Exploring Gene Expression Changes in Murine Female Genital Tract Tissues Following Single and Co-Infection with Nippostrongylus brasiliensis and Herpes Simplex Virus Type 2
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Experimental Models
2.2.1. Animals
2.2.2. Nippostrongylus brasiliensis (Nb) Maintenance and Infection
2.2.3. Virus
2.3. Total RNA Extraction, Quantification, and Quality Control
2.4. Library Preparation and RNA Sequencing
2.5. Bioinformatics Analysis
2.6. Differentially Expressed Gene Analysis
2.7. Identification of Immune-Related DEGs, Functional and Pathway Enrichment Analysis
2.8. Protein–Protein Interaction Network Construction, Hub Gene and Functional Module Identification
3. Results
3.1. Animal Infection
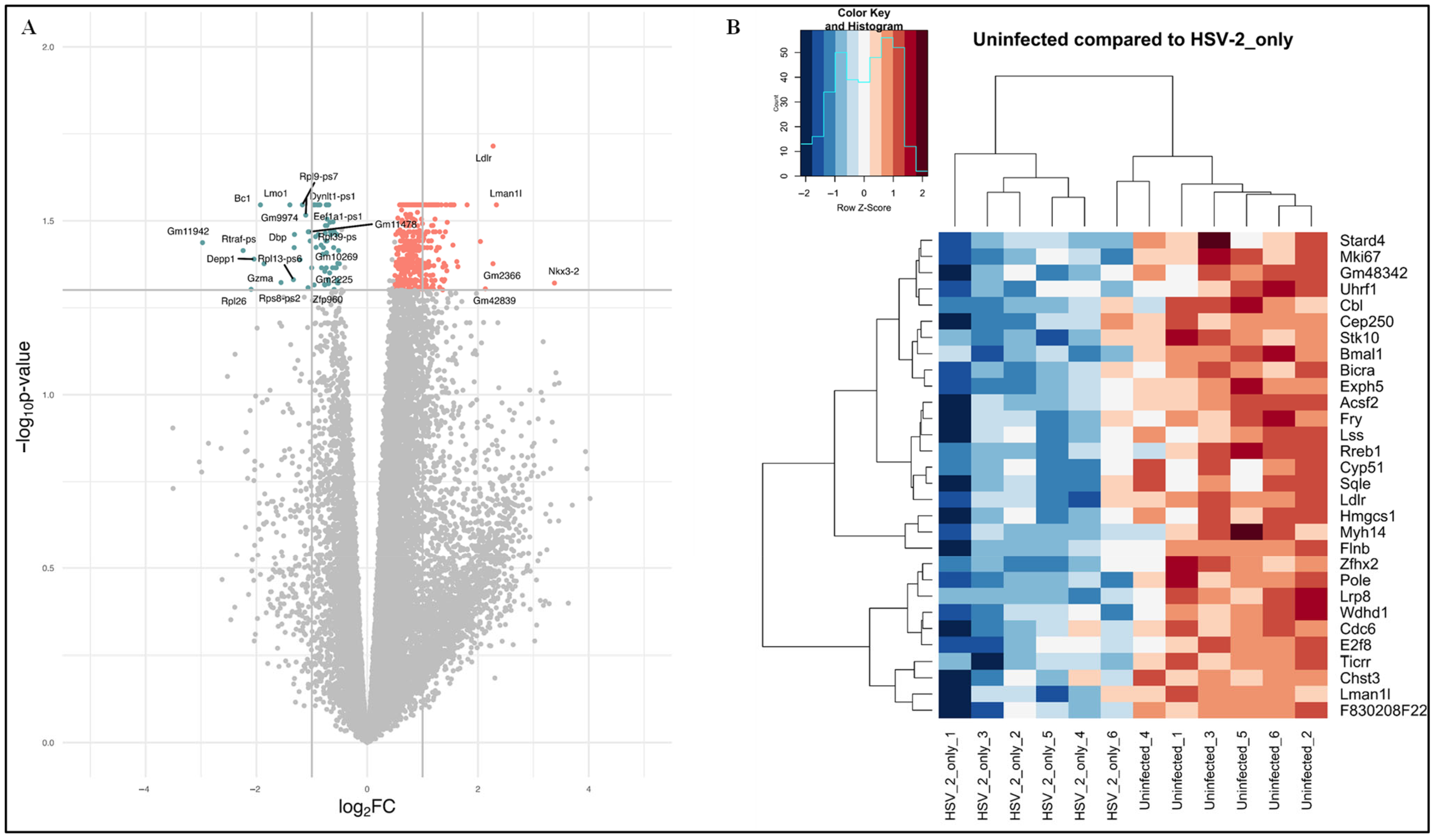
3.2. Identification of DEGs
3.3. Identification of Immune-Related DEGs
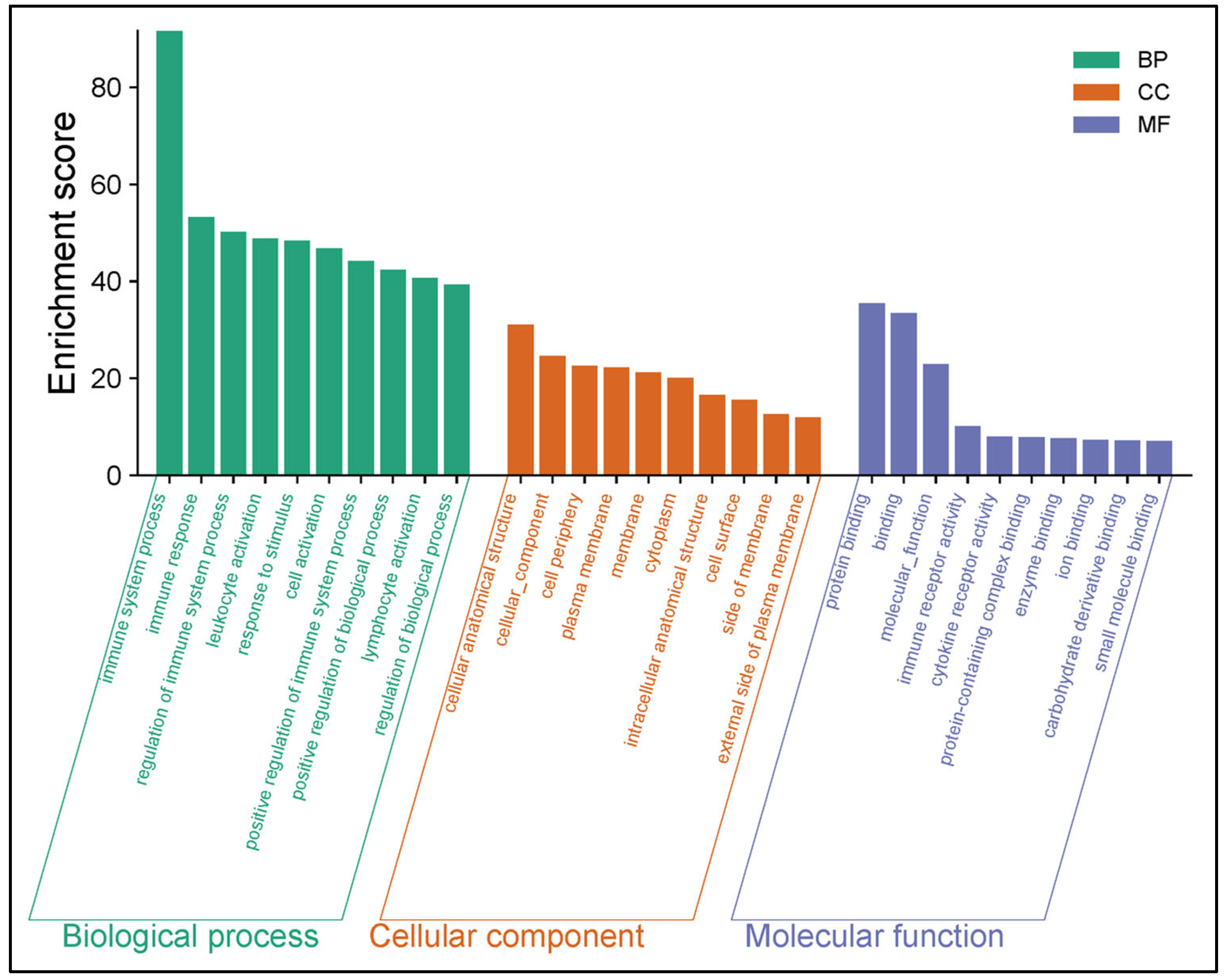
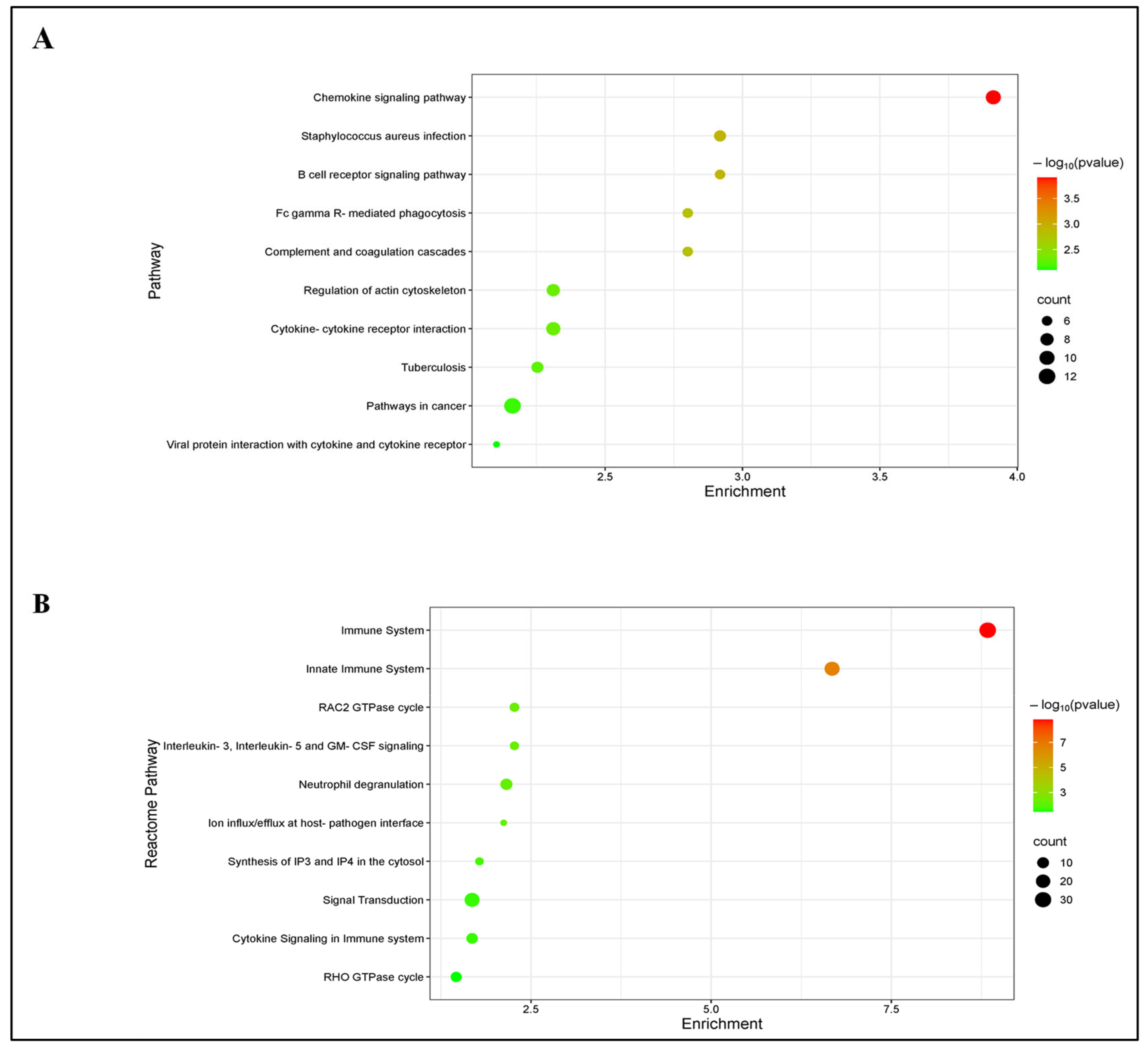
3.4. GO, KEGG and REACTOME Enrichment Analysis
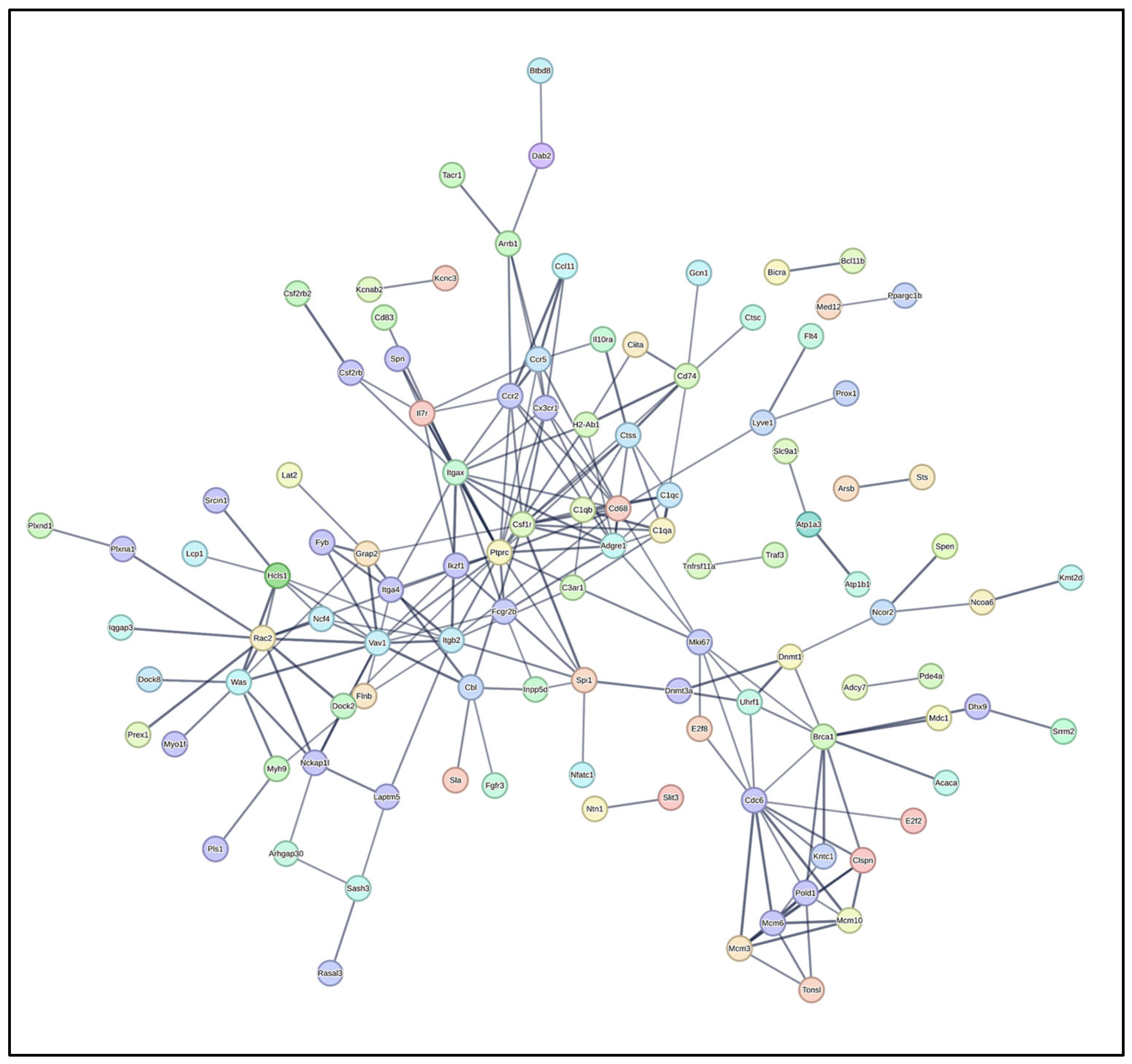
3.5. Protein–Protein Interaction (PPI) Network Analysis
4. Discussion
4.1. Animal Infection
4.2. DEGs Identified in the Comparison of Uninfected Versus Nb-Infected FGT Tissues
4.3. DEGs Identified in Comparison of Uninfected Versus HSV-2-Infected FGT Tissues
4.4. Limitations of the Current Study and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- James, C.; Harfouche, M.; Welton, N.J.; Turner, K.M.; Abu-Raddad, L.J.; Gottlieb, S.L.; Looker, K.J. Herpes simplex virus: Global infection prevalence and incidence estimates, 2016. Bull. World Health Organ. 2020, 98, 315–329. [Google Scholar] [CrossRef]
- World Health Organisation. Soil-Transmitted Helminth Infections. Available online: https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections (accessed on 20 June 2025).
- Hotez, P.J.; Kamath, A. Neglected Tropical Diseases in Sub-Saharan Africa: Review of Their Prevalence, Distribution, and Disease Burden. PLoS Neglected Trop. Dis. 2009, 3, e412. [Google Scholar] [CrossRef]
- Chetty, A.; Omondi, M.A.; Butters, C.; Smith, K.A.; Katawa, G.; Ritter, M.; Layland, L.; Horsnell, W. Impact of Helminth Infections on Female Reproductive Health and Associated Diseases. Front. Immunol. 2020, 11, 577516. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, J.T.; Corey, L. Rapid host immune response and viral dynamics in herpes simplex virus-2 infection. Nat. Med. 2013, 19, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.; Barra, N.G.; Lee, A.J.; Ashkar, A.A. Innate and adaptive immunity against herpes simplex virus type 2 in the genital mucosa. J. Reprod. Immunol. 2011, 88, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-P.; Muhammad, Z.S.; Wang, J.-G.; Lin, W.; Guo, S.-K.; Zhang, W. HSV-2 Vaccine: Current Status and Insight into Factors for Developing an Efficient Vaccine. Viruses 2014, 6, 371–390. [Google Scholar] [CrossRef]
- Chew, T.; Taylor, K.E.; Mossman, K.L. Innate and adaptive immune responses to herpes simplex virus. Viruses 2009, 1, 979–1002. [Google Scholar] [CrossRef]
- Harris, N.L.; Loke, P. Recent Advances in Type-2-Cell-Mediated Immunity: Insights from Helminth Infection. Immunity 2017, 47, 1024–1036. [Google Scholar] [CrossRef]
- Rapin, A.; Harris, N.L. Helminth-Bacterial Interactions: Cause and Consequence. Trends Immunol. 2018, 39, 724–733. [Google Scholar] [CrossRef]
- McSorley, H.J.; Maizels, R.M. Helminth infections and host immune regulation. Clin. Microbiol. Rev. 2012, 25, 585–608. [Google Scholar] [CrossRef]
- Mkhize-Kwitshana, Z.L.; Taylor, M.; Jooste, P.; Mabaso, M.L.H.; Walzl, G. The influence of different helminth infection phenotypes on immune responses against HIV in co-infected adults in South Africa. BMC Infect. Dis. 2011, 11, 273. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, O.A.; Yogeswaran, P.; Wright, G. Intestinal helminth infections amongst HIV-infected adults in Mthatha General Hospital, South Africa. Afr. J. Prim. Health Care Fam. Med. 2015, 7, 910. [Google Scholar] [CrossRef] [PubMed]
- Gravitt, P.E.; Marks, M.; Kosek, M.; Huang, C.; Cabrera, L.; Olortegui, M.P.; Medrano, A.M.; Trigoso, D.R.; Qureshi, S.; Bardales, G.S.; et al. Soil-Transmitted Helminth Infections Are Associated with an Increase in Human Papillomavirus Prevalence and a T-Helper Type 2 Cytokine Signature in Cervical Fluids. J. Infect. Dis. 2016, 213, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Holali Ameyapoh, A.; Katawa, G.; Ritter, M.; Tchopba, C.N.; Tchadié, P.E.; Arndts, K.; Kamassa, H.E.; Mazou, B.; Amessoudji, O.M.; N’djao, A.; et al. Hookworm Infections and Sociodemographic Factors Associated with Female Reproductive Tract Infections in Rural Areas of the Central Region of Togo. Front. Microbiol. 2021, 12, 738894. [Google Scholar] [CrossRef]
- Omondi, M.A.; Kamassa, E.H.; Katawa, G.; Tchopba, C.N.; Vogelbusch, C.; Parcina, M.; Tchadié, E.P.; Amessoudji, O.M.; Arndts, K.; Karou, S.D.; et al. Hookworm infection associates with a vaginal Type 1/Type 2 immune signature and increased HPV load. Front. Immunol. 2022, 13, 1009968. [Google Scholar] [CrossRef]
- Chetty, A.; Darby, M.G.; Vornewald, P.M.; Martín-Alonso, M.; Filz, A.; Ritter, M.; McSorley, H.J.; Masson, L.; Smith, K.; Brombacher, F.; et al. Il4ra-independent vaginal eosinophil accumulation following helminth infection exacerbates epithelial ulcerative pathology of HSV-2 infection. Cell Host Microbe 2021, 29, 579–593.e5. [Google Scholar] [CrossRef]
- Chin, C.-H.; Chen, S.-H.; Wu, H.-H.; Ho, C.-W.; Ko, M.-T.; Lin, C.-Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8, S11. [Google Scholar] [CrossRef]
- Camberis, M.; Le Gros, G.; Urban, J., Jr. Animal model of Nippostrongylus brasiliensis and Heligmosomoides polygyrus. Curr. Protoc. Immunol. 2003, 55, 12–19. [Google Scholar] [CrossRef]
- Shao, Q.; Wu, F.; Liu, T.; Wang, W.; Liu, T.; Jin, X.; Xu, L.; Ma, Y.; Huang, G.; Chen, Z. JieZe-1 Alleviates HSV-2 Infection-Induced Genital Herpes in Balb/c Mice by Inhibiting Cell Apoptosis via Inducing Autophagy. Front. Pharmacol. 2021, 12, 775521. [Google Scholar] [CrossRef]
- Merle, N.S.; Noe, R.; Halbwachs-Mecarelli, L.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part II: Role in Immunity. Front. Immunol. 2015, 6, 257. [Google Scholar] [CrossRef]
- Wira, C.R.; Fahey, J.V.; Ghosh, M.; Patel, M.V.; Hickey, D.K.; Ochiel, D.O. Sex hormone regulation of innate immunity in the female reproductive tract: The role of epithelial cells in balancing reproductive potential with protection against sexually transmitted pathogens. Am. J. Reprod. Immunol. 2010, 63, 544–565. [Google Scholar] [CrossRef]
- Agostinis, C.; Mangogna, A.; Bossi, F.; Ricci, G.; Kishore, U.; Bulla, R. Uterine Immunity and Microbiota: A Shifting Paradigm. Front. Immunol. 2019, 10, 2387. [Google Scholar] [CrossRef]
- Kraemer, L.; McKay, D.M.; Russo, R.C.; Fujiwara, R.T. Chemokines and chemokine receptors: Insights from human disease and experimental models of helminthiasis. Cytokine Growth Factor Rev. 2022, 66, 38–52. [Google Scholar] [CrossRef]
- Bachelerie, F.; Ben-Baruch, A.; Burkhardt, A.M.; Combadiere, C.; Farber, J.M.; Graham, G.J.; Horuk, R.; Sparre-Ulrich, A.H.; Locati, M.; Luster, A.D.; et al. International Union of Basic and Clinical Pharmacology. [corrected]. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol. Rev. 2014, 66, 1–79. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Appleton, J.A. Eosinophils in Helminth Infection: Defenders and Dupes. Trends Parasitol. 2016, 32, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Gurish, M.F.; Humbles, A.; Tao, H.; Finkelstein, S.; Boyce, J.A.; Gerard, C.; Friend, D.S.; Austen, K.F. CCR3 Is Required for Tissue Eosinophilia and Larval Cytotoxicity After Infection with Trichinella spiralis1. J. Immunol. 2002, 168, 5730–5736. [Google Scholar] [CrossRef] [PubMed]
- Dixon, H.; Blanchard, C.; deSchoolmeester, M.L.; Yuill, N.C.; Christie, J.W.; Rothenberg, M.E.; Else, K.J. The role of Th2 cytokines, chemokines and parasite products in eosinophil recruitment to the gastrointestinal mucosa during helminth infection. Eur. J. Immunol. 2006, 36, 1753–1763. [Google Scholar] [CrossRef]
- Warmington, K.S.; Boring, L.; Ruth, J.H.; Sonstein, J.; Hogaboam, C.M.; Curtis, J.L.; Kunkel, S.L.; Charo, I.R.; Chensue, S.W. Effect of C-C Chemokine Receptor 2 (CCR2) Knockout on Type-2 (Schistosomal Antigen-Elicited) Pulmonary Granuloma Formation: Analysis of Cellular Recruitment and Cytokine Responses. Am. J. Pathol. 1999, 154, 1407–1416. [Google Scholar] [CrossRef]
- deSchoolmeester, M.L.; Manku, H.; Else, K.J. The Innate Immune Responses of Colonic Epithelial Cells to Trichuris muris Are Similar in Mouse Strains That Develop a Type 1 or Type 2 Adaptive Immune Response. Infect. Immun. 2006, 74, 6280–6286. [Google Scholar] [CrossRef]
- Gazzinelli-Guimaraes, P.H.; de Queiroz Prado, R.; Ricciardi, A.; Bonne-Année, S.; Sciurba, J.; Karmele, E.P.; Fujiwara, R.T.; Nutman, T.B. Allergen presensitization drives an eosinophil-dependent arrest in lung-specific helminth development. J. Clin. Investig. 2019, 129, 3686–3701. [Google Scholar] [CrossRef]
- Rosbottom, A.; Knight, P.A.; McLachlan, G.; Thornton, E.M.; Wright, S.W.; Miller, H.R.; Scudamore, C.L. Chemokine and cytokine expression in murine intestinal epithelium following Nippostrongylus brasiliensis infection. Parasite Immunol. 2002, 24, 67–75. [Google Scholar] [CrossRef] [PubMed]
- deSchoolmeester, M.L.; Little, M.C.; Rollins, B.J.; Else, K.J. Absence of CC Chemokine Ligand 2 Results in an Altered Th1/Th2 Cytokine Balance and Failure to Expel Trichuris muris Infection1. J. Immunol. 2003, 170, 4693–4700. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Barnes, M.A.; Sureshchandra, S.; Menicucci, A.R.; Patel, J.J.; Messaoudi, I.; Nair, M.G. CX3CR1-Expressing Myeloid Cells Regulate Host–Helminth Interaction and Lung Inflammation. Adv. Biol. 2022, 6, 2101078. [Google Scholar] [CrossRef] [PubMed]
- Loredan, D.G.; Devlin, J.C.; Khanna, K.M.; Loke, P.N. Recruitment and Maintenance of CX3CR1+CD4+ T Cells during Helminth Infection. J. Immunol. 2024, 212, 632–644. [Google Scholar] [CrossRef]
- Cortés, A.; Muñoz-Antoli, C.; Esteban, J.G.; Toledo, R. Th2 and Th1 Responses: Clear and Hidden Sides of Immunity Against Intestinal Helminths. Trends Parasitol. 2017, 33, 678–693. [Google Scholar] [CrossRef]
- Zhu, S.; Viejo-Borbolla, A. Pathogenesis and virulence of herpes simplex virus. Virulence 2021, 12, 2670–2702. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Wang, P.; Guan, X.; He, S.; Luo, S.; Li, C.; Hu, K.; Jin, W.; Du, T. HSV-2 immediate-early protein US1 inhibits IFN-β production by suppressing association of IRF-3 with IFN-β promoter. J. Immunol. 2015, 194, 3102–3115. [Google Scholar] [CrossRef]
- Guan, X.; Zhang, M.; Fu, M.; Luo, S.; Hu, Q. Herpes simplex virus type 2 immediate early protein ICP27 inhibits IFN-β production in mucosal epithelial cells by antagonizing IRF3 activation. Front. Immunol. 2019, 10, 290. [Google Scholar] [CrossRef]
- Zhang, M.; Fu, M.; Li, M.; Hu, H.; Gong, S.; Hu, Q. Herpes simplex virus type 2 inhibits type I IFN signaling mediated by the novel E3 ubiquitin protein ligase activity of viral protein ICP22. J. Immunol. 2020, 205, 1281–1292. [Google Scholar] [CrossRef]
- Milligan, G.N.; Dudley-McClain, K.L.; Young, C.G.; Chu, C.F. T-cell-mediated mechanisms involved in resolution of genital herpes simplex virus type 2 (HSV-2) infection of mice. J. Reprod. Immunol. 2004, 61, 115–127. [Google Scholar] [CrossRef]
- Iijima, N.; Iwasaki, A. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science 2014, 346, 93–98. [Google Scholar] [CrossRef]
- Savage, K.I.; Harkin, D.P. BRCA1, a ‘complex’ protein involved in the maintenance of genomic stability. FEBS J. 2015, 282, 630–646. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.R.; Lu, T.M.; Chin, L.W.; Yang, C.C. Possible DNA viral factors of human breast cancer. Cancers 2010, 2, 498–512. [Google Scholar] [CrossRef] [PubMed]
- Giotti, B.; Chen, S.H.; Barnett, M.W.; Regan, T.; Ly, T.; Wiemann, S.; Hume, D.A.; Freeman, T.C. Assembly of a parts list of the human mitotic cell cycle machinery. J. Mol. Cell Biol. 2019, 11, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Liu, J.J.; Yeh, K.-C.; Knipe, D.M. Herpes Simplex Virus Infection Blocks Events in the G1 Phase of the Cell Cycle. Virology 2000, 267, 326–334. [Google Scholar] [CrossRef]
- Bagga, S.; Bouchard, M.J. Cell Cycle Regulation During Viral Infection. In Cell Cycle Control: Mechanisms and Protocols; Noguchi, E., Gadaleta, M.C., Eds.; Springer: New York, NY, USA, 2014; pp. 165–227. [Google Scholar]
- Zhang, B.; Li, Y.; Yang, P.; He, S.; Li, W.; Li, M.; Hu, Q.; Zhang, M. Herpes Simplex Virus Type 2 Blocks IFN-β Production through the Viral UL24 N-Terminal Domain-Mediated Inhibition of IRF-3 Phosphorylation. Viruses 2024, 16, 1601. [Google Scholar] [CrossRef]
- Wudiri, G.A.; Pritchard, S.M.; Li, H.; Liu, J.; Aguilar, H.C.; Gilk, S.D.; Nicola, A.V. Molecular Requirement for Sterols in Herpes Simplex Virus Entry and Infectivity. J. Virol. 2014, 88, 13918–13922. [Google Scholar] [CrossRef]
- Wudiri, G.A.; Nicola, A.V. Cellular Cholesterol Facilitates the Postentry Replication Cycle of Herpes Simplex Virus 1. J. Virol. 2017, 91, e00445-17. [Google Scholar] [CrossRef]
- Bommer, G.T.; MacDougald, O.A. Regulation of Lipid Homeostasis by the Bifunctional SREBF2-miR33a Locus. Cell Metab. 2011, 13, 241–247. [Google Scholar] [CrossRef]
- Xiao, H.; Lu, M.; Lin, T.Y.; Chen, Z.; Chen, G.; Wang, W.-C.; Marin, T.; Shentu, T.-p.; Wen, L.; Gongol, B. Sterol regulatory element binding protein 2 activation of NLRP3 inflammasome in endothelium mediates hemodynamic-induced atherosclerosis susceptibility. Circulation 2013, 128, 632–642. [Google Scholar] [CrossRef]
- Fowler, J.W.M.; Boutagy, N.E.; Zhang, R.; Horikami, D.; Whalen, M.B.; Romanoski, C.E.; Sessa, W.C. SREBP2 regulates the endothelial response to cytokines via direct transcriptional activation of KLF6. J. Lipid Res. 2023, 64, 100411. [Google Scholar] [CrossRef]
- York, A.G.; Williams, K.J.; Argus, J.P.; Zhou, Q.D.; Brar, G.; Vergnes, L.; Gray, E.E.; Zhen, A.; Wu, N.C.; Yamada, D.H. Limiting cholesterol biosynthetic flux spontaneously engages type I IFN signaling. Cell 2015, 163, 1716–1729. [Google Scholar] [CrossRef]
- National Health Research Ethics Council. South African Ethics in Health Research Guidelines: Principles, Processes and Structures; NdoH: Pretoria, South African, 2024; 137p. [Google Scholar]




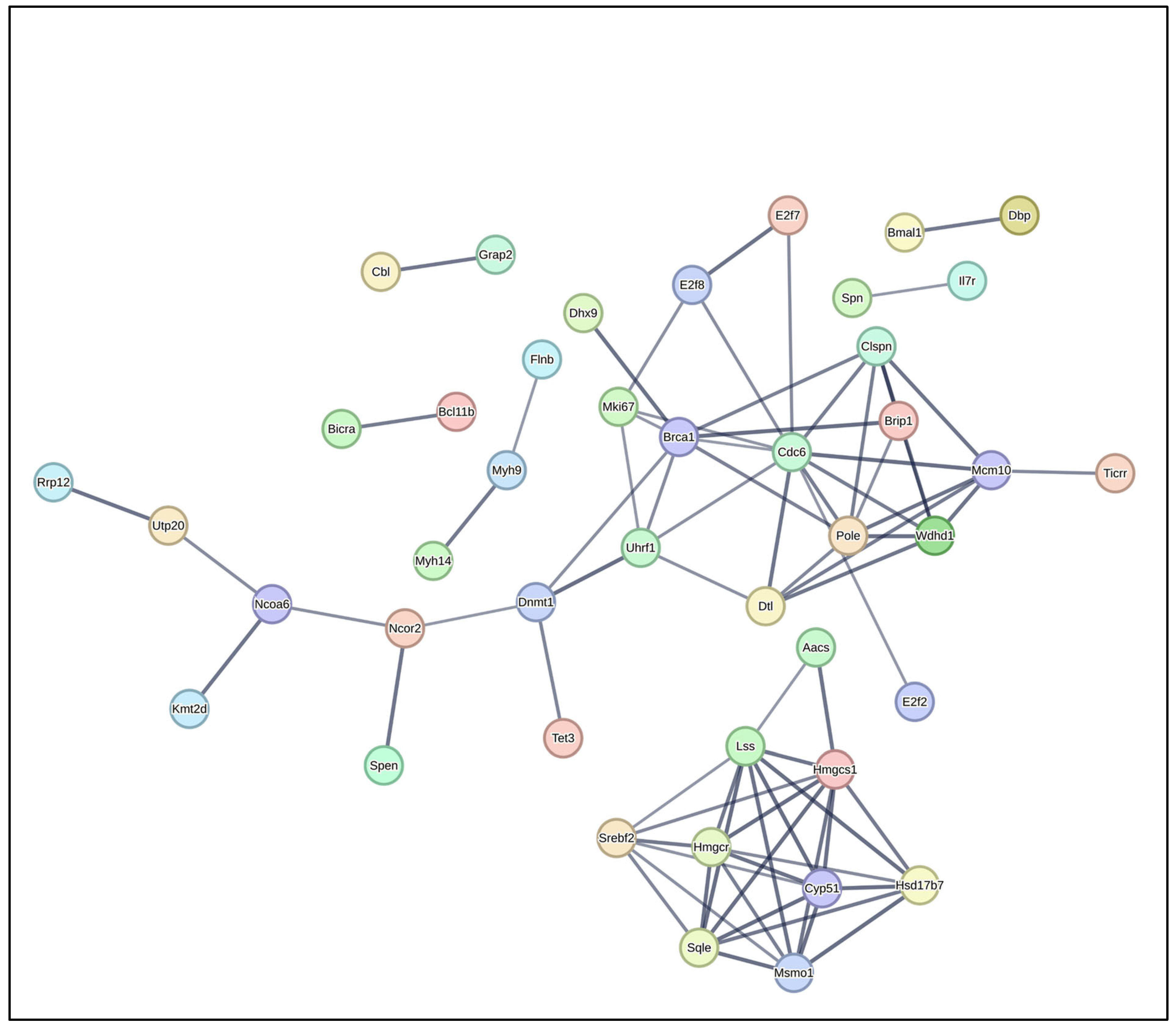
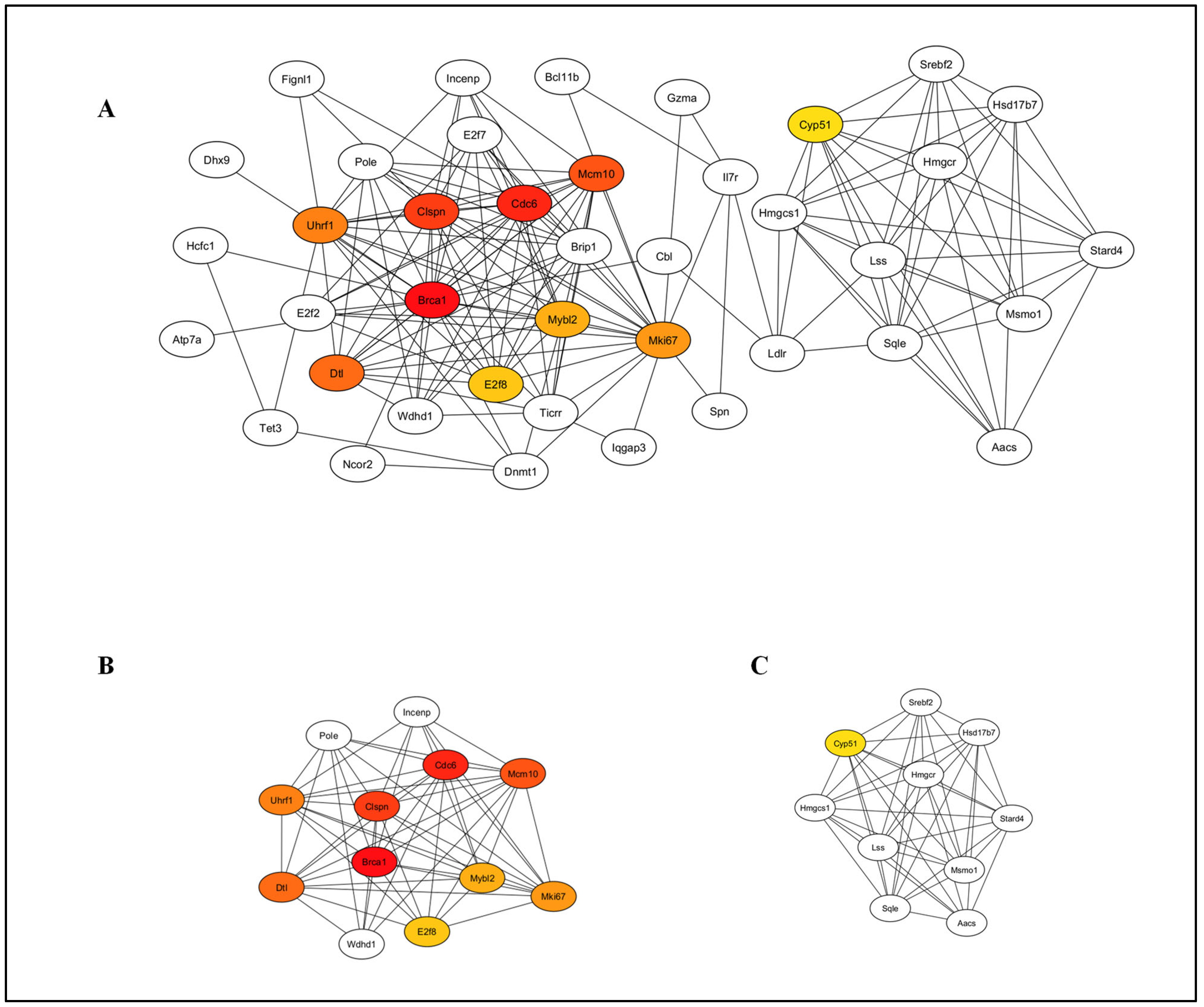
| Gene ID | Gene Name | logFC | p Value | Adjusted p Value |
|---|---|---|---|---|
| ENSMUSG00000033016.17 | Nfatc1 | 1.01 | 2.30 × 10−6 | 0.02 |
| ENSMUSG00000033220.8 | Rac2 | 1.30 | 2.60 × 10−6 | 0.02 |
| ENSMUSG00000025017.11 | Pik3ap1 | 1.55 | 6.32 × 10−6 | 0.02 |
| ENSMUSG00000029098.18 | Acox3 | 1.02 | 9.09 × 10−6 | 0.02 |
| ENSMUSG00000038644.15 | Pold1 | 1.02 | 1.63 × 10−5 | 0.02 |
| ENSMUSG00000039637.16 | Coro7 | 1.05 | 1.73 × 10−5 | 0.02 |
| ENSMUSG00000033083.17 | Tbc1d4 | 1.11 | 1.85 × 10−5 | 0.02 |
| ENSMUSG00000089960.2 | Ugt1a1 | 1.27 | 2.14 × 10−5 | 0.02 |
| ENSMUSG00000029547.11 | Ints1 | 1.16 | 2.14 × 10−5 | 0.02 |
| ENSMUSG00000041638.19 | Gcn1 | 1.04 | 2.57 × 10−5 | 0.02 |
| ENSMUSG00000000631.21 | Myo18a | 1.14 | 3.02 × 10−5 | 0.02 |
| ENSMUSG00000003882.6 | Il7r | 1.54 | 3.37 × 10−5 | 0.02 |
| ENSMUSG00000032536.13 | Trak1 | 1.08 | 3.37 × 10−5 | 0.02 |
| ENSMUSG00000028581.18 | Laptm5 | 1.68 | 4.39 × 10−5 | 0.02 |
| ENSMUSG00000004099.17 | Dnmt1 | 1.06 | 4.92 × 10−5 | 0.02 |
| ENSMUSG00000034584.4 | Exph5 | 1.56 | 5.28 × 10−5 | 0.02 |
| ENSMUSG00000031004.9 | Mki67 | 1.05 | 5.45 × 10−5 | 0.02 |
| ENSMUSG00000024621.17 | Csf1r | 1.68 | 5.55 × 10−5 | 0.02 |
| ENSMUSG00000026116.12 | Tmem131 | 1.10 | 5.85 × 10−5 | 0.02 |
| ENSMUSG00000030560.18 | Ctsc | 1.32 | 6.20 × 10−5 | 0.02 |
| ENSMUSG00000022946.11 | Dop1b | 1.12 | 7.49 × 10−5 | 0.02 |
| ENSMUSG00000032380.10 | Dapk2 | 1.37 | 7.63 × 10−5 | 0.02 |
| ENSMUSG00000020357.4 | Flt4 | 1.48 | 7.64 × 10−5 | 0.02 |
| ENSMUSG00000061607.16 | Mdc1 | 1.09 | 8.16 × 10−5 | 0.02 |
| ENSMUSG00000028931.13 | Kcnab2 | 1.47 | 8.23 × 10−5 | 0.02 |
| ENSMUSG00000120290.1 | Gm56959 | 1.08 | 8.40 × 10−5 | 0.02 |
| ENSMUSG00000026288.15 | Inpp5d | 1.67 | 8.97 × 10−5 | 0.02 |
| ENSMUSG00000022443.18 | Myh9 | 1.29 | 9.65 × 10−5 | 0.02 |
| ENSMUSG00000020100.16 | Slc29a3 | 1.16 | 9.69 × 10−5 | 0.02 |
| ENSMUSG00000048865.17 | Arhgap30 | 1.50 | 9.84 × 10−5 | 0.02 |
| Gene ID | Gene Name | logFC | p Value | Adjusted p Value |
|---|---|---|---|---|
| ENSMUSG00000032193.10 | Ldlr | 2.27 | 9.96 × 10−7 | 0.02 |
| ENSMUSG00000031004.9 | Mki67 | 1.24 | 5.82 × 10−6 | 0.03 |
| ENSMUSG00000039145.17 | Camk1d | 1.14 | 8.31 × 10−6 | 0.03 |
| ENSMUSG00000004356.9 | Utp20 | 1.04 | 9.39 × 10−6 | 0.03 |
| ENSMUSG00000046179.18 | E2f8 | 1.53 | 1.28 × 10−5 | 0.03 |
| ENSMUSG00000024251.11 | Thada | 1.02 | 1.44 × 10−5 | 0.03 |
| ENSMUSG00000028613.16 | Lrp8 | 1.28 | 2.26 × 10−5 | 0.03 |
| ENSMUSG00000055116.9 | Bmal1 | 1.27 | 2.49 × 10−5 | 0.03 |
| ENSMUSG00000024660.10 | Incenp | 1.01 | 2.54 × 10−5 | 0.03 |
| ENSMUSG00000114934.2 | Gm48342 | 1.52 | 3.35 × 10−5 | 0.03 |
| ENSMUSG00000024378.10 | Stard4 | 1.25 | 3.38 × 10−5 | 0.03 |
| ENSMUSG00000093930.3 | Hmgcs1 | 1.18 | 4.20 × 10−5 | 0.03 |
| ENSMUSG00000004099.17 | Dnmt1 | 1.04 | 5.12 × 10−5 | 0.03 |
| ENSMUSG00000073529.10 | F830208F22Rik | 1.80 | 5.91 × 10−5 | 0.03 |
| ENSMUSG00000022351.15 | Sqle | 1.42 | 6.44 × 10−5 | 0.03 |
| ENSMUSG00000039835.17 | Nhsl1 | 1.07 | 6.59 × 10−5 | 0.03 |
| ENSMUSG00000039840.9 | Epg5 | 1.11 | 6.98 × 10−5 | 0.03 |
| ENSMUSG00000000631.21 | Myo18a | 1.06 | 7.31 × 10−5 | 0.03 |
| ENSMUSG00000030739.20 | Myh14 | 1.23 | 7.35 × 10−5 | 0.03 |
| ENSMUSG00000021670.15 | Hmgcr | 1.05 | 7.51 × 10−5 | 0.03 |
| ENSMUSG00000035049.5 | Rrp12 | 1.13 | 8.00 × 10−5 | 0.03 |
| ENSMUSG00000039087.18 | Rreb1 | 1.17 | 8.25 × 10−5 | 0.03 |
| ENSMUSG00000022463.9 | Srebf2 | 1.02 | 8.47 × 10−5 | 0.03 |
| ENSMUSG00000013629.17 | Cad | 1.02 | 8.78 × 10−5 | 0.03 |
| ENSMUSG00000001228.15 | Uhrf1 | 1.18 | 9.33 × 10−5 | 0.03 |
| ENSMUSG00000046591.11 | Ticrr | 1.43 | 9.35 × 10−5 | 0.03 |
| ENSMUSG00000025278.10 | Flnb | 1.17 | 9.79 × 10−5 | 0.03 |
| ENSMUSG00000040721.10 | Zfhx2 | 1.22 | 9.81 × 10−5 | 0.03 |
| ENSMUSG00000115783.3 | Bc1 | −1.93 | 9.84 × 10−5 | 0.03 |
| ENSMUSG00000034584.4 | Exph5 | 1.42 | 1.01 × 10−4 | 0.03 |
| (A) Immune-Related DEGs: Uninfected Versus Nb-Infected FGT Tissues | (B) Immune-Related DEGs: Uninfected Versus HSV-2-Infected FGT Tissues |
|---|---|
| Nfatc1, Rac2, Pik3ap1, Myo18a, Il7r, Laptm5, Csf1r, Ctsc, Dapk2, Inpp5d, Myh9, Lcp1, Spn, Dock2, Itgb2, Cd74, Itgax, Nckap1l, Wdfy4, Csf2rb2, Ccr5, Ciita, Apbb1ip, Stk10, Cx3cr1, Chst3, Hcls1, Crtc3, Ptpro, Flnb, Ppargc1b, H2-Ab1, Cd180, Cbl, Adcy7, Vav1, Traf3, Pigr, Slc11a1, Dock8, Ccdc88b, Mpeg1, Zmiz1, C1qc, Prex1, Dtx1, Atp7a, Myo1f, Trim56, Elf4, Fgfr3, Trim62, Pld4, Bcl11b, Itga4, C1qb, Ctss, Wfdc15b, Tnfrsf11a, Cd68, Was, Ptprc, Adgre1, Ccr2, Dhx9, Lat2, C3ar1, Fcgr2b, Rab43, Naip2, Cd83, Tifab, Lyve1, Msmp, Plxna1, Spi1, Dtx4, Ikzf1, Itpkb, Rasal3, Csf2rb, Cd84, Sash3, Ccl11, C1qa, Myo1g, Epas1, Themis2, Tacr1 | Ldlr, Camk1d, Lrp8, Epg5, Myo18a, Flnb, Lmo1, Cbl, Stk10, Chst3, Bcl11b, Il7r, Spn, Atp7a, Foxn1, Zmiz1, Zbtb34, Dhx9, Myh9, Plec, Csf2rb2, Nkx3-2, Tnfrsf11a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pillay, R.; Naidoo, P.; Mkhize-Kwitshana, Z.L. Exploring Gene Expression Changes in Murine Female Genital Tract Tissues Following Single and Co-Infection with Nippostrongylus brasiliensis and Herpes Simplex Virus Type 2. Pathogens 2025, 14, 795. https://doi.org/10.3390/pathogens14080795
Pillay R, Naidoo P, Mkhize-Kwitshana ZL. Exploring Gene Expression Changes in Murine Female Genital Tract Tissues Following Single and Co-Infection with Nippostrongylus brasiliensis and Herpes Simplex Virus Type 2. Pathogens. 2025; 14(8):795. https://doi.org/10.3390/pathogens14080795
Chicago/Turabian StylePillay, Roxanne, Pragalathan Naidoo, and Zilungile L. Mkhize-Kwitshana. 2025. "Exploring Gene Expression Changes in Murine Female Genital Tract Tissues Following Single and Co-Infection with Nippostrongylus brasiliensis and Herpes Simplex Virus Type 2" Pathogens 14, no. 8: 795. https://doi.org/10.3390/pathogens14080795
APA StylePillay, R., Naidoo, P., & Mkhize-Kwitshana, Z. L. (2025). Exploring Gene Expression Changes in Murine Female Genital Tract Tissues Following Single and Co-Infection with Nippostrongylus brasiliensis and Herpes Simplex Virus Type 2. Pathogens, 14(8), 795. https://doi.org/10.3390/pathogens14080795







