Multisystemic Tuberculosis Masquerading as Aggressive Cardiac Tumor Causing Budd–Chiari Syndrome Disseminated to the Brain Resulting in Death of a Six-Year-Old Boy
Abstract
1. Introduction
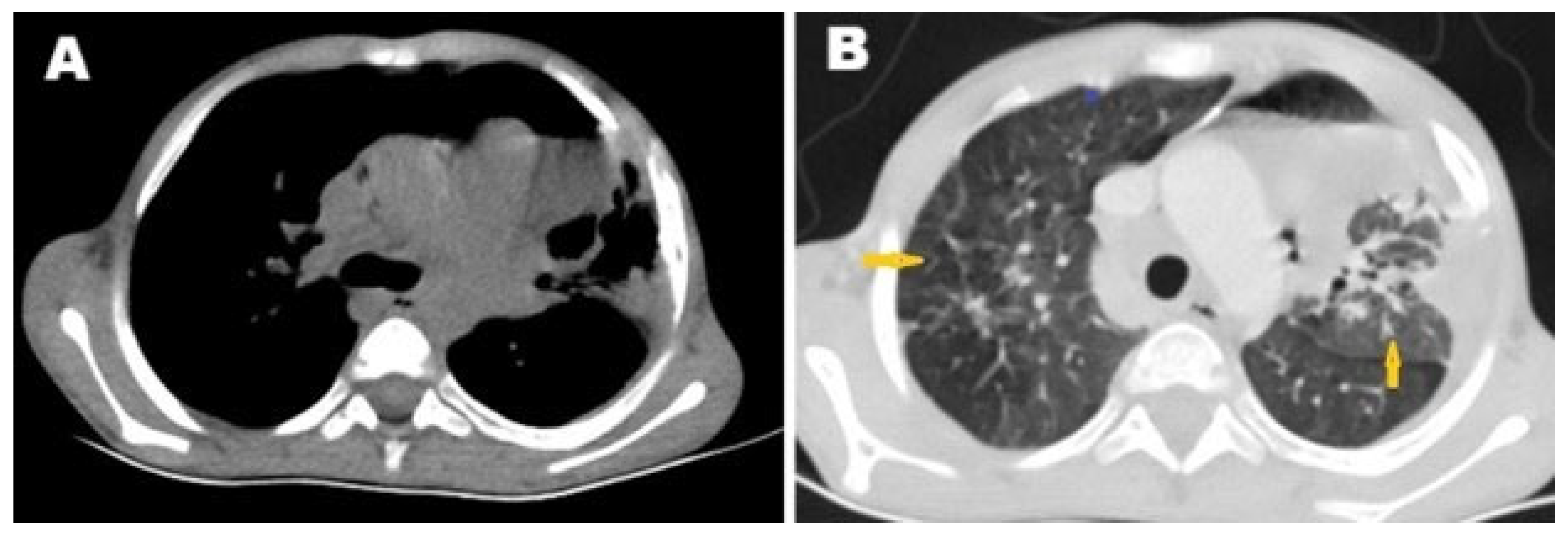
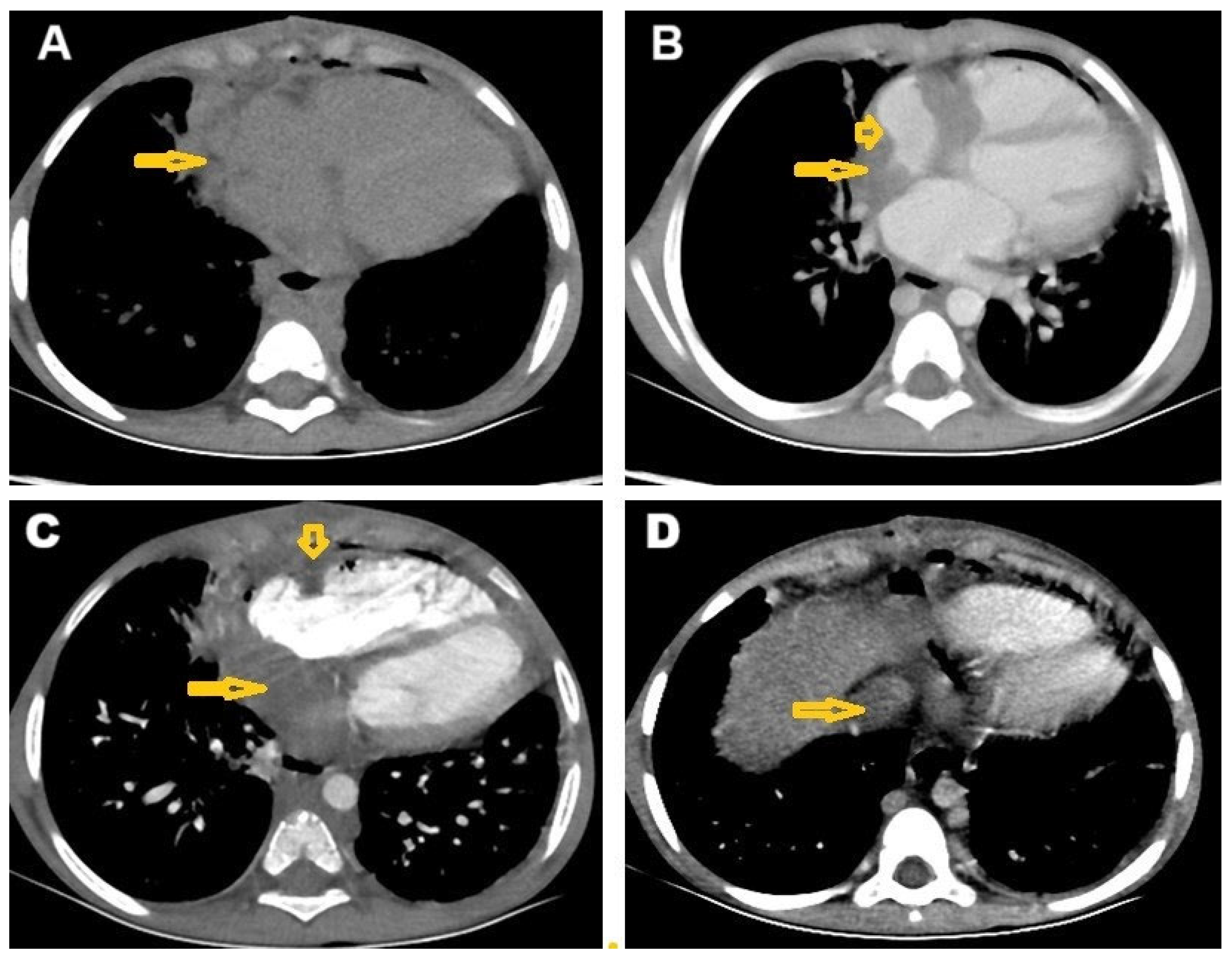
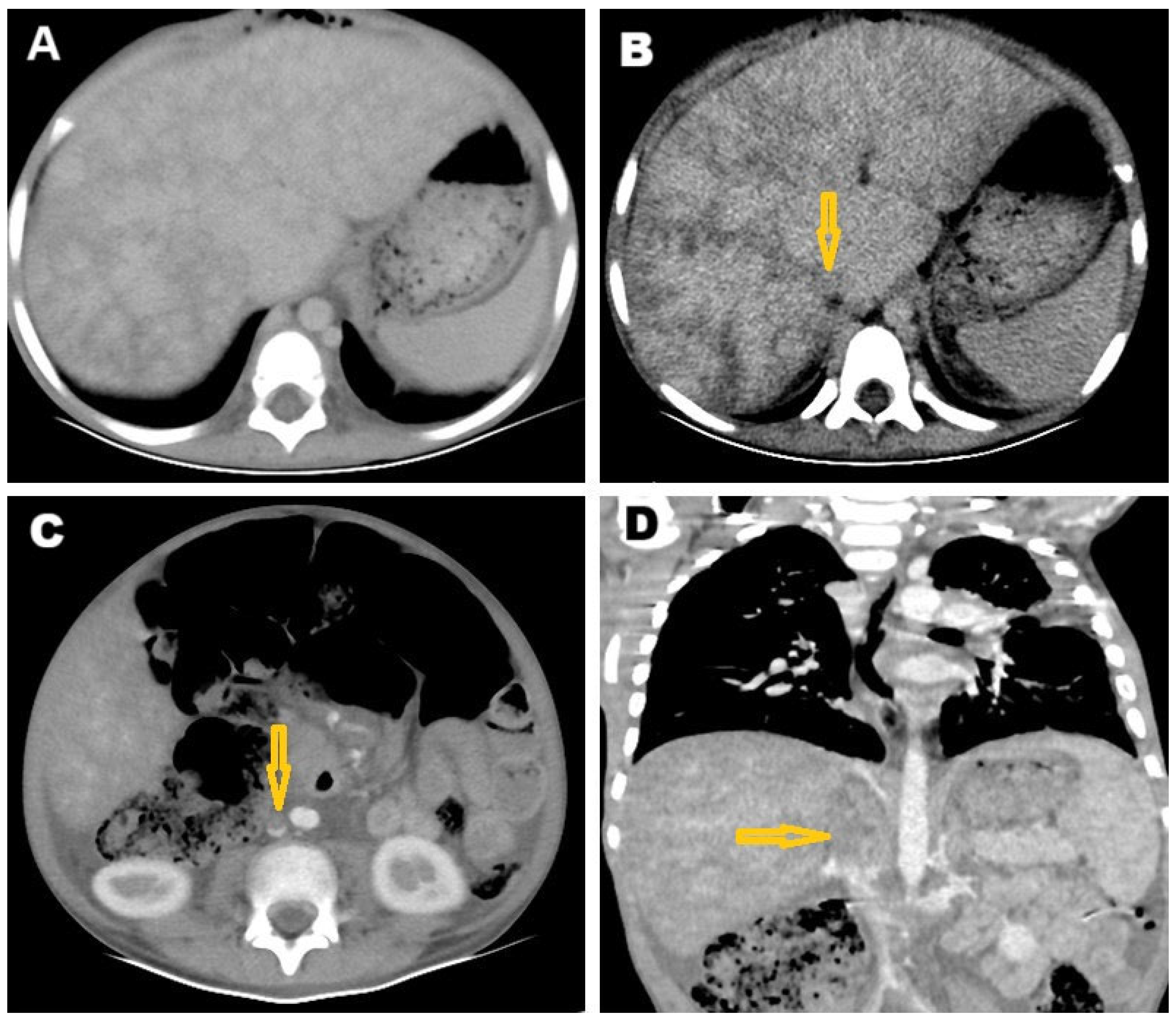
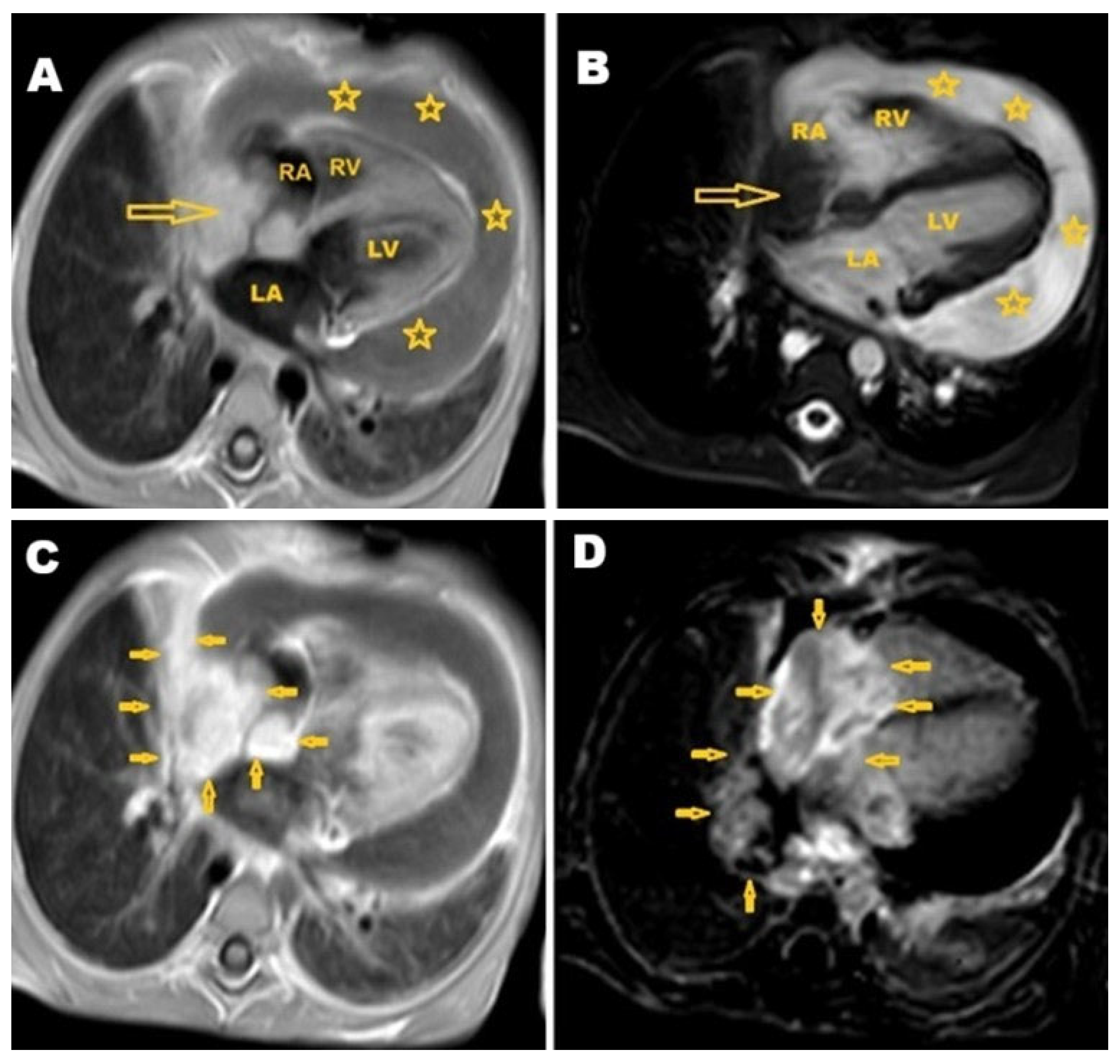
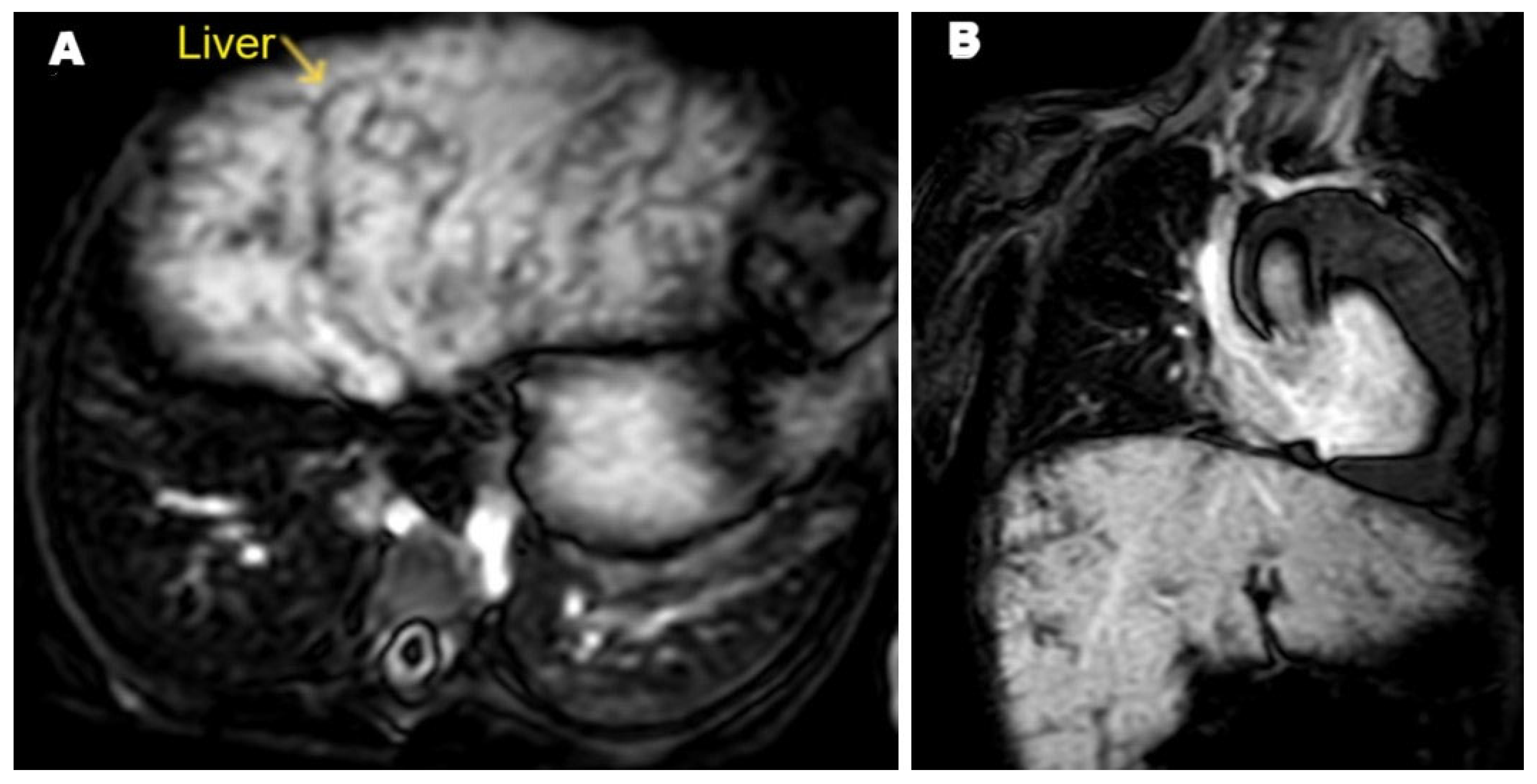
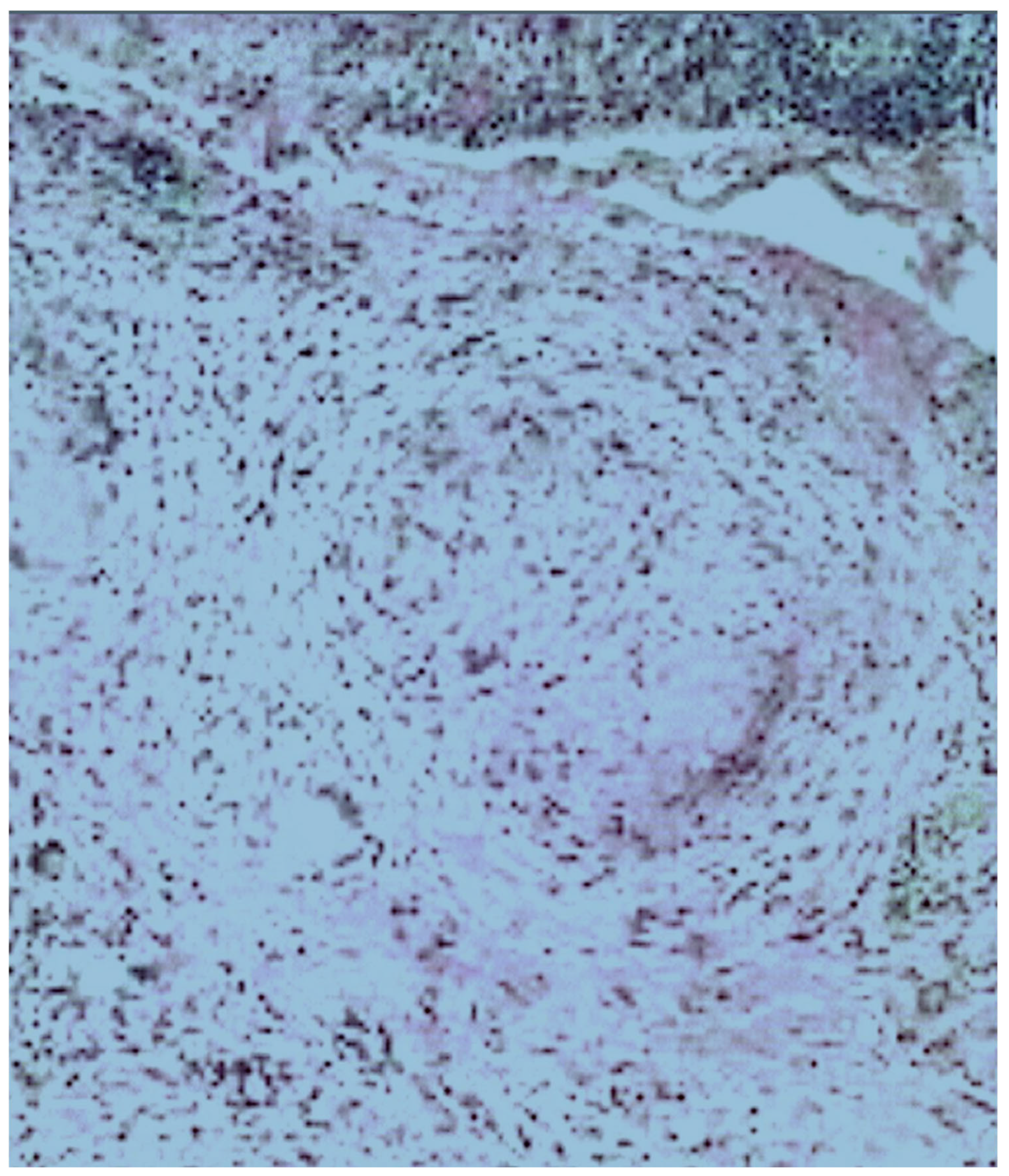
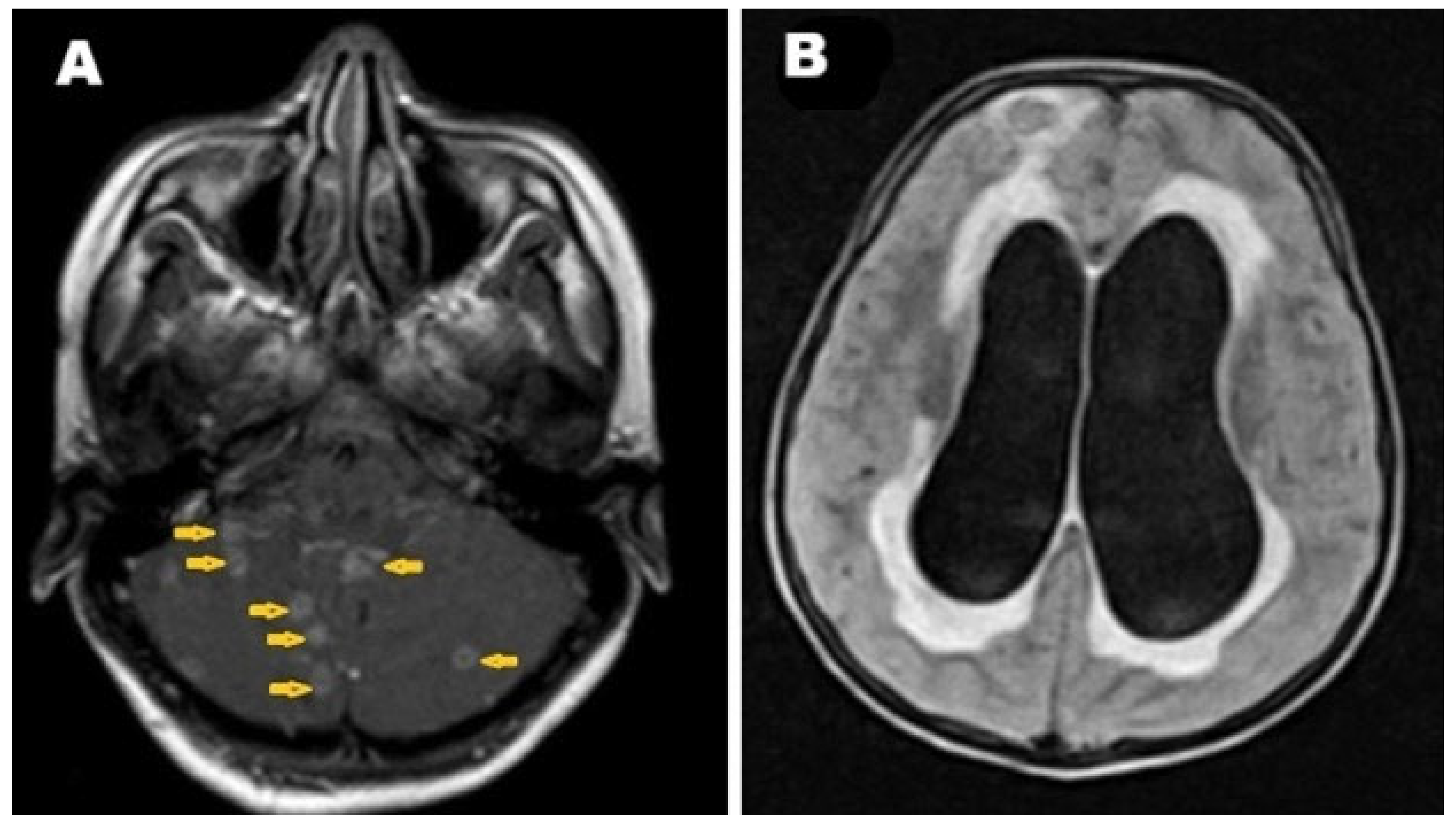
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADA | adenosine deaminase |
| AFB | acid-fast bacilli |
| CT | computed tomography |
| IVC | inferior vena cava |
| MRI | magnetic resonance imaging |
| RA | right atrium |
| TB | tuberculosis |
References
- Hatiya, M.; Merid, Y.; Mola, A.; Belayneh, F.; Ali, M.M. Prevalence of drug-resistant Mycobacterium tuberculosis and its associated factors among tuberculosis patients attending Dilla university referral hospital, Ethiopia. BMC Infect. Dis. 2025, 25, 797. [Google Scholar] [CrossRef] [PubMed]
- Girma, A.; Kassawmar, F.; Kassa, Y.; Asrat, Y. Nanoparticles as an Alternative Strategy for the Rapid Detection of Mycobacterium tuberculosis Complex (MTBC): A Systematic Literature Review of In Vitro Studies. IET Nanobiotechnol. 2025, 2025, 4639233. [Google Scholar] [CrossRef] [PubMed]
- Salvador, V.G.; Undurraga, E.A.; Escobar, N.; García, C.; Vergara, N.; Balcells, M.E. Short- and long-term increased risk of all-cause mortality in a tuberculosis cohort attributed to SARS-CoV-2 infection: A time-dependent survival analysis in Chile. Lancet Reg. Health Am. 2025, 46, 101119. [Google Scholar] [CrossRef]
- Khan, M.A.; Rahman, S.A.; Danish, M.; Afrose, R. A Rare Case of Tubercular Osteomyelitis of Mandible in a 5-year-old Child. Int. J. Clin. Pediatr. Dent. 2024, 17, 202–205. [Google Scholar] [CrossRef]
- Datta, S.; Evans, C.A. The uncertainty of tuberculosis diagnosis. Lancet Infect. Dis. 2020, 20, 1002–1004. [Google Scholar] [CrossRef]
- Alshoabi, S.A.; Almas, K.M.; Aldofri, S.A.; Hamid, A.M.; Alhazmi, F.H.; Alsharif, W.M.; Abdulaal, O.M.; Qurashi, A.A.; Aloufi, K.M.; Alsultan, K.D.; et al. The Diagnostic Deceiver: Radiological Pictorial Review of Tuberculosis. Diagnostics 2022, 12, 306. [Google Scholar] [CrossRef]
- Alshoabi, S.A. Tuberculous Meningitis, Vasculitis, and Pericarditis presented by deep coma. Pak. J. Med. Sci. 2018, 34, 1576–1578. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Q.; Wu, H. Primary cardiac tumor: A case report of right atrial angiosarcoma and review of the literature. Front. Oncol. 2023, 13, 1164153. [Google Scholar] [CrossRef]
- Kiwaki, T.; Ishihara, A.; Toyama, T.; Kusaka, H.; Kataoka, H. Primary cardiac angiosarcoma directly invading the right lung: An autopsy report. Clin. Case Rep. 2021, 9, e04582. [Google Scholar] [CrossRef]
- Yu, J.F.; Cui, H.; Ji, G.M.; Li, S.Q.; Huang, Y.; Wang, R.N.; Xiao, W.F. Clinical and imaging manifestations of primary cardiac angiosarcoma. BMC Med. Imaging 2019, 19, 16. [Google Scholar] [CrossRef] [PubMed]
- Jeoung, Y.; Lee, S.R.; Kim, H.; Kim, J.W.; Lee, J.J.; Chung, S.Y. Diffuse infiltrative primary cardiac lymphoma with delayed extracardiac involvement. Chonnam Med. J. 2014, 50, 27–30. [Google Scholar] [CrossRef]
- Huang, D.; Wu, C.; Xiao, L.; Lin, B.; He, C.; Du, X. Diffuse myocardial infiltration in secondary cardiac lymphoma: A case report. Oncol. Lett. 2025, 29, 263. [Google Scholar] [CrossRef] [PubMed]
- Kopcinovic, L.M.; Culej, J. Pleural, peritoneal and pericardial effusions—A biochemical approach. Biochem. Med. 2014, 24, 123–137. [Google Scholar] [CrossRef]
- Dybowska, M.; Błasińska, K.; Gątarek, J.; Klatt, M.; Augustynowicz-Kopeć, E.; Tomkowski, W.; Szturmowicz, M. Tuberculous Pericarditis-Own Experiences and Recent Recommendations. Diagnostics 2022, 12, 619. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Bertamino, M.; Guerisoli, L.; Stratoti, S.; Canale, C.; Spallarossa, P.; Porto, I.; Ameri, P. Pericardial effusion in oncological patients: Current knowledge and principles of management. Cardiooncology 2024, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- İnan, D.; Demirtola Mammadli, A.İ.; Gençol Akçay, G.; Tekin, A.F.; Mammadli, A. Incremental Diagnostic Value of Computed Tomography Attenuation in Differentiating Malignant Pericardial Effusion: A Retrospective Observational Study. Turk. Kardiyol. Dern. Ars. 2025, 53, 304–311. [Google Scholar] [CrossRef]
- Saleemi, S.A.; Alothman, B.; Alamer, M.; Alsayari, S.; Almogbel, A.; Mohammed, S. Tuberculosis presenting as metastatic lung cancer. Int. J. Mycobacteriol. 2021, 10, 327–329. [Google Scholar] [CrossRef]
- Gakidi, A.; Faniadou, E.; Ioannidou, D.; Boutou, A. A Rare Case of a Solitary Central Nervous System Tuberculoma Mimicking an Intracranial Tumor. Cureus 2025, 17, e78201. [Google Scholar] [CrossRef]
- Gameraddin, M.; Alshoabi, S. Tuberculous Granuloma of the Brain. Austin. J. Radiol. 2016, 3, 1048. [Google Scholar]
- Ni’ami, M.A.; Nurhayati, D. Giant cerebral tuberculoma mimicking tumor in a pediatric patient: A case report. Radiol. Case Rep. 2024, 19, 5908–5915. [Google Scholar] [CrossRef] [PubMed]
- Abdu, A.M.; Alshoabi, S.A.; Arafah, M.A.; Hamid, R.A.; Hamid, A.M.; Kocak, M. Intramedullary tuberculoma of the thoracic spine presented as paraparesis: A rare case report. Radiol. Case Rep. 2023, 19, 310–314. [Google Scholar] [CrossRef]
- Dey, S.; Misra, S.; Dutta, M. Primary Sinonasal Tuberculosis: A Diagnostic Challenge. Turk. Arch. Otorhinolaryngol. 2018, 56, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Alherabi, A.Z.; Marglani, O.A.; Gazzaz, M.J.; Abbas, M.M. Paraspinal tuberculosis mimicking malignancy. Saudi Med. J. 2013, 34, 1296–1298. [Google Scholar] [PubMed]
- Kale, A.; Patil, P.S.; Chhanchure, U.; Deodhar, K.; Kulkarni, S.; Mehta, S.; Tandon, S. Hepatic tuberculosis masquerading as malignancy. Hepatol. Int. 2022, 16, 463–472. [Google Scholar] [CrossRef]
- Benkabbou, A.; El Malki, H.O.; Mohsine, R.; Ifrine, L.; Belkouchi, A. Tuberculose isolée du pancréas et des ganglions péripancréatiques: Challenge diagnostique [Isolated tuberculosis of pancreas and peripancreatic lymph nodes: Diagnostic challenge]. Tunis Med. 2009, 87, 89–92. (In French) [Google Scholar]
- Suarez-Zamora, D.A.; Palau-Lazaro, M.A.; Rodriguez-Urrego, P.A. Esophageal tuberculosis in an HIV-positive patient mimicking a spindle cell tumor. Rev. Española Patol. 2019, 52, 199–201. [Google Scholar] [CrossRef]
- Chen, M.T.; Ong, F.; Choy, K.T.; Chakraborty, J. Colonic tuberculosis mimicking malignancy: A multidisciplinary medical and surgical approach. BMJ Case Rep. 2025, 18, e262626. [Google Scholar] [CrossRef]
- Resendes, M.S.; Santos, A.P.; Meireles, D.; Pereira, F.G. When Peritoneal Tuberculosis Mimics Carcinomatosis: A Diagnostic Enigma. Int. J. Mycobacteriol. 2025, 14, 204–207. [Google Scholar] [CrossRef]
- Al-Zughali, E.A.; Al-Shami, N.A.; Hamedat, A.W.; Al-Bustanji, S.; Almaletti, M.; Al-Shaibani, E.M.; Ba-Shammakh, S.A. Peritoneal Tuberculosis Mimicking Ovarian Malignancy: A Case Report. Cureus 2024, 16, e76231. [Google Scholar] [CrossRef]
- Harouachi, A.; Jabri, L.; Bouhout, T.; Serji, B. Ovarian tuberculosis mimicking ovarian malignancy in an unvaccinated patient: A case report. Int. J. Surg. Case Rep. 2024, 122, 110031. [Google Scholar] [CrossRef]
- Ifthikar, M.A.; Kamath, S.N.; Shetty, R.; Thippeswamy, H.G.; Biswas, S. Unmasking the Masquerade: Navigating the Diagnostic Enigma of Abdominal TB Mimicking Endometriosis. J. Obstet. Gynaecol. India 2025, 75 (Suppl. S1), 582–584. [Google Scholar] [CrossRef]
- Legesse, T.K.; Issa, S.A.; Yaynishet, Y.A.; Dessie, T.A.; Gebremariam, T.Y.; Reta, B.K. Isolated Prostate Tuberculosis Mimicking Prostate Cancer. Ethiop. J. Health Sci. 2024, 34, 67–72. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Akhali, E.S.; Alshoabi, S.A.; Muslem, H.F.; Alhazmi, F.H.; Alsaedi, A.F.; Alsultan, K.D.; Alzain, A.F.; Omer, A.M.; Elzaki, M.; Hamid, A.M. Multisystemic Tuberculosis Masquerading as Aggressive Cardiac Tumor Causing Budd–Chiari Syndrome Disseminated to the Brain Resulting in Death of a Six-Year-Old Boy. Pathogens 2025, 14, 772. https://doi.org/10.3390/pathogens14080772
Al-Akhali ES, Alshoabi SA, Muslem HF, Alhazmi FH, Alsaedi AF, Alsultan KD, Alzain AF, Omer AM, Elzaki M, Hamid AM. Multisystemic Tuberculosis Masquerading as Aggressive Cardiac Tumor Causing Budd–Chiari Syndrome Disseminated to the Brain Resulting in Death of a Six-Year-Old Boy. Pathogens. 2025; 14(8):772. https://doi.org/10.3390/pathogens14080772
Chicago/Turabian StyleAl-Akhali, Eman S., Sultan Abdulwadoud Alshoabi, Halah Fuad Muslem, Fahad H. Alhazmi, Amirah F. Alsaedi, Kamal D. Alsultan, Amel F. Alzain, Awatif M. Omer, Maisa Elzaki, and Abdullgabbar M. Hamid. 2025. "Multisystemic Tuberculosis Masquerading as Aggressive Cardiac Tumor Causing Budd–Chiari Syndrome Disseminated to the Brain Resulting in Death of a Six-Year-Old Boy" Pathogens 14, no. 8: 772. https://doi.org/10.3390/pathogens14080772
APA StyleAl-Akhali, E. S., Alshoabi, S. A., Muslem, H. F., Alhazmi, F. H., Alsaedi, A. F., Alsultan, K. D., Alzain, A. F., Omer, A. M., Elzaki, M., & Hamid, A. M. (2025). Multisystemic Tuberculosis Masquerading as Aggressive Cardiac Tumor Causing Budd–Chiari Syndrome Disseminated to the Brain Resulting in Death of a Six-Year-Old Boy. Pathogens, 14(8), 772. https://doi.org/10.3390/pathogens14080772





