Risk Factors for Latent Tuberculosis Identified Using Epidemiological Investigation in Congregate Settings of Gyeongsan City, Republic of Korea (2014–2023)
Abstract
1. Introduction
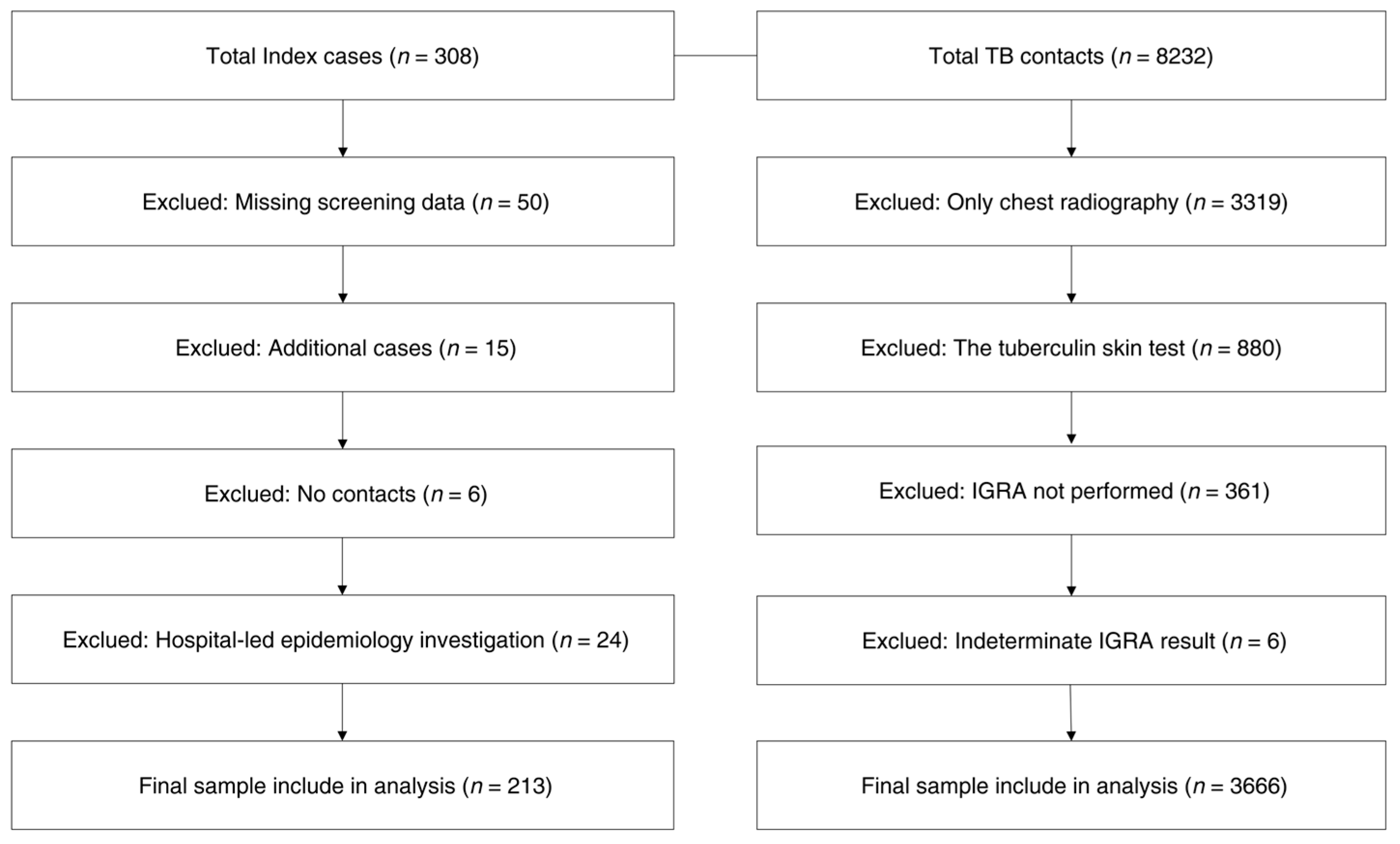
2. Materials and Methods
2.1. Population Data
2.2. Measures and Definitions
2.3. Data Collection
2.4. Data Analysis
2.5. Ethical Statement
3. Results
3.1. Characteristics of Index Cases
3.2. Characteristics of TB Contacts in Congregate Settings
3.3. Risk Factors Associated with LTBI Positivity
3.4. Temporal Trends in LTBI Positivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, S. Pathogenesis, immunology, and diagnosis of Latent Mycobacterium tuberculosis infection. J. Immunol. Res. 2011, 2011, 814943. [Google Scholar] [CrossRef]
- Kasambira, T.S.; Shah, M.; Adrian, P.V.; Holshouser, M.; Madhi, S.A.; Chaisson, R.E.; Martinson, N.A.; Dorman, S.E. QuantiFERON-TB Gold In-Tube for the detection of Mycobacterium tuberculosis infection in children with household tuberculosis contact. Int. J. Tuberc. Lung Dis. 2011, 15, 628–634. [Google Scholar] [CrossRef]
- Biraro, I.A.; Kimuda, S.; Egaesa, M.; Cose, S.; Webb, E.L.; Joloba, M.; Smith, S.G.; Elliott, A.M.; Dockrell, H.M.; Katamba, A. The Use of Interferon Gamma Inducible Protein 10 as a Potential Biomarker in the Diagnosis of Latent Tuberculosis Infection in Uganda. PLoS ONE 2016, 11, e0146098. [Google Scholar] [CrossRef]
- Houben, R.M.; Dodd, P.J. The global burden of latent tuberculosis infection: A re-estimation using mathematical modelling. PLoS Med. 2016, 13, e1002152. [Google Scholar] [CrossRef]
- World Health Organization. Global Tuberculosis Report 2013; World Health Organization: Geneva, Switzerland, 2013; Available online: https://iris.who.int/bitstream/handle/10665/91355/9789241564656_eng.pdf?sequence=1 (accessed on 10 March 2025).
- Korea Disease Control and Prevention Agency (KDCA). Status of Notified Tuberculosis in Korea, 2012; KDCA: Cheongju, Republic of Korea, 2013. Available online: https://www.kdca.go.kr/board/board.es?mid=a20607020000&bid=0034&list_no=21483&act=view (accessed on 11 March 2025).
- World Health Organization. Global Tuberculosis Report 2024; World Health Organization: Geneva, Switzerland, 2024; Available online: https://iris.who.int/bitstream/handle/10665/379339/9789240101531_eng.pdf?sequence=1 (accessed on 10 July 2025).
- Selassie, A.W.; Pozsik, C.; Wilson, D.; Ferguson, P.L. Why pulmonary tuberculosis recurs: A population-based epidemiological study. Ann. Epidemiol. 2005, 15, 519–525. [Google Scholar] [CrossRef]
- Cho, K.S. Tuberculosis control in the Republic of Korea. Epidemiol. Health 2018, 40, e2018036. [Google Scholar] [CrossRef]
- KDCA. National Tuberculosis (TB) Control Guidelines—South Korea, 2025; KDCA: Cheongju, Republic of Korea, 2025. Available online: https://www.kdca.go.kr/board/board.es?mid=a20607020000&bid=0034&act=view&list_no=731335 (accessed on 11 June 2025).
- Katz, M.H. Multivariable Analysis: A Practial Guide for Clinicial, 2nd ed.; Cambridge University Press: Cambridge, UK, 1999; p. 66. [Google Scholar]
- KDCA. Annual Report on the Notified Tuberculosis in Korea 2023; KDCA: Cheongju, Republic of Korea, 2024. Available online: https://tbzero.kdca.go.kr/tbzero/borad/boradView.do (accessed on 11 June 2025).
- Pai, M.; Zwerling, A.; Menzies, D. Systematic review: T-cell–based assays for the diagnosis latent tuberculosis infection: An update. Ann. Intern. Med. 2008, 149, 177–184. [Google Scholar] [CrossRef]
- Mazurek, G.H.; Jereb, J.; Vernon, A.; LoBue, P.; Goldberg, S.; Castro, K.; IGRA Expert Committee and Centers for Disease Control; Prevention (CDC). Updated Guidelines for Using Interferon Gamma Release Assays to Detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm. Rep. 2010, 59, 1–25. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5905a1.htm (accessed on 11 July 2025).
- KDCA. Korean Guidelines for Tuberculosis, 5th ed.; KDCA: Cheongju, Republic of Korea, 2023. Available online: https://www.kdca.go.kr (accessed on 11 July 2025).
- Fu, H.; Lin, H.H.; Hallett, T.B.; Arinaminpathy, N. Explaining age disparities in tuberculosis burden in Taiwan: A modelling study. BMC Infect. Dis. 2020, 20, 191. [Google Scholar] [CrossRef]
- Faiz, J.F.; Hadi, E.N. Risk Factors for Latent Tuberculosis Infection (LTBI) Among Household Contacts of TB Patients: A Systematic Review. Indones. J. Public Health 2024, 15, 288–302. [Google Scholar] [CrossRef]
- Cho, H.; Seok, J.; Park, Y.; Kim, H.J.; Lee, E.H.; Park, J.; Park, D.A.; Kang, Y.A.; Lee, J. Cost-Effectiveness of Age-Expanding Strategy of Latent Tuberculosis Infection Treatment in Household Contacts in The Republic of Korea. Yonsei Med. J. 2023, 64, 366–374. [Google Scholar] [CrossRef]
- Mori, T.; Leung, C.C. Tuberculosis in the global aging population. Infect. Dis. Clin. N. Am. 2010, 24, 751–768. [Google Scholar] [CrossRef]
- Kim, H.W.; Min, J.; Choi, J.Y.; Shin, A.Y.; Myong, J.-P.; Lee, Y.; Yim, H.W.; Jeong, H.; Bae, S.; Choi, H.; et al. Prevalence of latent tuberculosis infection among participants of the national LTBI screening program in South Korea—A problem of low coverage rate with current LTBI strategy. Front. Public Health 2023, 10, 1066269. [Google Scholar] [CrossRef]
- Shim, J.; Han, S.; Kim, J.; Choi, S.; Choi, B.; Lee, H.Y.; Lee, J.; Park, Y.; Park, Y.J. Results of the Tuberculosis Contact Investigation, 2023. Public Health Wkly. Rep. 2024, 17, 1519–1533. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Shim, J.; Choi, S.; Park, Y.J. Five-year Trends in the Results of Tuberculosis Contact Investigations in Congregate Settings, 2019–2023. Public Health Wkly. Rep. 2025, 18, 23–38. [Google Scholar] [CrossRef]
- Yeon, J.H.; Seong, H.; Hur, H.; Park, Y.; Kim, Y.A.; Park, Y.S.; Han, C.H.; Lee, S.M.; Seo, J.H.; Kang, J.G. Prevalence and risk factors of latent tuberculosis among Korean healthcare workers using whole-blood interferon-γ release assay. Sci. Rep. 2018, 8, 10113. [Google Scholar] [CrossRef]
- Esmail, H.; Barry, C.E.; Young, D.B.; Wilkinson, R.J. The ongoing challenge of latent tuberculosis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130437. [Google Scholar] [CrossRef]
- Tostmann, A.; Kik, S.V.; Kalisvaart, N.A.; Sebek, M.M.G.; Verver, S.; Boeree, M.J.; van Soolingen, D. Tuberculosis transmission by patients with smear-negative pulmonary tuberculosis in a large cohort in the Netherlands. Clin. Infect. Dis. 2008, 47, 1135–1142. [Google Scholar] [CrossRef]
- Park, S.Y.; Han, S.; Kim, Y.-M.; Kim, J.; Lee, S.; Yang, J.; Kim, U.-N.; Park, M.-S. Risk of active tuberculosis development in contacts exposed to infectious tuberculosis in congregate settings in Korea. Sci. Rep. 2020, 10, 1306. [Google Scholar] [CrossRef]
- Meregildo-Rodriguez, E.; Contreras-Pulache, H.; Gutierrez-Ajalcriña, M.; Sánchez-Sánchez, J.; Benites-Zapata, V.A. Latent tuberculosis infection in healthcare workers: A cross-sectional study at a northern Peruvian hospital. Front. Med. 2023, 10, 1295299. [Google Scholar] [CrossRef]
- Lee, M.-R.; Huang, Y.-P.; Kuo, Y.-T.; Luo, C.-H.; Shih, Y.-J.; Shu, C.-C.; Wang, J.-Y.; Ko, J.-C.; Yu, C.-J.; Lin, H.-H. Diabetes mellitus and latent tuberculosis infection: A systemic review and metaanalysis. Clin. Infect. Dis. 2016, 64, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.P.; Babu, S. Impact of diabetes mellitus on immunity to latent tuberculosis infection. Front. Clin. Diabetes Healthc. 2023, 4, 1095467. [Google Scholar] [CrossRef] [PubMed Central]
- Ugarte-Gil, C.; Carrillo-Larco, R.M.; Kirwan, D.E. Latent tuberculosis infection and non-infectious co-morbidities: Diabetes mellitus type 2, chronic kidney disease and rheumatoid arthritis. Int. J. Infect. Dis. 2019, 80, S29–S31. [Google Scholar] [CrossRef]
| Characteristics | Category | Number | Percentage (%) |
|---|---|---|---|
| Sex | Female | 97 | 45.5 |
| Male | 116 | 54.5 | |
| Age (years) | <20 | 17 | 8.0 |
| 20–29 | 56 | 26.2 | |
| 30–39 | 21 | 9.9 | |
| 40–49 | 17 | 8.0 | |
| 50–59 | 21 | 9.9 | |
| 60–69 | 13 | 6.1 | |
| 70–79 | 17 | 8.0 | |
| 80–89 | 45 | 21.1 | |
| ≥90 | 6 | 2.8 | |
| Nationality | Republic of Korea national | 194 | 91.1 |
| Foreign national | 19 | 8.9 | |
| Types of congregate settings | Educational facilities | 67 | 31.4 |
| Military camps | 1 | 0.5 | |
| Social welfare facilities | 44 | 20.7 | |
| Workplaces | 62 | 29.1 | |
| Healthcare facilities | 39 | 18.3 | |
| Respiratory symptoms | Negative | 63 | 29.6 |
| Positive | 150 | 70.4 | |
| Cavity on chest radiography | Negative | 159 | 74.6 |
| Positive | 54 | 25.4 | |
| Sputum smear status | Negative | 104 | 48.8 |
| Positive | 109 | 51.2 |
| Characteristics | Category | LTBI Positives (n, %) | LTBI Negatives (n, %) | Chi-Square (p-Value) |
|---|---|---|---|---|
| Sex | Female | 339 (19.4) | 1411 (80.6) | 3.77 (0.052) |
| Male | 421 (22.0) | 1495 (78.0) | ||
| Age (years) | <19 | 70 (11.0) | 569 (89.0) | 183.27 (<0.001) |
| 19–64 | 553 (20.1) | 2202 (79.9) | ||
| ≥65 | 137 (50.4) | 135 (49.6) | ||
| Types of congregate settings | Educational facilities | 118 (11.0) | 1517 (89.0) | 212.99 (<0.001) |
| Military camps | 13 (16.7) | 65 (83.3) | ||
| Social welfare facilities | 186 (31.3) | 409 (68.7) | ||
| Workplaces | 174 (24.3) | 542 (75.7) | ||
| Healthcare facilities | 199 (34.8) | 373 (65.2) | ||
| Number of contacts (cases) | <10 | 232 (18.6) | 1018 (81.4) | 52.3 (<0.001) |
| 10–29 | 104 (13.6) | 662 (86.4) | ||
| 30–49 | 320 (25.9) | 916 (74.1) | ||
| ≥50 | 104 (25.1) | 310 (74.9) | ||
| Respiratory symptoms | Negative | 249 (22.6) | 855 (77.4) | 3.20 (0.074) |
| Positive | 511 (19.9) | 2051 (80.1) | ||
| Cavity on chest radiography | Negative | 603 (20.4) | 2346 (79.6) | 0.74 (0.391) |
| Positive | 157 (21.9) | 560 (78.1) | ||
| Sputum smear status (PBS) | Negative | 398 (20.2) | 1573 (79.8) | 0.74 (0.386) |
| Positive | 362 (21.4) | 1333 (78.6) | ||
| TB symptoms or signs in index * | Negative | 139 (18.6) | 608 (21.4) | 2.57 (0.109) |
| Positive | 621 (21.3) | 2298 (78.7) | ||
| Total | 760 (20.7) | 2906 (79.3) |
| Variable Type | Category | OR * | 95% CI | p-Value |
|---|---|---|---|---|
| Age (years) | <19 | 1.0 | Ref | |
| 19–64 | 1.09 | 0.81–1.48 | 0.574 | |
| ≥65 | 2.93 | 1.95–4.39 | <0.001 | |
| Types of congregate settings | Educational facilities | 1.0 | Ref | |
| Military camps | 1.57 | 0.84–2.92 | 0.159 | |
| Social welfare facilities | 2.75 | 2.10–3.58 | <0.001 | |
| Workplaces | 2.42 | 1.88–3.10 | <0.001 | |
| Healthcare facilities | 3.42 | 2.63–4.43 | <0.001 |
| Year | No. of Contacts | LTBI-Positive | Positive Rate (%) |
|---|---|---|---|
| 2014 | 370 | 78 | 21.1 |
| 2015 | 572 | 93 | 16.3 |
| 2016 | 293 | 66 | 22.5 |
| 2017 | 275 | 48 | 17.5 |
| 2018 | 210 | 48 | 19.2 |
| 2019 | 672 | 130 | 19.3 |
| 2020 | 269 | 38 | 14.1 |
| 2021 | 208 | 52 | 25.0 |
| 2022 | 376 | 71 | 18.9 |
| 2023 | 446 | 144 | 32.3 |
| total | 3666 | 760 | 20.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Lee, K. Risk Factors for Latent Tuberculosis Identified Using Epidemiological Investigation in Congregate Settings of Gyeongsan City, Republic of Korea (2014–2023). Pathogens 2025, 14, 740. https://doi.org/10.3390/pathogens14080740
Park S, Lee K. Risk Factors for Latent Tuberculosis Identified Using Epidemiological Investigation in Congregate Settings of Gyeongsan City, Republic of Korea (2014–2023). Pathogens. 2025; 14(8):740. https://doi.org/10.3390/pathogens14080740
Chicago/Turabian StylePark, Seonyeong, and Kwan Lee. 2025. "Risk Factors for Latent Tuberculosis Identified Using Epidemiological Investigation in Congregate Settings of Gyeongsan City, Republic of Korea (2014–2023)" Pathogens 14, no. 8: 740. https://doi.org/10.3390/pathogens14080740
APA StylePark, S., & Lee, K. (2025). Risk Factors for Latent Tuberculosis Identified Using Epidemiological Investigation in Congregate Settings of Gyeongsan City, Republic of Korea (2014–2023). Pathogens, 14(8), 740. https://doi.org/10.3390/pathogens14080740







