) | 4.50 (±2.45) | 4.70 (±2.23) | 4.19 (±2.95) | 0.950 |
| Median forced expiratory volume in the first second, FEV1 z-score (range) | −2.46 (−4.46–1.2) | −2.26 (−4.46–(−0.10)) | −2.58 (−2.58–1.2) | 0.897 |
| Mean Resonant Frequency, Fres (SD) | 22.73 (±6.97) | 22.40 (±5.69) | 23.34 (±9.61) | 0.819 |
| Median reactance at 10 Hz % due value, 10x due value (range) | 212 (−365–1577) | 192 (−365–1577) | 212 (−47–270) | 1.000 |
| Mean area of reactance, AX (SD) | 2.262 (1.554) | 2.306 (1.703) | 2.184 (1.425) | 0.895 |
| Median total lung capacity, TLC (range) | 4.78 (2.96–6.03) | 5.22 (2.96–6.03) | 4.53 (4.38–4.68) | 0.487 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stachowiak, Z.; Andrzejewska, M.; Jończyk-Potoczna, K.; Narożna, B.; Musiał, A.; Wiesner, A.; Bręborowicz, A.; Szczepankiewicz, A.; Wojsyk-Banaszak, I. Viral Infection Correlates with a Better Clinical Outcome than Pulmonary Exacerbation of Bacterial Origin in Paediatric Patients with Cystic Fibrosis. Pathogens 2025, 14, 850. https://doi.org/10.3390/pathogens14090850
Stachowiak Z, Andrzejewska M, Jończyk-Potoczna K, Narożna B, Musiał A, Wiesner A, Bręborowicz A, Szczepankiewicz A, Wojsyk-Banaszak I. Viral Infection Correlates with a Better Clinical Outcome than Pulmonary Exacerbation of Bacterial Origin in Paediatric Patients with Cystic Fibrosis. Pathogens. 2025; 14(9):850. https://doi.org/10.3390/pathogens14090850
Chicago/Turabian StyleStachowiak, Zuzanna, Marta Andrzejewska, Katarzyna Jończyk-Potoczna, Beata Narożna, Anna Musiał, Anna Wiesner, Anna Bręborowicz, Aleksandra Szczepankiewicz, and Irena Wojsyk-Banaszak. 2025. "Viral Infection Correlates with a Better Clinical Outcome than Pulmonary Exacerbation of Bacterial Origin in Paediatric Patients with Cystic Fibrosis" Pathogens 14, no. 9: 850. https://doi.org/10.3390/pathogens14090850
APA StyleStachowiak, Z., Andrzejewska, M., Jończyk-Potoczna, K., Narożna, B., Musiał, A., Wiesner, A., Bręborowicz, A., Szczepankiewicz, A., & Wojsyk-Banaszak, I. (2025). Viral Infection Correlates with a Better Clinical Outcome than Pulmonary Exacerbation of Bacterial Origin in Paediatric Patients with Cystic Fibrosis. Pathogens, 14(9), 850. https://doi.org/10.3390/pathogens14090850








