Abstract
Bovine endometritis is a common postpartum disease that significantly impairs reproductive performance and reduces economic sustainability in dairy and beef cattle. It is primarily caused by gram-negative and -positive bacteria, triggering strong inflammatory responses in the endometrium. Serum amyloid A (SAA) is an acute-phase protein and precursor of amyloid A (AA) in AA amyloidosis. In cattle, multiple SAA isoforms have been identified; however, the biological functions of SAA3 remain unclear. Hence, this study investigated the role of SAA3 in bovine endometrial epithelial cells (BEnEpCs) following stimulation with gram-negative or -positive bacterial antigens. BEnEpCs were treated with lipopolysaccharide (LPS) and lipoteichoic acid (LTA) and, subsequently, the expression levels of SAA3 and SAA1 mRNA were compared by real-time PCR. To further investigate protein-level changes, immunocytochemistry (ICC) was performed to assess the expressions of SAA3 and SAA1. These analyses revealed that SAA3 mRNA expression was significantly enhanced by LPS and LTA, whereas SAA1 mRNA remained undetectable or showed only minimal responsiveness. Notably, only SAA3 protein expression increased in response to stimulation. These results indicate that SAA3 plays a crucial role in the innate immune response of BEnEpCs against gram-negative bacteria. Our in vitro findings may facilitate understanding of the innate immune activity in bovine uterus.
1. Introduction
Endometritis is a common postpartum disorder in cattle, caused by bacterial infections and characterized by inflammation of the endometrial uterine lining [1,2]. It is a significant cause of reproductive inefficiency in both dairy and beef cattle, causing reduced conception rates, prolonged calving intervals, reduced milk yield, and increased culling rates [1,2,3]. Whereas the disease may be less frequently monitored in beef cattle due to differences in herd management, its effect on reproductive performance and economic sustainability is catastrophic [4]. Bovine endometritis pathogenesis involves several gram-negative and gram-positive bacteria, such as Escherichia coli and Trueperella pyogenes, being frequently isolated from the infected uterus [5,6]. These pathogens disrupt the normal postpartum uterine repair processes and elicit strong inflammatory responses that compromise fertility. The condition is worsened by subclinical infections, which are difficult to detect but significantly impair reproductive outcomes [7].
The innate immune system of the bovine mucosal epithelial cells is essential for recognizing and responding to microbial invasion. Among the innate defense molecules, serum amyloid A3 (SAA3) is upregulated in response to bacterial infection and may contribute to mucosal immune defense [8,9]. SAA is an acute-phase protein and precursor of amyloid A (AA) in the AA amyloidosis that occurs in response to chronic infections or chronic inflammatory disorders, such as rheumatoid arthritis and juvenile inflammatory arthritis [10]. Additionally, SAA is regarded as a major acute-phase protein in the plasma of various animal species, including humans, and is recognized as a diagnostic marker for infection and inflammation [11]. Based on differences in amino acid sequences, multiple SAA isoforms—SAA1, 2, 3, and 4—have been identified in several mammalian species, including humans, mice, and rabbits [12]. SAA1 and SAA2 are predominantly produced in the liver and are the primary circulating isoforms in the plasma [12]. The concentration of SAAs in plasma, primarily SAA1, significantly increases up to 1000-fold during inflammation [12]. In contrast, SAA3 is primarily expressed in extrahepatic tissues, such as the intestine and mammary glands [13,14]. In mice, SAA3 expression increases on the colonic surface in the presence of microbiota [15]. In a previous in vitro study, lipopolysaccharide (LPS) treatments strongly induced SAA3 mRNA expression in mouse colonic epithelial CMT-93 cells [15]. LPS is a membranous antigen of gram-negative bacteria, such as E. coli. Additionally, treatment of tumor necrosis factor α (TNFα), a pro-inflammatory cytokine that is responsible to LPS, induced SAA3 expression in adipocytes [16] and CMT-93 cells [15]. Moreover, systemic administration of LPS to mice in vivo induced SAA3 expression in the brain [17]. In bovines, SAA isoforms have been identified, with bovine SAA3 being differentially regulated based on the expression site [18]. LPS stimulation increases SAA3 expression in bovine mammary epithelial cells in vitro [18]. Moreover, both SAA3 mRNA and protein have been detected in the intestine, mammary glands, lungs, and uterus in vivo [19,20]. However, SAA3 expression and its biological function in the endometrial epithelia of the uterus remain unclear. Therefore, to facilitate understanding of the role of SAA3 in innate immune activity in the bovine uterus, SAA3 and SAA1 expression levels were compared in bovine endometrial epithelial cells (BEnEpCs) in vitro by stimulation with LPS and lipopolysaccharide (LTA), which are the membranous antigens of the major endometritis-causing gram-negative and -positive bacteria, respectively. We performed real-time PCR to measure SAA3 and SAA1 in BEnEpCs after treatments with LPS and LTA. Moreover, the protein expressions of SAA3 and SAA1 were analyzed using immunocytochemistry (ICC).
2. Materials and Methods
2.1. Cells
BEnEpCs were purchased from Cell Applications, Inc. (B932-05; San Diego, CA, USA) and cultured in BEnEpC basal medium (B910-400; Cell Applications Inc.) supplemented with 20% growth supplement (B911-GS; Cell Applications Inc.) in a 10 cm collagen-coated dish (NCO430167; Corning, Corning, NY, USA). Madin−Darby bovine kidney (MDBK) cells were cultured in Dulbecco’s modified Eagle’s minimal essential medium (044-29765, Wako, Osaka, Japan) with 5% fetal bovine serum (PAA Laboratories, Pasching, Austria), 100 U/mL penicillin, and 100 μg/mL streptomycin (15140-122, Gibco, Grand Island, NY, USA).
2.2. Treatment with LPS and LTA for mRNA Expression Analysis
Cells were seeded at a density of 3 × 105 cells/well in six-well collagen-coated plates (4810-010N; IWAKI, Shizuoka, Japan) and incubated for 24 h at 37 °C in a 5% CO2 atmosphere. After rinsing the cells with phosphate-buffered saline (PBS; 045-29795; Wako), the cells were treated with LPS (L2630; Sigma-Aldrich, St. Louis, MO, USA) or LTA (L3265, Sigma-Aldrich), which are outer membrane proteins of gram-negative bacteria, E. coli O111:B4, or gram-positive bacteria, Bacillus subtilis, respectively. LPS and LTA were diluted to 0, 0.01, 0.1, 1, 10, and 100 µg/mL in BEnEpC basal medium without growth supplements. BEnEpC treated with LPS or LTA were incubated for 4 h at 37 °C for mRNA expression analysis.
2.3. RNA Extraction and cDNA Synthesis
RNA was extracted from LPS- or LTA-treated cells using the RNeasy Mini Kit (74106; Qiagen, Hilden, Germany) following the manufacturer’s protocol, and the RNA concentration was quantified using a NanoDropLite spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). DNA contamination in the extracted RNA was eliminated by treatment with DNaseI (18068-015; Invitrogen, Carlsbad, CA, USA), and cDNA was synthesized using PrimeScript RT Master Mix (RR036A; Takara, Kusatsu, Japan).
2.4. Cloning and Sequencing
Cloning was performed to obtain plasmid clones containing target genes to generate standard curves for absolute quantitative real-time PCR (qPCR).
PCR was performed on a Veriti thermal cycler (Applied Biosystems, Foster City, CA, USA) using GoTaq Hot Start Green Master Mix (M7122; Promega, Madison, WI, USA). SAA-specific PCR primers were designed (SAA1 mRNA F, 5′-GATCAGCACAATGAAGCTCTTCACAGGGCC-3′; SAA3 mRNA F, 5′-CACGGCCACAGGATGAACCTTTCCACGGGCA-3′; and SAA1,3 mRNA R, 5′-GAGAGGCAGCTCAGTACTTCTCAGGC-3′) based on bovine SAA1 (GenBank accession no. BC109787) and SAA3 (NM181016) sequences and used to amplify bovine SAA1 and SAA3 genes from the cDNA. Thermal cycling was performed for 2 min at 95 °C, followed by 40 cycles at 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s. The PCR products were purified using the QIAquick PCR Purification Kit (28106; Qiagen, Valencia, CA, USA) and ligated into the plasmid pTAC-2 using a DynaExpress TA PCR cloning kit (DS126L; BioDynamics Laboratory, Tokyo, Japan), following the manufacturer’s protocol. The recombinant plasmids were transformed into ECOS competent E. coli DH5α (310-06236; NipponGene, Toyama, Japan) following the manufacturer’s protocol. The recombinant bacterial colonies were selected on Luria–Bertani plates with 100 µg/mL ampicillin and confirmed through colony PCR using M13 primers (BioDynamics Laboratory). The PCR products were purified using a QIAquick PCR Purification Kit before their nucleotide sequences were determined by direct sequencing using a BigDye Terminator v3.1 cycle sequencing Kit (4337449; Applied Biosystems). Sequence analysis was performed using the Genetyx-Win software version 13 (Genetyx, Tokyo, Japan). Plasmids positive for amplicons were extracted using the QIAprep Spin MiniPrep Kit (27106; Qiagen) following the manufacturer’s protocol to generate standard curves in absolute qPCR.
2.5. Determination of Linear Range Using Plasmid-Derived Gene Standards
The plasmid concentration was measured using a NanoDropLite spectrophotometer (Thermo Fisher Scientific), and the plasmid gene copy number was calculated using the following equation:
where X and Y represent the amplicon quantity in nanograms and nucleotide length of the plasmid with its specific target insert, respectively. Each plasmid contained only one amplicon, thereby acting as a proxy for the gene copy number. To establish a linear detection range, the plasmids were initially diluted to a stock concentration of 1 × 1010 copies/µL and, subsequently, serially diluted 10-fold to a lower value of 1 × 101 copies/µL.
(X × [6.0221 × 1023 molecules/mol])/([Y × 660 g/mol] × [1 × 109 ng/g])
2.6. Absolute Quantification in qPCR
To measure the mRNA expression levels in cells, qPCR was performed in 96-well plates using 300 nmol each of forward and reverse primers, 10 ng of synthesized cDNA, and PowerUp SYBR Green Master Mix (A25742; Applied Biosystems) on a QuantStudio 3 real-time PCR system (Applied Biosystems). Thermal cycling was conducted for 2 min at 50 °C and 2 min at 95 °C, followed by 40 cycles at 95 °C for 3 s and 60 °C for 30 s. Specific primers were used to assess the mRNA expression levels of SAA1 (5′-AGTCCACAGCCAGTGGATGT-3′ and 5′-ATCTCTGAATATTTTCTCTGGCATC-3′) [19] and SAA3 (5′-CCTCAAGGAAGCTGGTCAAG-3′ and 5′-TACCTGGTCCCTGGTCATAC-3′) [21]. The SAA1 and SAA3 plasmid dilutions described above were used to generate standard curves to determine the absolute gene copy numbers of SAA1 and SAA3 mRNA in LPS- or LTA-treated cells. The cycle threshold values of the samples were compared with their respective standard curves to interpolate the gene copy number for each sample. The mean gene copy number with the standard error of the mean was plotted for each gene region under each condition. All experiments were independently repeated at least thrice.
2.7. Standard Curves for Measuring SAA1 and SAA3 mRNA Copy Numbers Through qPCR
Standard curves for qPCR were constructed using 10-fold serial dilutions (1 × 106–1 × 101 copies/µL) of SAA1 and SAA3 plasmid stocks to confirm the linearity of amplification and detection ranges. The copy numbers of SAA1 and SAA3 mRNA in BEnEpCs induced by LPS and LTA were determined based on these standard curves (Figure A1).
2.8. Treatment with LPS and LTA for ICC
BEnEpCs were seeded at a density of 1.5 × 105 cells/well in eight-well collagen-coated chamber slides (SCS-N38; Matsunami Glass, Kishiwada, Japan) and incubated for 24 h at 37 °C in a 5% CO2 atmosphere. After rinsing the cells with PBS, the cells were treated with LPS or LTA. BEnEpCs treated with 0, 0.01, 1, and 100 µg/mL LPS or LTA were incubated for 4 h at 37 °C for ICC. MDBK cells treated with 100 µg/mL LPS were used as positive controls for SAA1.
2.9. ICC
ICC was performed to assess the SAA1 and SAA3 protein expression levels in LPS- and LTA-stimulated cells. LPS- or LTA-treated cells were washed with PBS and fixed with 4% paraformaldehyde for 20 min at room temperature and 100% methanol for 20 min at −20 °C. After washing with PBS, endogenous peroxidase activity was inhibited using 3% hydrogen peroxide for 20 min at room temperature and blocked non-specific antibody binding with 1% bovine serum albumin (BSA) in PBS for 30 min at room temperature. Anti-SAA1 monoclonal antibody 25BF12 (1:2000) [22] or anti-SAA3 monoclonal antibody 231G7 (1:1000) [19] were used as primary antibodies. The cells were incubated overnight at 4 °C with the antibody diluted in PBS containing 1% BSA. The following day, the cells were washed with PBS and incubated with horseradish peroxidase-conjugated anti-mouse IgG polyclonal secondary antibody (414311; Nichirei Biosciences Inc., Kyozan, Japan) for 30 min at room temperature. The cells were then washed with PBS, incubated with peroxidase in the ImmPACT DAB Substrate Kit (SK-4105; Vector Laboratories, Newark, CA, USA), and the wells were stripped from the slides. After counterstaining the nuclei with hematoxylin, the slides were dried and mounted using Mount Quick (DM01; DhythSangyo, Toda, Japan).
2.10. Statistical Analysis
Data were collected from at least three independent experiments, expressed as means ± standard deviations and analyzed for statistical significance using one-way analysis of variance and Bonferroni’s post hoc analysis.
3. Results
3.1. mRNA Expressions of SAA1 and SAA3 in BEnEpCs Treated with LPS and LTA
SAA1 mRNA expression was not detected in the controls but became detectable following LPS treatment without showing a concentration-dependent increase (Figure 1A). Conversely, LPS treatment enhanced SAA3 mRNA expression in a concentration-dependent manner (Figure 1B), with significant upregulation at 0.1, 1, 10, and 100 µg/mL, peaking at 100 µg/mL.
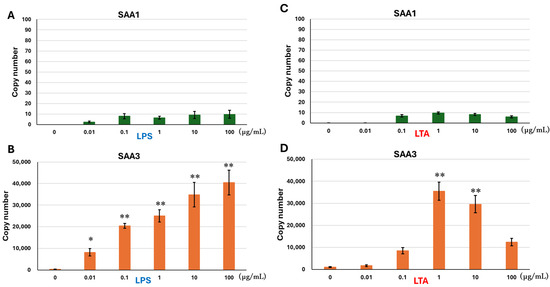
Figure 1.
mRNA expressions of SAA1 and SAA3 in BEnEpCs treated with LPS and LTA. BEnEpCs were treated with 0–100 µg/mL of LPS (A,B) or LTA (C,D) at 37 °C for 4 h. mRNA expressions of SAA1 (A,C) and SAA3 (B,D) are determined by copy number using the standard curve (Figure A1). Data are represented as the mean ± standard deviation from three independent experiments. * p < 0.05 and ** p < 0.01. SAA, serum amyloid A; BEnEpCs, bovine endometrial epithelial cells; LPS, lipopolysaccharide; LTA, lipoteichoic acid.
Similar to the LPS treatment, SAA1 mRNA expression was not detected in the controls but became detectable following LTA treatment without showing a concentration-dependent increase (Figure 1C). LTA treatment enhanced SAA3 mRNA expression (Figure 1D). LTA at 1 and 10 µg/mL significantly stimulated SAA3 mRNA expression, with the greatest enhancement observed at 1 µg/mL. SAA3 mRNA expression was higher in the LPS-treated cells than that in the LTA-treated cells, excluding 1 µg/mL (Figure 1B,D).
3.2. Protein Expressions of SAA1 and SAA3 in BEnEpCs Treated with LPS and LTA
According to ICC results, BEnEpCs were minimally stained with an anti-SAA1 monoclonal antibody, regardless of LPS treatment, whereas LPS-treated MDBK cells were strongly stained (Figure 2). In contrast, BEnEpCs were strongly stained with the anti-SAA3 monoclonal antibody at 1 and 100 µg/mL LPS treatment (Figure 2).
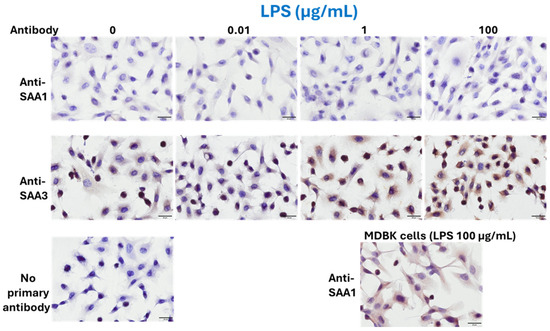
Figure 2.
Protein expressions of SAA1 and SAA3 in BEnEpCs treated with LPS. BEnEpCs were treated with 0, 0.01, 1, and 100 µg/mL of LPS at 37 °C for 4 h and stained with anti-SAA1 or anti-SAA3 monoclonal antibodies, 25BF12 or 231G7. Cells are minimally stained with anti-SAA1 antibody with or without LPS treatment. Conversely, cells are strongly stained with anti-SAA3 antibody at 1 or 100 µg/mL of LPS. Madin–Darby bovine kidney (MDBK) cells were used for positive control of the staining of anti-SAA1 antibody. Scale bar = 20 µmm. BEnEpCs, bovine endometrial epithelial cells; SAA, serum amyloid A; LPS, lipopolysaccharide.
BEnEpCs were minimally stained with the anti-SAA1 monoclonal antibody, regardless of LTA treatment (Figure 3). In contrast, BEnEpCs were strongly stained with anti-SAA3 monoclonal antibody at 1 µg/mL LTA treatment and weakly stained at a higher concentration (100 µg/mL). The dose-dependent expressions of SAA1 and SAA3 proteins in BEnEpCs were consistent with their mRNA expression patterns.
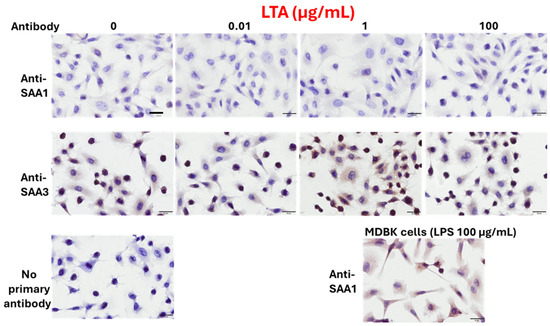
Figure 3.
Protein expressions of SAA1 and SAA3 in BEnEpCs treated with LTA. BEnEpCs were treated with 0, 0.01, 1, and 100 µg/mL of LTA at 37 °C for 4 h and stained with anti-SAA1 or anti-SAA3 monoclonal antibodies, 25BF12 or 231G7. Similar to LPS treatment, cells are minimally stained with anti-SAA1 antibody with or without LTA treatment. Conversely, cells are strongly stained with anti-SAA3 antibody at 1 µg/mL of LTA. Madin–Darby bovine kidney (MDBK) cells were used for positive control of the staining of anti-SAA1 antibody. Scale bar = 20 µm. BEnEpCs, bovine endometrial epithelial cells; SAA, serum amyloid A; LTA, lipoteichoic acid; LPS, lipopolysaccharide.
4. Discussion
Previously, we showed that bovine SAA3 mRNA expression was dose-dependently upregulated by LPS in bovine small intestinal epithelial cells and mammary epithelial cells, wherein SAA1/2 mRNA expression was unaffected [19]. Therefore, we hypothesized that LPS may induce SAA3 mRNA expression in other bovine epithelial cells. Herein, LPS stimulation induced SAA3 mRNA expression levels in BEnEpCs but had only a slight effect on SAA1 mRNA expression. Kiku et al. [23] analyzed gene expression profiles in primary bovine mammary epithelial cells after LTA stimulation and revealed that SAA3 was not among the top 20 regulated genes. In this study, SAA3 mRNA expression in bovine epithelial cells increased in response to LTA stimulation. However, its expression was lower than that observed in LPS-stimulated cells. The results of this study and those of a previous report indicate that SAA3 mRNA expression is predominantly induced by gram-negative bacterial infection [23].
The dose-dependent increase in protein expression was consistent with the mRNA expression results following LPS and LTA stimulation, indicating that the expressed SAA3 mRNA was translated into protein without inhibitory effect. In contrast, SAA1 protein levels were not increased by LPS and LTA stimulation, consistent with the mRNA expression results.
As the expression levels of inflammatory cytokines such as interleukin (IL)-6, IL-8, and tumor necrosis factor-alpha are significantly increased in BEnEpCs in response to E. coli stimulation [24], SAA3 may also be involved in defense against bacterial infection in the cattle uterus, alongside these cytokines. Transcriptome analysis by Zhou et al. [25] demonstrated that 36 genes were upregulated in bovine endometrial epithelial cells after LPS stimulation, in which SAA3 mRNA was significantly increased. A previous study showed that recombinant caprine SAA3 treatment reduced the infection rate of mastitis-causing bacteria in primary bovine mammary epithelial cells in vitro [26]. Therefore, SAA3 would play a role in activating innate immunity against bacterial infections in bovine epithelia, together with various cytokines. Bacteria in the uterus of cattle are typically eliminated within 1–3 months after calving; however, cattle with reproductive issues retain uterine bacteria for a prolonged period [27]; compromised uterine defense mechanisms result in post-calving infections [28]. To confirm the relationship between SAA3 and the host’s innate immunity, we must assess whether SAA3 stimulates the expression of genes involved in innate immunity, both in vitro and in vivo.
In summary, the results of this study indicate that SAA3 mRNA and protein are expressed in bovine endometrial epithelial cells due to bacterial infections, being particularly sensitive to gram-negative bacteria. These in vitro results suggest that similar responses may occur in vivo and are involved in innate immunity to bacterial infections.
Author Contributions
Conceptualization, Y.I.; methodology, Y.I.; formal analysis, K.A. and K.O.; investigation, K.A. and K.O.; writing—original draft preparation, K.A.; writing—review and editing, A.O., K.O., H.S. and Y.I.; project administration, Y.I.; funding acquisition, Y.I. All authors have read and agreed to the published version of the manuscript.
Funding
The Kieikai Research Foundation (grant number 2024C006) funded this research.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article and Appendix A.
Acknowledgments
We thank the members of the Laboratory of Veterinary Pathology, Gifu University, Japan, for their technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AA | Amyloid A |
| BEnEpC | Bovine endometrial epithelial cell |
| BME | Bovine mammary epithelial |
| BSA | Bovine serum albumin |
| ICC | Immunocytochemistry |
| IL | Interleukin |
| LPS | Lipopolysaccharide |
| LTA | Lipoteichoic acid |
| MDBK | Madin–Darby bovine kidney |
| MUC2 | Mucin2 |
| PBS | Phosphate-buffered saline |
| PCR | Polymerase chain reaction |
| qPCR | Quantitative real-time polymerase chain reaction |
| SAA | Serum amyloid A |
Appendix A
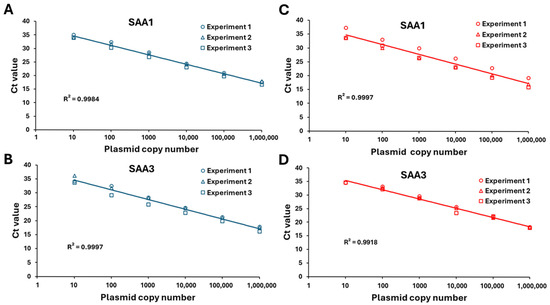
Figure A1.
Standard curves for measuring SAA1 and SAA3 mRNA copy numbers in BEnEpCs treated with LPS (A,B) or LTA (C,D) using quantitative real-time PCR. SAA1 and SAA3 plasmid stocks are serially diluted 10-fold from 1 × 106 to 10 copies to establish the linearity of amplification and detection range. To measure the within-assay variability, all reactions are performed in triplicate. Copy numbers are plotted against cycle threshold (Ct) values for SAA1 and SAA3 to determine R2 values. BEnEpCs, bovine endometrial epithelial cells; SAA, serum amyloid A; LPS, lipopolysaccharide; LTA, lipoteichoic acid.
References
- Sheldon, I.M.; Lewis, G.S.; LeBlanc, S.; Gilbert, R.O. Defining postpartum uterine disease in cattle. Theriogenology 2006, 65, 1516–1530. [Google Scholar] [CrossRef]
- LeBlanc, S.J. Postpartum uterine disease and dairy herd reproductive performance: A review. Vet. J. 2008, 176, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Aitken, S.L.; Corl, C.M.; Sordillo, L.M. Immunopathology of the postpartum cow: Insights into endometrial and mammary inflammation. J. Dairy Sci. 2016, 99, 2930–2940. [Google Scholar]
- Bicalho, M.L.S.; Machado, V.S.; Higgins, C.H.; Lima, F.S.; Bicalho, R.C. Genetic and functional analysis of the bovine uterine microbiota. Vet. Microbiol. 2010, 142, 379–385. [Google Scholar]
- Gilbert, R.O.; Shin, S.T.; Guard, C.L.; Erb, H.N.; Frajblat, M. Prevalence of endometritis and its effects on reproductive performance of dairy cows. Theriogenology 2005, 64, 1879–1888. [Google Scholar] [CrossRef]
- Kasimanickam, R.; Cornwell, J.M.; Nebel, R.L. Fertility following postpartum uterine infection in beef cattle. Theriogenology 2004, 61, 1285–1299. [Google Scholar]
- Patterson, D.J.; Perry, R.C.; Kiracofe, G.H.; Bellows, R.A.; Staigmiller, R.B.; Corah, L.R. Management considerations in beef heifer development and puberty. J. Anim. Sci. 2013, 70, 4018–4035. [Google Scholar] [CrossRef]
- Urieli-Shoval, S.; Meek, R.L.; Hanson, R.H.; Eriksen, N.; Benditt, E.P. Human serum amyloid A genes are expressed in monocyte/macrophage cell lines. Am. J. Pathol. 2000, 157, 269–277. [Google Scholar]
- Wagener, K.; Prunner, I.; Pothmann, H. A review of the ongoing discussion about definition, diagnosis and pathomechanisms of bovine endometritis. Theriogenology 2015, 84, 1372–1383. [Google Scholar]
- Obici, L.; Merlini, G. AA amyloidosis: Basic knowledge, unmet needs and future treatments. Swiss Med. Wkly. 2012, 142, w13580. [Google Scholar] [CrossRef]
- Eckersall, P.D.; Bell, R. Acute phase proteins: Biomarkers of infection and inflammation in veterinary medicine. Vet. J. 2010, 185, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Uhlar, C.M.; Whitehead, A.S. Serum amyloid A, the major vertebrate acute-phase reactant. Eur. J. Biochem. 1999, 265, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Meek, R.L.; Benditt, E.P. Amyloid A gene family expression in different mouse tissues. J. Exp. Med. 1986, 164, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- McDonald, T.L.; Larso, M.A.; Mack, D.R.; Weber, A. Elevated extrahepatic expression and secretion of mammary-associated serum amyloid A 3 (M-SAA3) into colostrum. Vet. Immunol. Immunopathol. 2001, 83, 203–211. [Google Scholar] [CrossRef]
- Reigstad, C.S.; Lundén, G.O.; Felin, J.; Backhed, F. Regulation of serum amyloid A3 (SAA3) in mouse colonic epithelium and adipose tissue by the intestinal microbiota. PLoS ONE 2009, 4, e5842. [Google Scholar] [CrossRef]
- Lin, Y.; Rajala, M.W.; Berger, J.P.; Moller, D.E.; Barzilai, N.; Scherer, P.E. Hyperglycemia-induced production of acute phase reactants in adipose tissue. J. Biol. Chem. 2001, 276, 42077–42083. [Google Scholar] [CrossRef]
- Liu, J.; Wang, D.; Li, S.Q.; Ye, R.D. Suppression of LPS-induced tau heperphosphorylation by serum amyloid A. J. Neuroinflamm. 2016, 13, 28. [Google Scholar] [CrossRef]
- Larson, M.A.; Weber, A.; Weber, A.T.; McDonald, T.L. Differential expression and secretion of bovine serum amyloid A3 (SAA3) by mammary epithelial cells stimulated with prolactin or lipopolysaccharide. Vet. Immunol. Immunopathol. 2005, 107, 255–264. [Google Scholar] [CrossRef]
- Murata, E.; Kozaki, S.; Murakami, T.; Shimizu, K.; Okada, A.; Ishiguro, N.; Inoshima, Y. Differential expression of serum amyloid A1 and A3 in bovine epithelia. J. Vet. Med. Sci. 2020, 82, 1409–1412. [Google Scholar] [CrossRef]
- Saremi, B.; Mielenz, M.; Rahman, M.M.; Hosseini, A.; Kopp, C.; Dänicke, S.; Ceciliani, F. Hepatic and extrahepatic expression of serum amyloid A3 during lactation in dairy cow. J. Dairy Sci. 2013, 96, 6944–6954. [Google Scholar] [CrossRef]
- Green, B.B.; Kerr, D.E. Epigenetic contribution to individual variation in response to lipopolysaccharide in bovine dermal fibroblasts. Vet. Immunol. Immunopathol. 2014, 157, 49–58. [Google Scholar] [CrossRef]
- Taira, Y.; Inoshima, Y.; Ishiguro, N.; Murakami, T.; Matsui, T. Isolation and characterization of monoclonal antibodies against bovine serum amyloid A1 protein. Amyloid 2009, 16, 215–220. [Google Scholar] [CrossRef]
- Kiku, Y.; Nagasawa, Y.; Tanabe, F.; Sugawara, K.; Watanabe, A.; Hata, E.; Ozawa, T.; Nakajima, K.I.; Arai, T.; Hayashi, T. The cell wall component lipoteichoic acid of Staphylococcus aureus induces chemokine gene expression in bovine mammary epithelial cells. J. Vet. Med. Sci. 2016, 78, 1505–1510. [Google Scholar] [CrossRef]
- Chapwanya, A.; Meade, K.G.; Doherty, L.M.; Callanan, J.J.; O’Farrelly, C. Endometrial epithelial cells are potent producers of tracheal antimicrobial peptide and serum amyloid A3 gene expression in response to E. coli stimulation. Vet. Immunol. Immunopathol. 2013, 151, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.W.; Shen, P.X.; Sun, Y.; Zhang, X.; Yan, C.B.; Yu, J.C.; Liu, F.; Yang, D.X.; Xu, E.B.; Wang, Y.Z.; et al. Transcriptome profiling of bovine endometrial epithelial cells induced by lipopolysaccharides in vitro. Anim. Biotechnol. 2023, 34, 4588–4599. [Google Scholar] [CrossRef] [PubMed]
- Parés, S.; Fàbregas, F.; Bach, A.; Garcia-Fruitós, E.; Prado, A.; Arís, A. Short communication: Recombinant mammary serum amyloid A3 as a potential strategy for preventing intramammary infections in dairy cows at dryoff. J. Dairy Sci. 2020, 103, 3615–3621. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Bacteria within the uterus and therapy of bacterial uterine disease. J. Farm. Anim. Infect. Dis. 2012, 1, 97–103. [Google Scholar]
- Osawa, T. Predisposing factors, diagnostic and therapeutic aspects of persistent endometritis in postpartum cows. J. Reprod. Dev. 2021, 67, 291–299. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).