Role and Contribution of Serological Surveillance in Animals and Exposed Humans to the Study of Zoonotic Influenza Disease Epidemiology: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
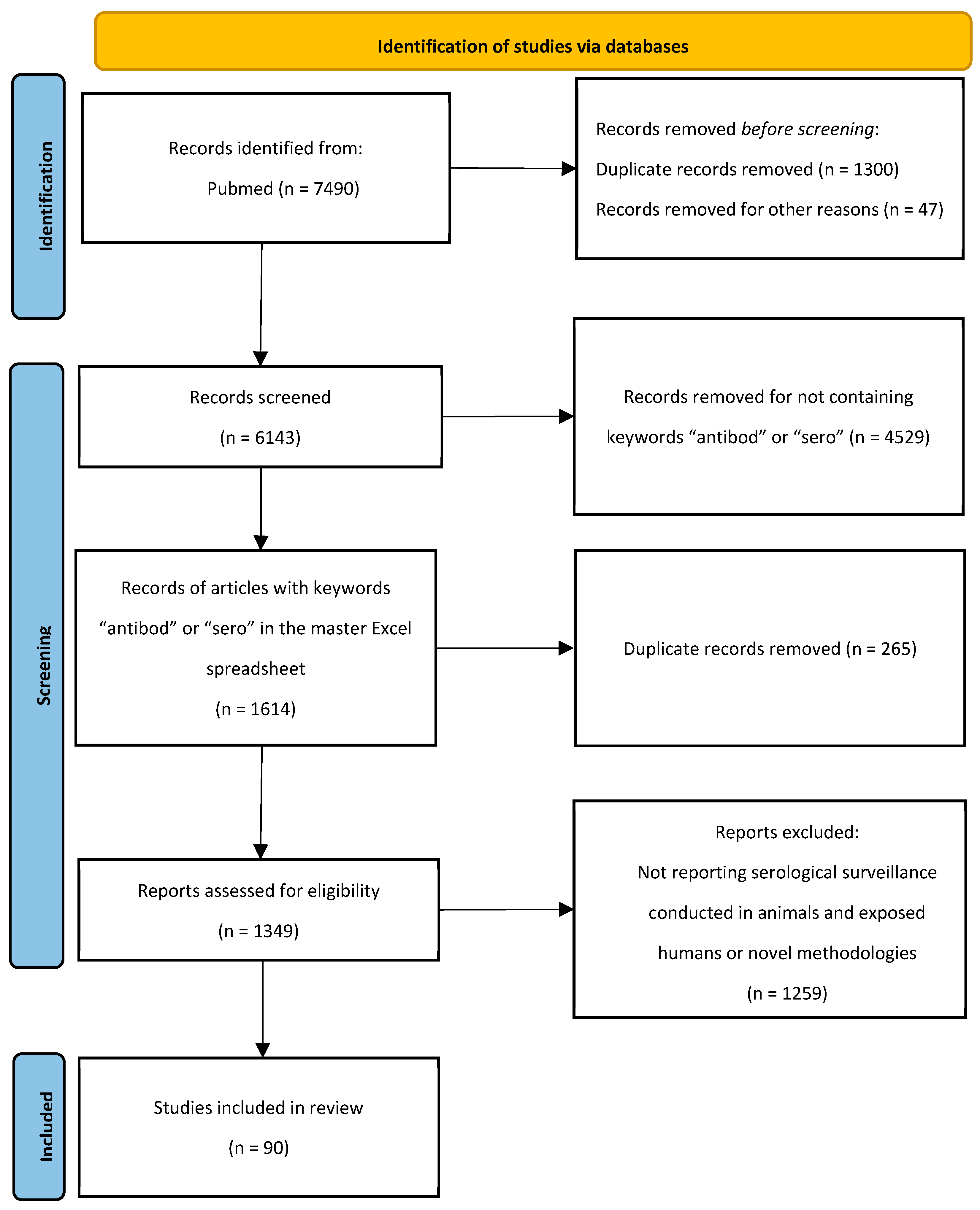
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Collection Process
2.4. Synthesis of Results
3. Findings
3.1. Serological Surveillance of Zoonotic Influenza Viruses in Domestic Animal Husbandry
3.1.1. Serological Surveillance of Avian Influenza Virus in Domestic Birds
3.1.2. Serological Surveillance of Avian Influenza Virus in Domestic Mammals
3.1.3. Serological Surveillance of Swine Influenza Virus in Swine and Pigs
3.1.4. Serological Surveillance of Canine Influenza Virus in Domestic Animal Husbandry
3.1.5. Serological Surveillance of Influenza D Virus in Domestic Animal Husbandry
3.2. Serological Surveillance of Zoonotic Influenza Viruses in Wild Animals
3.2.1. Serological Surveillance of Zoonotic Influenza Virus in Wild Birds
3.2.2. Serological Surveillance of Zoonotic Influenza Virus in Wild Mammals
3.2.3. Serological Surveillance of Swine Influenza Virus
3.3. Serological Surveillance at the Animal–Human Interface
3.3.1. Serological Surveillance at the Bird–Human Interface
3.3.2. Serological Surveillance at the Mammalian–Human Interface
3.4. Novel Methodologies, Technical Limitations, and Implementation Challenges
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdelwhab, E.M.; Mettenleiter, T.C. Zoonotic Animal Influenza Virus and Potential Mixing Vessel Hosts. Viruses 2023, 15, 980. [Google Scholar] [CrossRef]
- Cheng, X.; Tan, Y.; He, M.; Lam, T.T.; Lu, X.; Viboud, C.; He, J.; Zhang, S.; Lu, J.; Wu, C.; et al. Epidemiological dynamics and phylogeography of influenza virus in southern China. J. Infect. Dis. 2013, 207, 106–114. [Google Scholar] [CrossRef]
- Laurie, K.L.; Huston, P.; Riley, S.; Katz, J.M.; Willison, D.J.; Tam, J.S.; Mounts, A.W.; Hoschler, K.; Miller, E.; Vandemaele, K.; et al. Influenza serological studies to inform public health action: Best practices to optimise timing, quality and reporting. Influenza Other Respir. Viruses 2013, 7, 211–224. [Google Scholar] [CrossRef]
- Poen, M.J.; Verhagen, J.H.; Manvell, R.J.; Brown, I.; Bestebroer, T.M.; van der Vliet, S.; Vuong, O.; Scheuer, R.D.; van der Jeugd, H.P.; Nolet, B.A. Lack of virological and serological evidence for continued circulation of highly pathogenic avian influenza H5N8 virus in wild birds in the Netherlands, 14 November 2014 to 31 January 2016. Eurosurveillance 2016, 21, 30349. [Google Scholar] [CrossRef]
- Haselbeck, A.H.; Im, J.; Prifti, K.; Marks, F.; Holm, M.; Zellweger, R.M. Serology as a Tool to Assess Infectious Disease Landscapes and Guide Public Health Policy. Pathogens 2022, 11, 732. [Google Scholar] [CrossRef]
- Katz, J.M.; Hancock, K.; Xu, X. Serologic assays for influenza surveillance, diagnosis and vaccine evaluation. Expert Rev. Anti-Infect. Ther. 2011, 9, 669–683. [Google Scholar] [CrossRef]
- Clancey, E.; Nuismer, S.L.; Seifert, S.N. Using serosurveys to optimize surveillance for zoonotic pathogens. biorXiv 2024. [Google Scholar] [CrossRef]
- Villaamil, F.J.; Arnaiz, I.; Allepuz, A.; Molins, M.; Lazaro, M.; Benavides, B.; Moya, S.J.; Fabrega, J.C.; Yus, E.; Dieguez, F.J. A survey of biosecurity measures and serological status for bovine viral diarrhoea virus and bovine herpesvirus 1 on dairy cattle farms in north-west and north-east Spain. Vet. Rec. Open 2020, 7, e000399. [Google Scholar] [CrossRef]
- Cutts, F.T.; Hanson, M. Seroepidemiology: An underused tool for designing and monitoring vaccination programmes in low- and middle-income countries. Trop. Med. Int. Health 2016, 21, 1086–1098. [Google Scholar] [CrossRef]
- Metcalf, C.J.; Farrar, J.; Cutts, F.T.; Basta, N.E.; Graham, A.L.; Lessler, J.; Ferguson, N.M.; Burke, D.S.; Grenfell, B.T. Use of serological surveys to generate key insights into the changing global landscape of infectious disease. Lancet 2016, 388, 728–730. [Google Scholar] [CrossRef]
- Mast, E.E.; Margolis, H.S.; Fiore, A.E.; Brink, E.W.; Goldstein, S.T.; Wang, S.A.; Moyer, L.A.; Bell, B.P.; Alter, M.J. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: Immunization of infants, children, and adolescents. MMWR Recomm. Rep. Morb. Mortal. Wkly. Rep. 2005, 54, 1–31. [Google Scholar]
- Arnold, B.F.; Scobie, H.M.; Priest, J.W.; Lammie, P.J. Integrated Serologic Surveillance of Population Immunity and Disease Transmission. Emerg. Infect. Dis. 2018, 24, 1188–1194. [Google Scholar] [CrossRef]
- Mina, M.J.; Metcalf, C.J.E.; McDermott, A.B.; Douek, D.C.; Farrar, J.; Grenfell, B.T. A Global lmmunological Observatory to meet a time of pandemics. eLife 2020, 9, e58989. [Google Scholar] [CrossRef]
- World Health Organization. Preparing for Containment and Mitigation of Pandemic H5N1 Influenza, Uses of Statistical and Mathematical Modeling; World Health Organization: Geneva, Switzerland, 2024; Available online: https://www.who.int/news-room/events/detail/2024/11/14/default-calendar/preparing-for-containment-and-mitigation-of-pandemic-h5n1-influenza--uses-of-statistical-and-mathematical-modeling (accessed on 8 July 2025).
- Abdelaziz, A.M.; Mohamed, M.H.A.; Fayez, M.M.; Al-Marri, T.; Qasim, I.; Al-Amer, A.A. Molecular survey and interaction of common respiratory pathogens in chicken flocks (field perspective). Vet. World 2019, 12, 1975–1986. [Google Scholar] [CrossRef]
- Hassan, M.M.; Islam, A.; Hasan, R.B.; Rahman, M.K.; Webby, R.J.; Hoque, M.A.; El Zowalaty, M.E. Prevalence and Distribution of Avian Influenza Viruses in Domestic Ducks at the Waterfowl-Chicken Interface in Wetlands. Pathogens 2020, 9, 953. [Google Scholar] [CrossRef]
- Gupta, S.D.; Hoque, M.A.; Fournie, G.; Henning, J. Patterns of Avian Influenza A (H5) and A (H9) virus infection in backyard, commercial broiler and layer chicken farms in Bangladesh. Transbound. Emerg. Dis. 2021, 68, 137–151. [Google Scholar] [CrossRef]
- Gupta, S.D.; Fournie, G.; Hoque, M.A.; Henning, J. Farm-Level Risk Factors Associated With Avian Influenza A (H5) and A (H9) Flock-Level Seroprevalence on Commercial Broiler and Layer Chicken Farms in Bangladesh. Front. Vet. Sci. 2022, 9, 893721. [Google Scholar] [CrossRef]
- Rahman, M.A.; Belgrad, J.P.; Sayeed, M.A.; Abdullah, M.S.; Barua, S.; Chisty, N.N.; Mohsin, M.A.S.; Foysal, M.; Hossain, M.E.; Islam, A.; et al. Prevalence and risk factors of Avian Influenza Viruses among household ducks in Chattogram, Bangladesh. Vet. Res. Commun. 2022, 46, 471–480. [Google Scholar] [CrossRef]
- Bourscheid, C.L.P.; Moreira, R.B.; Reischak, D.; Negreiros, R.L.; Mascarenhas, L.A.; Muniz, G.G.S.; Muniz, M.V.B.; Aguiar, D.M. Surveillance of avian influenza and Newcastle disease viruses in backyard poultry raised near migratory bird sites in Mato Grosso state, Brazil. Rev. Sci. Et Tech. 2020, 39, 907–922. [Google Scholar] [CrossRef]
- Yuyun, I.; Wibawa, H.; Setiaji, G.; Kusumastuti, T.A.; Nugroho, W.S. Determining highly pathogenic H5 avian influenza clade 2.3.2.1c seroprevalence in ducks, Purbalingga, Central Java, Indonesia. Vet. World 2020, 13, 1138–1144. [Google Scholar] [CrossRef]
- Di Pillo, F.; Baumberger, C.; Salazar, C.; Galdames, P.; Ruiz, S.; Sharp, B.; Freiden, P.; Tan, S.; Schultz-Cherry, S.; Hamilton-West, C.; et al. Novel Low Pathogenic Avian Influenza H6N1 in Backyard Chicken in Easter Island (Rapa Nui), Chilean Polynesia. Viruses 2022, 14, 718. [Google Scholar] [CrossRef]
- Fagrach, A.; Arbani, O.; Karroute, O.; El-Ftouhy, F.Z.; Kichou, F.; Bouslikhane, M.; Fellahi, S. Prevalence of major infectious diseases in backyard chickens from rural markets in Morocco. Vet. World 2023, 16, 1897–1906. [Google Scholar] [CrossRef]
- Agha, A.S.K.; Benlashehr, I.; Naffati, K.M.; Bshina, S.A.; Khashkhosha, A.A. Correlation of avian influenza-H9N2 with high mortality in broiler flocks in the southwest of Tripoli, Libya. Open Vet. J. 2023, 13, 715–722. [Google Scholar] [CrossRef]
- Simbizi, V.; Moerane, R.; Ramsay, G.; Mubamba, C.; Abolnik, C.; Gummow, B. A study of rural chicken farmers, diseases and remedies in the Eastern Cape province of South Africa. Prev. Vet. Med. 2021, 194, 105430. [Google Scholar] [CrossRef]
- European Food Safety, A.; Brouwer, A.; Gonzales, J.; Huneau, A.; Mulatti, P.; Kuiken, T.; Staubach, C.; Stegeman, A.; Antoniou, S.E.; Baldinelli, F.; et al. Annual Report on surveillance for avian influenza in poultry and wild birds in Member States of the European Union in 2018. EFSA J. 2019, 17, e05945. [Google Scholar] [CrossRef]
- European Food Safety, A.; Baldinelli, F.; Papanikolaou, A.; Stoicescu, A.; Van der Stede, Y.; Aznar, I. Annual Report on surveillance for Avian Influenza in poultry and wild birds in Member States of the European Union in 2019. EFSA J. 2020, 18, e06349. [Google Scholar] [CrossRef]
- European Food Safety, A.; Aznar, I.; Baldinelli, F.; Papanikolaou, A.; Stoicescu, A.; Van der Stede, Y. Annual Report on surveillance for avian influenza in poultry and wild birds in Member States of the European Union in 2020. EFSA J. 2021, 19, e06953. [Google Scholar] [CrossRef]
- European Food Safety, A.; Aznar, I.; Baldinelli, F.; Stoicescu, A.; Kohnle, L. Annual report on surveillance for avian influenza in poultry and wild birds in Member States of the European Union in 2021. EFSA J. 2022, 20, e07554. [Google Scholar] [CrossRef]
- European Food Safety, A.; Aznar, I.; Kohnle, L.; Stoicescu, A.; van Houtum, A.; Zancanaro, G. Annual report on surveillance for avian influenza in poultry and wild birds in Member States of the European Union in 2022. EFSA J. 2023, 21, e8480. [Google Scholar] [CrossRef]
- Herve, S.; Schmitz, A.; Briand, F.X.; Gorin, S.; Queguiner, S.; Niqueux, E.; Paboeuf, F.; Scoizec, A.; Le Bouquin-Leneveu, S.; Eterradossi, N.; et al. Serological Evidence of Backyard Pig Exposure to Highly Pathogenic Avian Influenza H5N8 Virus during 2016–2017 Epizootic in France. Pathogens 2021, 10, 621. [Google Scholar] [CrossRef]
- Jallow, M.M.; Barry, M.A.; Fall, A.; Ndiaye, N.K.; Kiori, D.; Sy, S.; Goudiaby, D.; Niang, M.N.; Fall, G.; Fall, M.; et al. Influenza A Virus in Pigs in Senegal and Risk Assessment of Avian Influenza Virus (AIV) Emergence and Transmission to Human. Microorganisms 2023, 11, 1961. [Google Scholar] [CrossRef]
- Rosone, F.; Bonfante, F.; Sala, M.G.; Maniero, S.; Cersini, A.; Ricci, I.; Garofalo, L.; Caciolo, D.; Denisi, A.; Napolitan, A.; et al. Seroconversion of a Swine Herd in a Free-Range Rural Multi-Species Farm against HPAI H5N1 2.3.4.4b Clade Virus. Microorganisms 2023, 11, 1162. [Google Scholar] [CrossRef]
- Yu, Z.; Cheng, K.; Wu, J. Serological evidence of the infection of H7 virus and the co-infection of H7 and H9 viruses in farmed fur-bearing animals in eastern China. Braz. J. Microbiol 2020, 51, 2163–2167. [Google Scholar] [CrossRef]
- Moreno, A.; Bonfante, F.; Bortolami, A.; Cassaniti, I.; Caruana, A.; Cottini, V.; Cereda, D.; Farioli, M.; Fusaro, A.; Lavazza, A.; et al. Asymptomatic infection with clade 2.3.4.4b highly pathogenic avian influenza A(H5N1) in carnivore pets, Italy, April 2023. Eurosurveillance 2023, 28, 2300441. [Google Scholar] [CrossRef]
- Alnaeem, A.A.; Al-Shabeb, A.; Hemida, M.G. Evaluation of the immune status of birds and domestic and companion animals for the influenza A virus in Eastern Saudi Arabia. Vet. World 2020, 13, 1966–1969. [Google Scholar] [CrossRef]
- Villanueva-Saz, S.; Martinez, M.; Rueda, P.; Perez, M.D.; Lacasta, D.; Marteles, D.; Ruiz, H.; Gonzalez, A.; Verde, M.T.; Pardo, J.; et al. Serological exposure to influenza A in cats from an area with wild birds positive for avian influenza. Zoonoses Public Health 2024, 71, 324–330. [Google Scholar] [CrossRef]
- Chumsang, S.; Na Lampang, K.; Srikitjakarn, L.; Pringproa, K. Seroprevalence of the viral pig diseases among backyard pigs in Chiang Mai, Thailand. Prev. Vet. Med. 2021, 190, 105330. [Google Scholar] [CrossRef]
- Harima, H.; Okuya, K.; Kajihara, M.; Ogawa, H.; Simulundu, E.; Bwalya, E.; Qiu, Y.; Mori-Kajihara, A.; Munyeme, M.; Sakoda, Y.; et al. Serological and molecular epidemiological study on swine influenza in Zambia. Transbound. Emerg. Dis. 2022, 69, e931–e943. [Google Scholar] [CrossRef]
- Nurhayati; Wibawa, H.; Mahawan, T.; Zenal, F.C.; Schoonman, L.; Pfeiffer, C.N.; Stevenson, M.; Punyapornwithaya, V. Herd-Level Risk Factors for Swine Influenza (H1N1) Seropositivity in West Java and Banten Provinces of Indonesia (2016–2017). Front. Vet. Sci. 2020, 7, 544279. [Google Scholar] [CrossRef]
- Tialla, D.; Sausy, A.; Cisse, A.; Sagna, T.; Ilboudo, A.K.; Ouedraogo, G.A.; Hubschen, J.M.; Tarnagda, Z.; Snoeck, C.J. Serological evidence of swine exposure to pandemic H1N1/2009 influenza A virus in Burkina Faso. Vet. Microbiol. 2020, 241, 108572. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, L.; Chen, T.; Sang, H.; Ding, G.; Li, Y.; Wang, B.; Qin, L.; Liu, S.; Hou, Y.; et al. Serological Surveillance of the H1N1 and H3N2 Swine Influenza A Virus in Chinese Swine between 2016 and 2021. BioMed Res. Int. 2022, 2022, 5833769. [Google Scholar] [CrossRef]
- Fraiha, A.L.S.; Matos, A.C.D.; Cunha, J.L.R.; Santos, B.; Peixoto, M.V.C.; Oliveira, A.G.G.; Galinari, G.C.F.; Nascimento, H.I.J.; Guedes, M.; Machado, A.M.V.; et al. Swine influenza A virus subtypes circulating in Brazilian commercial pig herds from 2012 to 2019. Braz. J. Microbiol. 2021, 52, 2421–2430. [Google Scholar] [CrossRef]
- Serafini Poeta Silva, A.P.; de Freitas Costa, E.; Sousa, E.S.G.; Souza, C.K.; Schaefer, R.; da Silva Vaz, I., Jr.; Corbellini, L.G. Biosecurity practices associated with influenza A virus seroprevalence in sows from southern Brazilian breeding herds. Prev. Vet. Med. 2019, 166, 1–7. [Google Scholar] [CrossRef]
- Papatsiros, V.G.; Papakonstantinou, G.I.; Meletis, E.; Koutoulis, K.; Athanasakopoulou, Z.; Maragkakis, G.; Labronikou, G.; Terzidis, I.; Kostoulas, P.; Billinis, C. Seroprevalence of Swine Influenza A Virus (swIAV) Infections in Commercial Farrow-to-Finish Pig Farms in Greece. Vet. Sci. 2023, 10, 599. [Google Scholar] [CrossRef]
- Milicevic, V.; Glisic, D.; Sapundzic, Z.Z.; Milovanovic, B.; Maletic, J.; Jezdimirovic, N.; Kureljusic, B. Seroprevalence of Viral Enzootic Diseases in Swine Backyard Farms in Serbia. Animals 2023, 13, 3409. [Google Scholar] [CrossRef]
- Almeida, H.M.; Storino, G.Y.; Pereira, D.A.; Gatto, I.R.; Mathias, L.A.; Montassier, H.J.; de Oliveira, L.G. A cross-sectional study of swine influenza in intensive and extensive farms in the northeastern region of the state of Sao Paulo, Brazil. Trop. Anim. Health Prod. 2017, 49, 25–30. [Google Scholar] [CrossRef]
- Chauhan, R.P.; Gordon, M.L. A systematic review of influenza A virus prevalence and transmission dynamics in backyard swine populations globally. Porc. Health Manag. 2022, 8, 10. [Google Scholar] [CrossRef]
- Baraldi, T.G.; Cruz, N.R.N.; Pereira, D.A.; Galdeano, J.V.B.; Gatto, I.R.H.; Silva, A.F.D.; Panzardi, A.; Linhares, D.C.L.; Mathias, L.A.; de Oliveira, L.G. Antibodies against Actinobacillus pleuropneumoniae, Mycoplasma hyopneumoniae and influenza virus and their relationships with risk factors, clinical signs and lung lesions in pig farms with one-site production systems in Brazil. Prev. Vet. Med. 2019, 171, 104748. [Google Scholar] [CrossRef]
- Su, W.; Kinoshita, R.; Gray, J.; Ji, Y.; Yu, D.; Peiris, J.S.M.; Yen, H.L. Seroprevalence of dogs in Hong Kong to human and canine influenza viruses. Vet. Rec. Open 2019, 6, e000327. [Google Scholar] [CrossRef]
- Kwasnik, M.; Smreczak, M.; Rola, J.; Urbaniak, K.; Rozek, W. Serologic investigation of influenza A virus infection in dogs in Poland. J. Vet. Diagn. Investig. 2020, 32, 420–422. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Liu, Y.; Feng, Y.; Wang, T.; Ge, Y.; Kong, Y.; Sun, H.; Xiang, H.; Zhou, B.; et al. Characterization of Canine Influenza Virus A (H3N2) Circulating in Dogs in China from 2016 to 2018. Viruses 2021, 13, 2279. [Google Scholar] [CrossRef]
- Shen, H.X.; Ge, F.F.; Li, X.; Liu, J.; Ju, H.B.; Yang, D.Q.; Yang, X.C.; Wang, J.; Zhao, H.J. Epidemiological survey and genetic evolution of H3N2 subtype influenza viruses from stray dogs in Shanghai, China. Virus Genes 2020, 56, 329–338. [Google Scholar] [CrossRef]
- O’Donovan, T.; Donohoe, L.; Ducatez, M.F.; Meyer, G.; Ryan, E. Seroprevalence of influenza D virus in selected sample groups of Irish cattle, sheep and pigs. Ir. Vet. J. 2019, 72, 11. [Google Scholar] [CrossRef]
- Sanogo, I.N.; Kouakou, C.; Batawui, K.; Djegui, F.; Byarugaba, D.K.; Adjin, R.; Adjabli, K.; Wabwire-Mangen, F.; Erima, B.; Atim, G.; et al. Serological Surveillance of Influenza D Virus in Ruminants and Swine in West and East Africa, 2017–2020. Viruses 2021, 13, 1749. [Google Scholar] [CrossRef]
- Alvarez, I.; Hägglund, S.; Näslund, K.; Eriksson, A.; Ahlgren, E.; Ohlson, A.; Ducatez, M.F.; Meyer, G.; Valarcher, J.F.; Zohari, S. Detection of Influenza D-Specific Antibodies in Bulk Tank Milk from Swedish Dairy Farms. Viruses 2023, 15, 829. [Google Scholar] [CrossRef]
- Gorin, S.; Fablet, C.; Queguiner, S.; Barbier, N.; Paboeuf, F.; Herve, S.; Rose, N.; Simon, G. Assessment of Influenza D Virus in Domestic Pigs and Wild Boars in France: Apparent Limited Spread within Swine Populations Despite Serological Evidence of Breeding Sow Exposure. Viruses 2019, 12, 25. [Google Scholar] [CrossRef]
- Lam, S.S.; Tjornlov, R.S.; Therkildsen, O.R.; Christensen, T.K.; Madsen, J.; Daugaard-Petersen, T.; Ortiz, J.M.C.; Peng, W.; Charbonneaux, M.; Rivas, E.I.; et al. Seroprevalence of avian influenza in Baltic common eiders (Somateria mollissima) and pink-footed geese (Anser brachyrhynchus). Environ. Int. 2020, 142, 105873. [Google Scholar] [CrossRef]
- Lee, M.M.; Jaspers, V.L.B.; Gabrielsen, G.W.; Jenssen, B.M.; Ciesielski, T.M.; Mortensen, A.K.; Lundgren, S.S.; Waugh, C.A. Evidence of avian influenza virus in seabirds breeding on a Norwegian high-Arctic archipelago. BMC Vet. Res. 2020, 16, 48. [Google Scholar] [CrossRef]
- Aziz, U.R.; Shabbir, M.A.B.; Iqbal, M.Z.; Yasin, R.; Ishaq, H.M.; Mehmood, A.; Yousaf, F.; Rasheed, M.; Rasul, S.; Usman, M.; et al. Seroprevalence of Newcastle disease virus and avian influenza virus in poultry and captive wild birds in poultry-dense regions of Pakistan. Vet. Ital. 2023, 59, 1–10. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, H.; Guo, W.; Liu, C.; Zhao, L.; Gu, Y.; Li, S.; Chen, X. Surveillance of multiple subtype specific antibodies against avian influenza viruses among egg yolk in wild ducks from northeast China, 2017–2019. Microb. Pathog. 2021, 152, 104618. [Google Scholar] [CrossRef]
- Brown Jordan, A.; Narang, D.; Essen, S.C.; Brookes, S.M.; Brown, I.H.; Oura, C. Serological Evidence for Influenza A Virus Exposure in Wild Birds in Trinidad & Tobago. Vet. Sci. 2018, 5, 50. [Google Scholar] [CrossRef]
- Carter, D.L.; Link, P.; Tan, G.; Stallknecht, D.E.; Poulson, R.L. Influenza A Viruses in Whistling Ducks (Subfamily Dendrocygninae). Viruses 2021, 13, 192. [Google Scholar] [CrossRef]
- Christie, K.F.; Poulson, R.L.; Seixas, J.S.; Hernandez, S.M. Avian Influenza Virus Status and Maternal Antibodies in Nestling White Ibis (Eudocimus albus). Microorganisms 2021, 9, 2468. [Google Scholar] [CrossRef]
- Malekian, M.; Shagholian, J.; Hosseinpour, Z. Pathogen Presence in Wild Birds Inhabiting Landfills in Central Iran. Ecohealth 2021, 18, 76–83. [Google Scholar] [CrossRef]
- Gobbo, F.; Fornasiero, D.; De Marco, M.A.; Zecchin, B.; Mulatti, P.; Delogu, M.; Terregino, C. Active Surveillance for Highly Pathogenic Avian Influenza Viruses in Wintering Waterbirds in Northeast Italy, 2020–2021. Microorganisms 2021, 9, 2188. [Google Scholar] [CrossRef]
- Hassan, M.M.; Hoque, M.A.; Debnath, N.C.; Yamage, M.; Klaassen, M. Are Poultry or Wild Birds the Main Reservoirs for Avian Influenza in Bangladesh? Ecohealth 2017, 14, 490–500. [Google Scholar] [CrossRef]
- Uher-Koch, B.D.; Spivey, T.J.; Van Hemert, C.R.; Schmutz, J.A.; Jiang, K.; Wan, X.F.; Ramey, A.M. Serologic Evidence for Influenza A Virus Exposure in Three Loon Species Breeding in Alaska, USA. J. Wildl. Dis. 2019, 55, 862–867. [Google Scholar] [CrossRef]
- Severo, D.R.T.; Werlang, R.A.; Mori, A.P.; Baldi, K.R.A.; Mendes, R.E.; Surian, S.R.S.; Coldebella, A.; Kramer, B.; Trevisol, I.M.; Gomes, T.M.A.; et al. Health profile of free-range wild boar (Sus scrofa) subpopulations hunted in Santa Catarina State, Brazil. Transbound. Emerg. Dis. 2021, 68, 857–869. [Google Scholar] [CrossRef]
- Adamu, A.M.; Furlong, M.; Ogunlade, S.; Adikwu, A.A.; Anyang, A.S.; Malgwi, A.; Abdulrahman, A.M.; Bida, N.A.; Owolodun, O.A.; Adegboye, O.A. Seroprevalence of Influenza A Virus in Dromedaries in North-Western Nigeria. Pathogens 2022, 11, 1476. [Google Scholar] [CrossRef]
- Chestakova, I.V.; van der Linden, A.; Bellido Martin, B.; Caliendo, V.; Vuong, O.; Thewessen, S.; Hartung, T.; Bestebroer, T.; Dekker, J.; Jonge Poerink, B.; et al. High number of HPAI H5 virus infections and antibodies in wild carnivores in the Netherlands, 2020–2022. Emerg. Microbes Infect. 2023, 12, 2270068. [Google Scholar] [CrossRef]
- Postel, A.; King, J.; Kaiser, F.K.; Kennedy, J.; Lombardo, M.S.; Reineking, W.; de le Roi, M.; Harder, T.; Pohlmann, A.; Gerlach, T.; et al. Infections with highly pathogenic avian influenza A virus (HPAIV) H5N8 in harbor seals at the German North Sea coast, 2021. Emerg. Microbes Infect. 2022, 11, 725–729. [Google Scholar] [CrossRef]
- Mirolo, M.; Pohlmann, A.; Ahrens, A.K.; Kuhl, B.; Rubio-Garcia, A.; Kramer, K.; Meinfelder, U.; Rosenberger, T.; Morito, H.L.; Beer, M.; et al. Highly pathogenic avian influenza A virus (HPAIV) H5N1 infection in two European grey seals (Halichoerus grypus) with encephalitis. Emerg. Microbes Infect. 2023, 12, e2257810. [Google Scholar] [CrossRef]
- Paungpin, W.; Thongdee, M.; Ketchim, N.; Chaiwattanarungruengpaisan, S.; Saechin, A.; Sariya, L.; Kaewchot, S.; Puthavathana, P.; Wiriyarat, W. Evidence of Influenza A Virus Infection in Cynomolgus Macaques, Thailand. Vet. Sci. 2022, 9, 132. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Inoue, H.; Ozawa, M.; Matsuu, A. Serological survey of influenza A virus infection in Japanese wild boars (Sus scrofa leucomystax). Microbiol. Immunol. 2019, 63, 517–522. [Google Scholar] [CrossRef]
- Schulein, A.; Ritzmann, M.; Christian, J.; Schneider, K.; Neubauer-Juric, A. Exposure of wild boar to Influenza A viruses in Bavaria: Analysis of seroprevalences and antibody subtype specificity before and after the panzootic of highly pathogenic avian influenza viruses A (H5N8). Zoonoses Public Health 2021, 68, 503–515. [Google Scholar] [CrossRef]
- Gomaa, M.R.; El Rifay, A.S.; Abu Zeid, D.; Elabd, M.A.; Elabd, E.; Kandeil, A.; Shama, N.M.A.; Kamel, M.N.; Marouf, M.A.; Barakat, A.; et al. Incidence and Seroprevalence of Avian Influenza in a Cohort of Backyard Poultry Growers, Egypt, August 2015-March 2019. Emerg. Infect. Dis. 2020, 26, 2129–2136. [Google Scholar] [CrossRef]
- Tahir, M.F.; Abbas, M.A.; Ghafoor, T.; Dil, S.; Shahid, M.A.; Bullo, M.M.H.; Ain, Q.U.; Ranjha, M.A.; Khan, M.A.; Naseem, M.T. Seroprevalence and risk factors of avian influenza H9 virus among poultry professionals in Rawalpindi, Pakistan. J. Infect. Public Health 2019, 12, 482–485. [Google Scholar] [CrossRef]
- Matilda, A.A.; Juergen, M.; Krumkamp, R.; Timm, H.; Eva, M. Molecular and serological prevalence of influenza A viruses in poultry and poultry farmers in the Ashanti region, Ghana. Infect. Ecol. Epidemiol. 2019, 9, 1698904. [Google Scholar] [CrossRef][Green Version]
- Ryu, S.; Kim, C.K.; Kim, K.; Woo, S.H.; Chun, B.C. Serosurveillance of avian influenza A/H5N6 virus infection in poultry farmers, Gyeonggi Province, Republic of Korea, 2016–2017. Int. J. Infect. Dis. 2018, 75, 49–51. [Google Scholar] [CrossRef]
- Sirawan, A.; Berry, A.; Badra, R.; El Bazzal, B.; Dabaja, M.; Kataya, H.; Kandeil, A.; Gomaa, M.R.; Ali, M.; Kayali, G. Avian influenza surveillance at the human-animal interface in Lebanon, 2017. East. Mediterr. Health J. 2020, 26, 774–778. [Google Scholar] [CrossRef]
- Borkenhagen, L.K.; Wang, G.L.; Simmons, R.A.; Bi, Z.Q.; Lu, B.; Wang, X.J.; Wang, C.X.; Chen, S.H.; Song, S.X.; Li, M.; et al. High Risk of Influenza Virus Infection Among Swine Workers: Examining a Dynamic Cohort in China. Clin. Infect. Dis. 2020, 71, 622–629. [Google Scholar] [CrossRef]
- Ayim-Akonor, M.; Mertens, E.; May, J.; Harder, T. Exposure of domestic swine to influenza A viruses in Ghana suggests unidirectional, reverse zoonotic transmission at the human-animal interface. Zoonoses Public Health 2020, 67, 697–707. [Google Scholar] [CrossRef]
- El Zowalaty, M.E.; Abdelgadir, A.; Borkenhagen, L.K.; Ducatez, M.F.; Bailey, E.S.; Gray, G.C. Influenza A viruses are likely highly prevalent in South African swine farms. Transbound. Emerg. Dis. 2022, 69, 2373–2383. [Google Scholar] [CrossRef]
- Poirot, E.; Levine, M.Z.; Russell, K.; Stewart, R.J.; Pompey, J.M.; Chiu, S.; Fry, A.M.; Gross, L.; Havers, F.P.; Li, Z.N.; et al. Detection of Avian Influenza A(H7N2) Virus Infection Among Animal Shelter Workers Using a Novel Serological Approach-New York City, 2016–2017. J. Infect. Dis. 2019, 219, 1688–1696. [Google Scholar] [CrossRef]
- Wibowo, M.H.; Tarigan, S.; Sumarningsih; Artanto, S.; Indriani, R.; Anggoro, D.; Putra, C.P.; Idris, S.; Untari, T.; Asmara, W.; et al. Use of M2e ELISAs for longitudinal surveillance of commercial poultry in Indonesia vaccinated against highly pathogenic avian influenza. J. Virol. Methods 2017, 249, 181–188. [Google Scholar] [CrossRef]
- Moreno, A.; Lelli, D.; Lavazza, A.; Sozzi, E.; Zanni, I.; Chiapponi, C.; Foni, E.; Capucci, L.; Brocchi, E. MAb-based competitive ELISA for the detection of antibodies against influenza D virus. Transbound. Emerg. Dis. 2019, 66, 268–276. [Google Scholar] [CrossRef]
- Li, Y.; Ye, H.; Liu, M.; Song, S.; Chen, J.; Cheng, W.; Yan, L. Development and evaluation of a monoclonal antibody-based competitive ELISA for the detection of antibodies against H7 avian influenza virus. BMC Vet. Res. 2021, 17, 64. [Google Scholar] [CrossRef]
- Dong, J.; Fan, J.; Wang, Y.; Zhang, Q.; Yang, Y.; Jia, Y.; Ming, F.; Zhang, X.; Yao, R.; Li, S.; et al. Development and evaluation of a C-ELISA for rapid detection of antibody to AIV- H7. Anal. Biochem. 2019, 572, 52–57. [Google Scholar] [CrossRef]
- Luo, L.; Nishi, K.; MacLeod, E.; Sabara, M.I. Expression and Characterization of HA1 Protein of Highly Pathogenic H5N1 Avian Influenza Virus for Use in a Serodiagnostic Assay. Transbound. Emerg. Dis. 2017, 64, 432–441. [Google Scholar] [CrossRef]
- Du, T.; Zhu, G.; Wu, X.; Fang, J.; Zhou, E.M. Biotinylated Single-Domain Antibody-Based Blocking ELISA for Detection of Antibodies Against Swine Influenza Virus. Int. J. Nanomed. 2019, 14, 9337–9349. [Google Scholar] [CrossRef]
- Ji, P.; Wang, K.; Zhang, L.; Yan, Z.; Kong, M.; Sun, X.; Zhang, Q.; Zhou, N.; Liu, B.; Zhou, E.M.; et al. A new nanobody-enzyme fusion protein-linked immunoassay for detecting antibodies against influenza A virus in different species. J. Biol. Chem. 2022, 298, 102709. [Google Scholar] [CrossRef]
- Saczynska, V.; Florys-Jankowska, K.; Porebska, A.; Cecuda-Adamczewska, V. A novel epitope-blocking ELISA for specific and sensitive detection of antibodies against H5-subtype influenza virus hemagglutinin. Virol. J. 2021, 18, 91. [Google Scholar] [CrossRef]
- Wang, H.; Cong, F.; Guan, J.; Xiao, L.; Zhu, Y.; Lian, Y.; Huang, R.; Chen, M.; Guo, P. Establishment of xMAP for the simultaneous detection of antibodies to Newcastle disease virus and avian influenza virus. Poult. Sci. 2019, 98, 1494–1499. [Google Scholar] [CrossRef]
- Germeraad, E.; Achterberg, R.; Venema, S.; Post, J.; de Leeuw, O.; Koch, G.; van der Wal, F.J.; Beerens, N. The development of a multiplex serological assay for avian influenza based on Luminex technology. Methods 2019, 158, 54–60. [Google Scholar] [CrossRef]
- Zhao, N.; Grund, C.; Beer, M.; Wang, G.; Harder, T.C. Tetraplex Fluorescent Microbead-Based Immunoassay for the Serodiagnosis of Newcastle Disease Virus and Avian Influenza Viruses in Poultry Sera. Pathogens 2022, 11, 1059. [Google Scholar] [CrossRef]
- Jian, D.; Wang, B.; Huang, H.; Meng, X.; Liu, C.; Xue, L.; Liu, F.; Wang, S. Sunlight based handheld smartphone spectrometer. Biosens. Bioelectron. 2019, 143, 111632. [Google Scholar] [CrossRef]
- Wang, B.; Li, B.; Huang, H.; Yang, S.; Jian, D.; Liu, J.; Yan, K.; Shan, Y.; Wang, S.; Liu, F. Sensitive antibody fluorescence immunosorbent assay (SAFIA) for rapid on-site detection on avian influenza virus H9N2 antibody. Anal. Chim. Acta 2021, 1164, 338524. [Google Scholar] [CrossRef]
- Delgadillo-Gutierrez, K.; Castelan-Vega, J.A.; Jimenez-Alberto, A.; Fernandez-Lizarraga, M.D.C.; Aparicio-Ozores, G.; Monterrubio-Lopez, G.P.; Ribas-Aparicio, R.M. Characterization and use in neutralization assays of avian influenza codon-optimized H5 and H7 retroviral pseudotypes. J. Virol. Methods 2022, 300, 114391. [Google Scholar] [CrossRef]
- Nishiyama, K.; Takeda, Y.; Maeki, M.; Ishida, A.; Tani, H.; Shigemura, K.; Hibara, A.; Yonezawa, Y.; Imai, K.; Ogawa, H.; et al. Rapid detection of anti-H5 avian influenza virus antibody by fluorescence polarization immunoassay using a portable fluorescence polarization analyzer. Sens. Actuators B Chem. 2020, 316, 128160. [Google Scholar] [CrossRef]
- Xiao, Q.; Bi, Z.; Yao, L.; Lei, J.; Yan, Y.; Zhou, J.; Yan, L. Novel protein microarray for the detection of avian influenza virus antibodies and simultaneous distinction of antibodies against H5 and H7 subtypes. Avian Pathol. 2019, 48, 528–536. [Google Scholar] [CrossRef]
- Loreck, K.; Mitrenga, S.; Meemken, D.; Heinze, R.; Reissig, A.; Mueller, E.; Ehricht, R.; Engemann, C.; Greiner, M. Development of a miniaturized protein microarray as a new serological IgG screening test for zoonotic agents and production diseases in pigs. PLoS ONE 2019, 14, e0217290. [Google Scholar] [CrossRef]
- Yang, F.; Feng, S.; Li, Y.; He, Y.; Jin, X.; Wang, X.; Zhou, Z.; Xiao, Y.; Bi, D. Development of immunochromatographic test strips for rapid, quantitative detection of H9AIV antibodies. J. Chromatogr. B 2018, 1095, 59–64. [Google Scholar] [CrossRef]
- Wong, C.L.; Chua, M.; Mittman, H.; Choo, L.X.; Lim, H.Q.; Olivo, M. A Phase-Intensity Surface Plasmon Resonance Biosensor for Avian Influenza A (H5N1) Detection. Sensors 2017, 17, 2363. [Google Scholar] [CrossRef]
- World Health Organization. What Research is Important to Prepare and Respond to H5N1 Influenza Outbreaks? World Health Organization: Geneva, Switzerland, 2025; Available online: https://www.who.int/news-room/events/detail/2025/03/19/default-calendar/what-research-is-important-to-prepare-and-respond-to-h5n1-influenza-outbreaks (accessed on 8 July 2025).
| Country | Species | Sample Size | Virus Tested for | Serology Test Used | Seroprevalence | Reference |
|---|---|---|---|---|---|---|
| Saudi Arabia | Chicken | 359 | AIV | Commercial ELISA kit AI (IDEXX IAV Antibody test) | 12.7% * | [15] |
| Bangladesh | Ducks | 947 | AIV | Commercial cELISA kit (ID.vet ID Screen) | 63.8% (95% CI: 60.6–66.8) | [16] |
| Bangladesh | Ducks and chicken | 144 backyard, 106 broiler, and 113 layer chicken farms | AIV H5N1 and H9N2 | Commercial ELISA kits (IDEXX AI MultiS-Screen ELISA; ID Screen Influenza A Antibody Competition Multi-Species ELISA; IDEXX AI ELISA) | H5: 14.2% (95% CI: 10.0–19.8%) in ducks, 4.2% (95% CI: 2.8–6.1%) in chickens H9: 15.7% (95% CI: 11.3–21.4%) in ducks, 16% (95% CI: 13.2–19.2%) in chickens | [17] |
| Bangladesh | Chicken | 106 commercial broiler and 113 commercial layer chicken farms | AIV H5N1 and H9N2 | Commercial ELISA kits (IDEXX AI, ID Screen Influenza A Antibody Competition Multi-Species ELISA) | H5: 9.4% * in broiler flocks, 3% * in layer flocks H9: 5.7% * in broiler flocks, 22% * in layer flocks | [18] |
| Bangladesh | Ducks | 281 | AIV | Commercial cELISA kit, HI assay | 57.7% (95% CI: 51.6–63.3) | [19] |
| Brazil | Poultry | 2041 | AIV | Commercial ELISA kits (IDEXX Influenza A Ab Test kit, Multispecies Influenza A Antibody Test kit), and HI assay | 0.7% (95% CI: 0.0–2.0) in 2016 and 0.5% (95% CI: 0.0–1.4) in 2019 in Araguaiana 0.8% (95% CI: 0.2–1.3) in 2016 and 7.0% (95% CI: 5.2–8.8) in 2019 in Cáceres | [20] |
| Indonesia | Ducks | 245 | AIV H5 | HI assay | 54.69% * | [21] |
| Polynesia | Chicken | 135 | AIV | Commercial ELISA kits (NP-ELISA) and HI assay | 46% (95% CI: 35.1–58) | [22] |
| Morocco | Poultry | 712 | AIV H9N2 | Commercial ELISA kits (ID Screen IBV Indirect ELISA kit, PROFLOK ® Plus IBD Ab test kit) and HI assay | 63.5% (95% CI: 53–72%) | [23] |
| Libya | Chicken | 453 | AIV H9N2 | Commercial ELISA kits (X-OvO Limited) and HI assay | 53.4% * and 46.8% * in Twisha and other farms | [24] |
| South Africa | Chicken | 1007 | AIV | Commercial ELISA kits AI (IDEXX IAV Antibody test) | 1.8% (95% CI: 0.2−3.4%) | [25] |
| EU countries | Chicken | 18,596 | AIV | NA | 43/18,596 * poultry establishments seropositive for H5 2/18,596 * poultry establishments seropositive for H7 | [26] |
| EU countries | Chicken | 24,419 | AIV | NA | 87/24,419 * poultry establishments seropositive for H5 22/24,419 * seropositive for H7 | [27] |
| EU countries | Chicken | 24,768 | AIV | NA | 46/24,768 * poultry establishments seropositive for H5 7/24,768 * seropositive for H7 | [28] |
| EU countries | Chicken | 24,290 | AIV | NA | 27/24,290 * poultry establishments seropositive for H5 4/24,290 * seropositive for H7 | [29] |
| EU countries | Chicken | 18,490 | AIVs | NA | 15/18,490 * poultry establishments seropositive for H5 | [30] |
| Country | Species | Sample Size | Virus Tested For | Seroprevalence | Reference |
|---|---|---|---|---|---|
| France | Pig | 10 pig herds | AIV H5 | 1 backyard pig in 1/10 pig herds * had antibodies against H5 clade 2.3.4.4b | [31] |
| Senegal | Swine | 1636 | AIV | 83.5% (95%CI: 81.6–85.3) seroprevalence of antibodies against either H9N2, H5N1, H7N7, or H5N2 | [32] |
| Italy | Pig | 67 | AIV H5N1 | 73% * in swine | [33] |
| China | Mink and fox | 347 minks and 195 foxes | AIV | 6.6% * and 96.2% * seropositivity rates for H7 and H9 in mink samples, 16.4% * and 10.3% * seropositivity rates for H7 and H9 in fox samples | [34] |
| Italy | Dog and cat | 5 dogs and 1 cat | AIV H5N1 | Seroconversion was detected in five * asymptomatic domestic dogs and one cat | [35] |
| Saudi Arabia | Camel, sheep, goat, dog, and cat | 195 dromedary camels, sheep, goats, dogs, and cats | IAV | Overall IAV seropositivity rate of 4% * among unvaccinated dogs | [36] |
| Spain | Cat | 183 | IAV | 2.2% (95% CI: 0.85–5.48%) seropositivity rate for anti-influenza A antibodies | [37] |
| Thailand | Pig | 237 | IAV | Absence of antibodies against IAV | [38] |
| Country | Species | Sample Size | Virus Tested For | Seroprevalence | Reference |
|---|---|---|---|---|---|
| Zambia | Pig | 246 | Human A(H1N1)pdm09 | 32% * in sera collected in 2011 5.3% * in sera collected in 2012 and 2018 | [39] |
| Indonesia | Pig | 649 | IAV-S | 26% (95% CI: 20–33) | [40] |
| Burkina Faso | Pig | 600 | Human A(H1N1)pdm09 | 6.8% * | [41] |
| China | Swine | 649 | IAV-S (Eurasian avian-like H1N1, pandemic H1N1, and H3N2) | IAV-S: 48.8% * Eurasian avian-like H1N1: 24.7% * Pandemic swine H1N1: 7.9% * H3N2: 0.1% * | [42] |
| Brazil | Pig | 1631 | IAV-S (H1N1 and H3N2) | IAV-S: 75% * | [43] |
| Brazil | Swine | 233 | IAV-S (H1N1, H1N2, and H3N2) | Swine H1N1: 51.9% * Codetection H1N1 and H1N2: 38.1% * H1N2: 8.6% * Codetection H1N1 and H3N2: 0.6% * | [44] |
| Greece | Pig | 1416 | IAV-S (H1N1, H1N2, and H3N2) | Vaccinated pig farms: 54% * Unvaccinated pig farms: 23% * | [45] |
| Serbia | Pig | 222 | Swine viral diseases | 0% | [46] |
| Brazil | Pig | 962 | IAV-S | 24.1% (95% CI: 20.71–27.55) of samples from intensive herds No positive samples in extensive rearing herds | [47] |
| Global | Swine | 16,328 | IAV | IAV: 18.28% * Human A(H1N1)pdm09: 18.92% * | [48] |
| Brazil | Pig | 21 pig herds | IAV-S | 29.2 to 51.8% * throughout the raising phases | [49] |
| Method | Application | Technical Limitations and Implementation Challenges (When Identified) |
|---|---|---|
| ELISA methods | ||
| Single M2e peptide (sM2e) ELISA [86] | Identify antibodies against AIV M2e protein | |
| Tetrameric M2e peptide (tM2e) ELISA [86] | Identify antibodies against AIV M2e protein | |
| MAb-based competitive ELISA [87] | Detect antibodies against influenza D virus | |
| Competitive ELISA immunoassay (cELISA) [88] | Detect antibodies against H7 based on mAb 2F8 against recombinant H7-HA1 protein | Not suitable for outbreak diagnosis |
| Competitive ELISA immunoassay (cELISA) [89] | Detect antibodies against H7 based on a monoclonal antibody (mAb) directed against hemagglutinin (HA) gene neutralizing epitopes | |
| HA1 protein-based ELISA [90] | Detect antibodies against H5 | |
| single-domain antibodies (sdAbs) ELISA [91] | Detect antibodies against swine influenza virus | Higher false-positive rate |
| Nanobody-based competitive ELISA [92] | Detect anti-IAV antibodies in different species | |
| Epitope-blocking ELISA (H5 EB-ELISA) [93] | Detect H5 antibodies in chicken sera | Less sensitive than the commercial test in detecting anti-H5 HA antibodies in H5 influenza virus-infected chicken |
| Assays methods | ||
| Duplex xMAP assay [94] | Detect antibodies against Newcastle disease virus and AIV | |
| Multiplex serological assay using Luminex xMAP [95] | Detect subtype AIV antibodies in poultry sera | Sophisticated equipment needed for the analysis of data |
| tetraplex inhibition fluorescent microsphere immunoassay (4plex iFMIA) [96] | Detect antibodies against Newcastle disease virus, AIV H5, and AIV H7 in a single serum sample |
|
| Sunlight-based handheld smartphone spectrometer [97] | Detect H7N9 antibodies | Lower sensitivity than that of the commercial microplate reader |
| sensitive antibody fluorescence immunosorbent assay (SAFIA) [98] | Detect H9N2 antibodies | Low detection rate |
| Pseudotype neutralization assay carrying H5 and H7 hemagglutinins [99] | Detect neutralizing antibodies and show specificity for H5 and H7 subtypes | |
| Fluorescence polarization immunoassay (FPIA) [100] | Detect antibodies against H5 | Improving sensitivity is needed |
| Microarray methods | ||
| Triplex protein microarray assay [88] | Detect antibodies against influenza B virus, Newcastle disease virus, and AIV | weakly positive serum samples need confirmation of results by other more sensitive serodiagnosis testing |
| Blocking protein microarray [101] | Detect AIV antibodies and simultaneously distinguish between H5 and H7 subtypes | |
| Protein microarray-based assay [102] | Simultaneously detect IgG antibodies against pathogens in pigs | |
| Quantitative test strips [103] | Detect antibodies against H9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badra, R.; Zhang, W.; Tam, J.S.L.; Webby, R.; van der Werf, S.; Nikisins, S.; Cullinane, A.; Gharaibeh, S.; Njouom, R.; Peiris, M.; et al. Role and Contribution of Serological Surveillance in Animals and Exposed Humans to the Study of Zoonotic Influenza Disease Epidemiology: A Scoping Review. Pathogens 2025, 14, 739. https://doi.org/10.3390/pathogens14080739
Badra R, Zhang W, Tam JSL, Webby R, van der Werf S, Nikisins S, Cullinane A, Gharaibeh S, Njouom R, Peiris M, et al. Role and Contribution of Serological Surveillance in Animals and Exposed Humans to the Study of Zoonotic Influenza Disease Epidemiology: A Scoping Review. Pathogens. 2025; 14(8):739. https://doi.org/10.3390/pathogens14080739
Chicago/Turabian StyleBadra, Rebecca, Wenqing Zhang, John S. L. Tam, Richard Webby, Sylvie van der Werf, Sergejs Nikisins, Ann Cullinane, Saad Gharaibeh, Richard Njouom, Malik Peiris, and et al. 2025. "Role and Contribution of Serological Surveillance in Animals and Exposed Humans to the Study of Zoonotic Influenza Disease Epidemiology: A Scoping Review" Pathogens 14, no. 8: 739. https://doi.org/10.3390/pathogens14080739
APA StyleBadra, R., Zhang, W., Tam, J. S. L., Webby, R., van der Werf, S., Nikisins, S., Cullinane, A., Gharaibeh, S., Njouom, R., Peiris, M., Kayali, G., & Heraud, J.-M. (2025). Role and Contribution of Serological Surveillance in Animals and Exposed Humans to the Study of Zoonotic Influenza Disease Epidemiology: A Scoping Review. Pathogens, 14(8), 739. https://doi.org/10.3390/pathogens14080739








