Vector-Borne Agents in Species of Silky Anteater (Cyclopes Gray, 1821) from South America
Abstract
1. Introduction
2. Materials and Methods
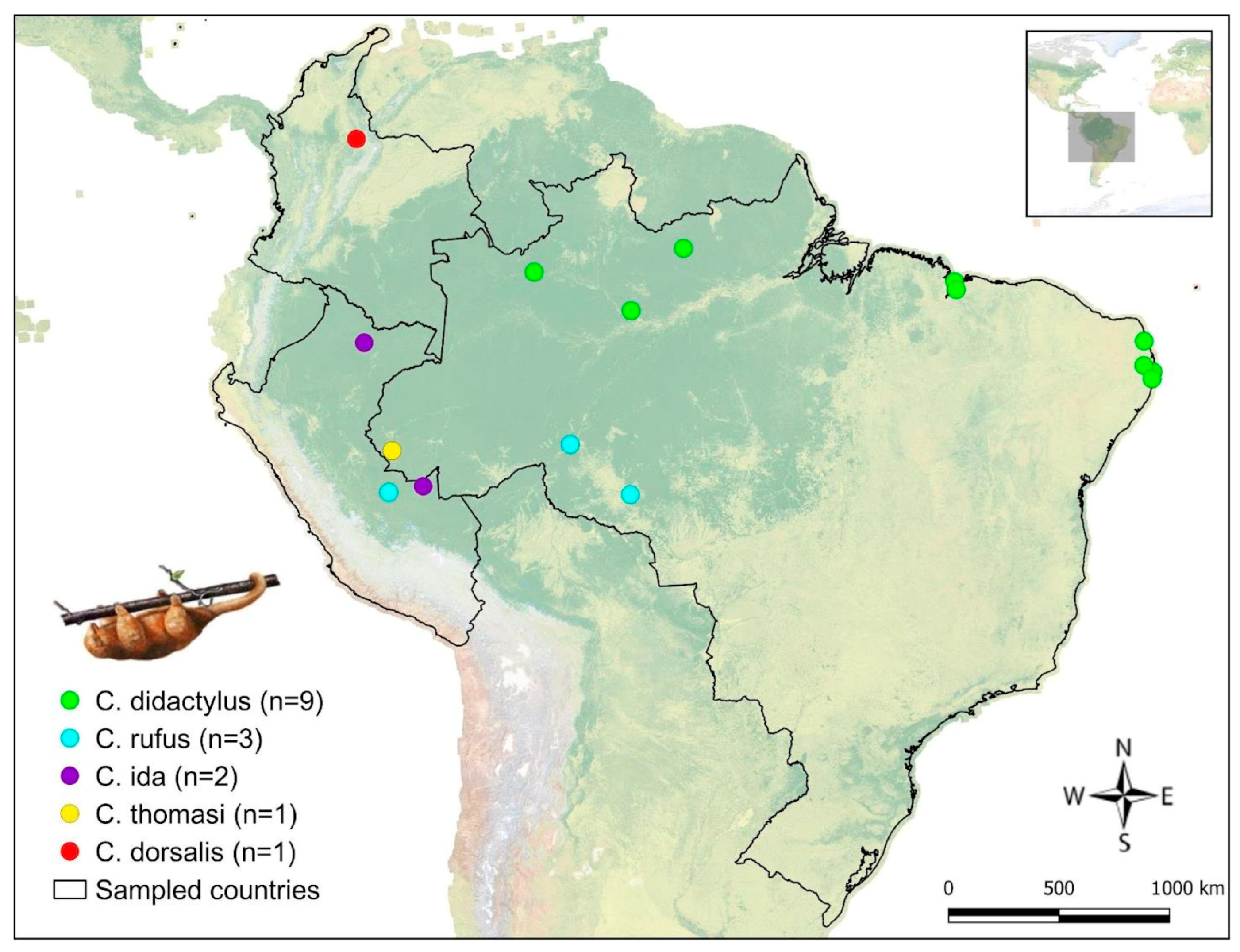
2.1. Sampling
2.2. Molecular Analysis
2.3. Genetic Sequencing
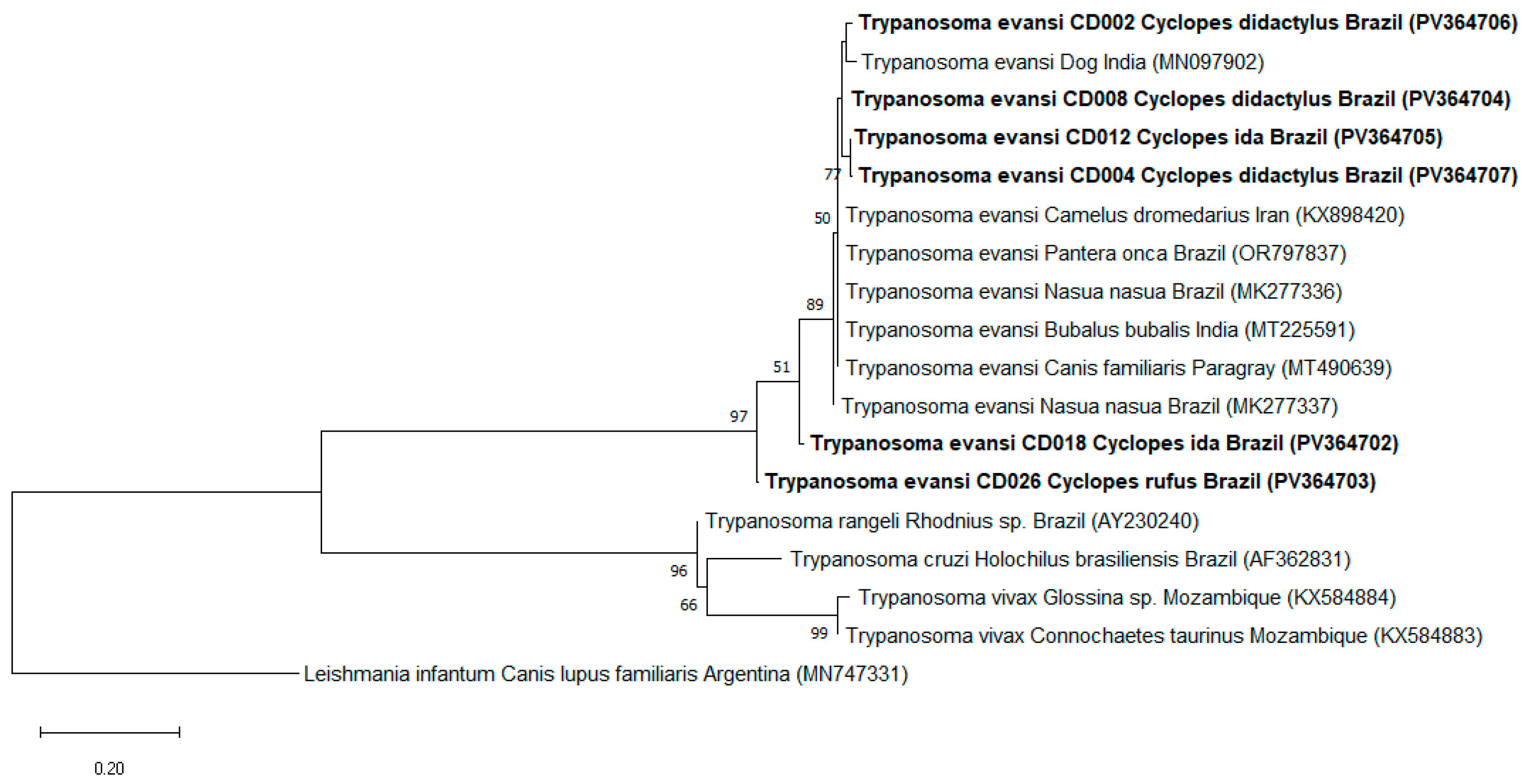
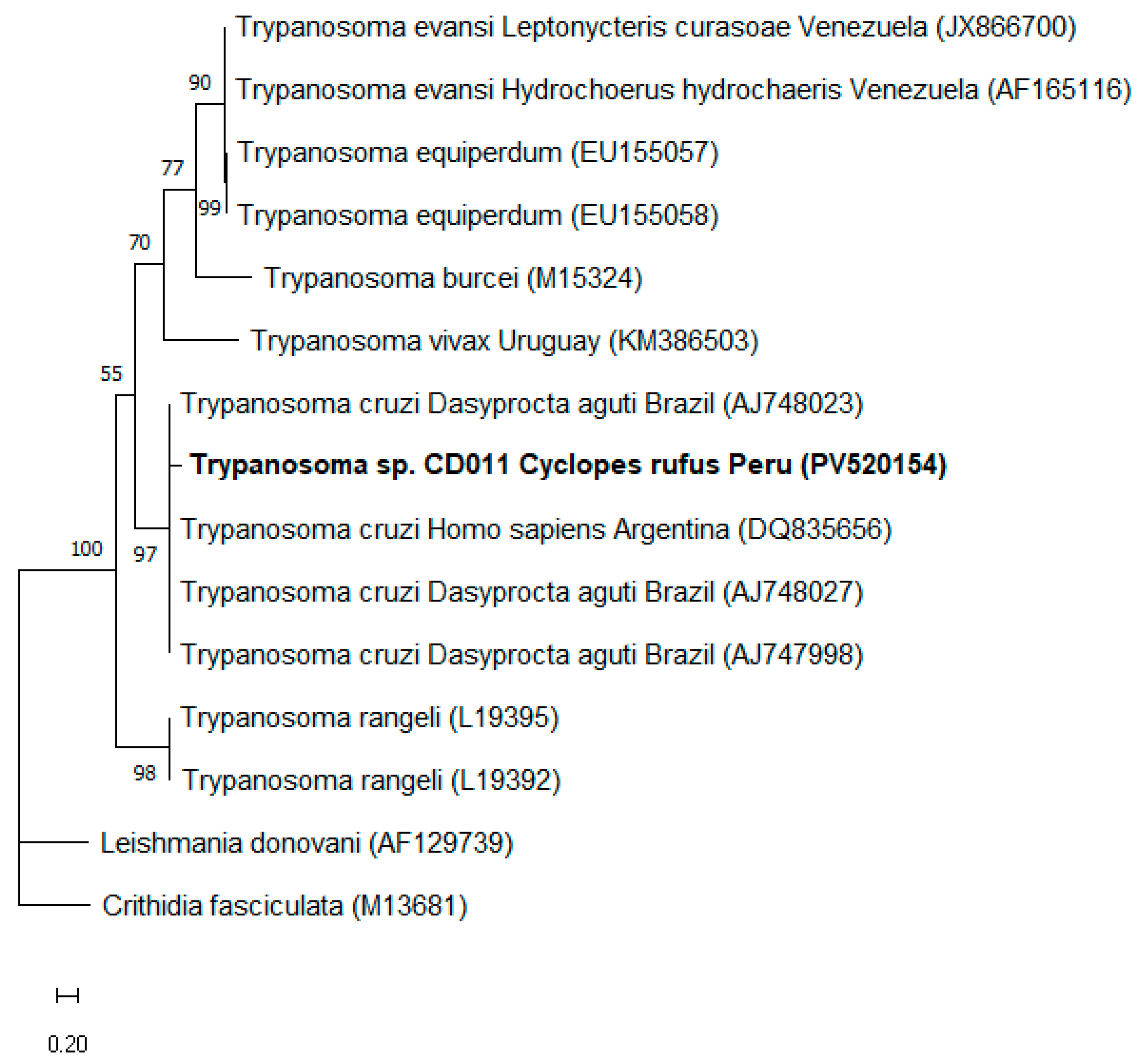
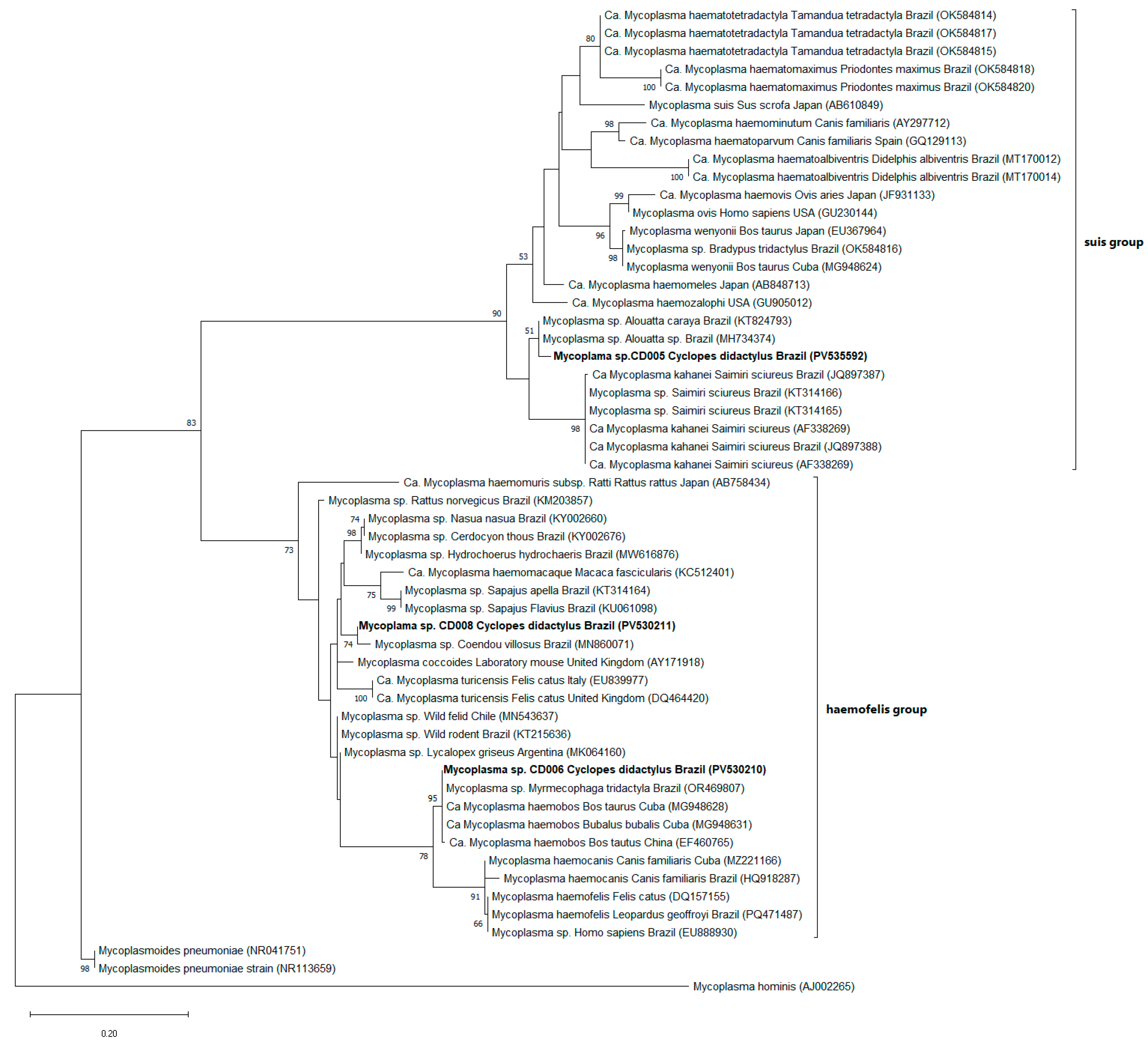
2.4. Phylogenetic Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miranda, F.R.; Casali, D.M.; Perini, F.A.; Machado, F.A.; Santos, F.R. Taxonomic review of the genus Cyclopes Gray, 1821 (Xenarthra: Pilosa), with the revalidation and description of new species. Zool. J. Linn. Soc. 2018, 183, 687–721. [Google Scholar] [CrossRef]
- Machado, A.F.; Miranda, F.R. The potential distribution of Cyclopes didactylus, a silky anteater, reveals a likely unknown population and urgent need for forest conservation in Northeast Brazil. J. Trop. Ecol. 2022, 38, 454–461. [Google Scholar] [CrossRef]
- McNab, B.K. Energetics, population biology, and distribution of Xenarthrans, living and extinct. In The Evolution and Ecology of Armadillos, Sloths, and Vermilinguas; Montgomery, G.G., Ed.; Smithsonian Institution Press: Washington, DC, USA; London, OH, USA, 1985; pp. 219–232. [Google Scholar]
- Wetzel, R.M. Systematics, distribution, ecology, and conservation of South American edentates. In The Pymatuning Symposia in Ecology, Volume 6: Mammalian Biology in South America; Mares, M.A., Genoways, H.H., Eds.; University of Pittsburgh: Pittsburgh, PA, USA, 1982; pp. 345–375. [Google Scholar]
- Paglia, A.P.; Fonseca, G.A.B.; Rylands, A.B.; Herrmann, G.; Aguiar, L.M.S.; Chiarello, A.G.; Leite, Y.L.; Costa, L.P.; Siciliano, S.; Kierulff, M.C. Lista Anotada dos Mamíferos do Brasil, 2nd ed.; Occasional Papers in Conservation Biology 6; Conservation International: Arlington, TX, USA, 2012; pp. 1–76. [Google Scholar]
- Nowak, R.M. Walker’s Mammals of the World, 6th ed.; The Johns Hopkins University Press: Baltimore, MD, USA; London, OH, USA, 1999; Volume 1. [Google Scholar]
- Miranda, F.; Superina, M. New distribution records of the silky anteater Cyclopes didactylus (Mammalia, Pilosa, Cyclopedidae) in coastal northeastern Brazil. Mastozool. Neotrop. 2010, 17, 381–384. [Google Scholar]
- Eisenberg, J.F.; Redford, K.H. Mammals of the Neotropics: The Central Neotropics. Ecuador, Peru, Bolivia, Brazil; The University of Chicago Press: Chicago, CA, USA, 1999; Volume 3. [Google Scholar]
- Fonseca, G.A.B.; Herrmann, G.; Leite, Y.L.R.; Mittermeier, R.A.; Rylands, A.B.; Patton, J.L. Lista Anotada dos Mamíferos do Brasil; Occasional Papers in Conservation Biology 4; Conservation International: Arlington, TX, USA, 1996; pp. 1–38. [Google Scholar]
- Montgomery, G.G. Impacts of vermilinguas (Cyclopes, Tamandua: Xenarthra = Edentata) on arboreal ant populations. In The Evolution and Ecology of Armadillos, Sloths, and Vermilinguas; Montgomery, G.G., Ed.; Smithsonian Institution Press: Washington, DC, USA; London, OH, USA, 1985; pp. 351–363. [Google Scholar]
- Miranda, F.; Meritt, D.A.; Tirira, D.G.; Arteaga, M. Cyclopes didactylus. The IUCN Red List of Threatened Species. 2014. Available online: https://www.iucnredlist.org/species/6019/47440020 (accessed on 27 March 2025).
- Miranda, F.R.; Chiarello, A.G.; Röhe, F.; Miranda, G.H.B.; Vaz, S.M.; Avaliação do Risco de Extinção de Cyclopes didactylus (Linnaeus, 1758). Processo de Avaliação do Risco de Extinção dos Xenarthros Brasileiros, ICMBio. 2015. Available online: https://www.researchgate.net/profile/Alessandra-Bertassoni/publication/323457425_Avaliacao_do_Risco_de_Extincao_de_Tamandua_tetradactyla_Linnaeus_1758_no_Brasil_Evaluation_of_the_extinction_risk_of_the_Southern_Anteater_Tamandua_tetradactyla_Linnaeus_1758_in_Brazil/links/5a970536aca27214056b3456/Avaliacao-do-Risco-de-Extincao-de-Tamandua-tetradactyla-Linnaeus-1758-no-Brasil-Evaluation-of-the-extinction-risk-of-the-Southern-Anteater-Tamandua-tetradactyla-Linnaeus-1758-in-Brazil.pdf (accessed on 18 July 2025).
- Castillo, A.P.; Colácio, N.; Rodrigues, P.H.C.; Miranda, J.V.O.; Lima, P.C.S.; Motta, R.O.C.; Tinoco, H.P.; Coelho, C.M.; da Silveira, J.A.G. Parasitic Protozoa and Other Vector-Borne Pathogens in Captive Mammals from Brazil. J. Zool. Bot. Gard. 2024, 5, 754–773. [Google Scholar] [CrossRef]
- Ceylan, O.; Ma, Z.; Ceylan, C.; Ider, M.; Evci, A.; Mavinehir, A.; Xuan, X.; Sevinc, F. Feline vector-borne haemopathogens in Türkiye: The first molecular detection of Mycoplasma wenyonii and ongoing Babesia ovis DNA presence in unspecific hosts. BMC Vet. Res. 2024, 20, 365. [Google Scholar] [CrossRef] [PubMed]
- Baldacchino, F.; Desquesnes, M.; Mihok, S.; Foil, L.D.; Duvallet, G.; Jittapalapong, S. Tabanids: Neglected subjects of research, but important vectors of disease agents! Infect. Genet. Evol. 2024, 28, 596–615. [Google Scholar] [CrossRef] [PubMed]
- Muita, J.W.; Bargul, J.L.; Makwatta, J.O.; Ngatia, E.M.; Tawich, S.K.; Masiga, D.K.; Getahun, M.N. Stomoxys flies (Diptera, Muscidae) are competent vectors of Trypanosoma evansi, Trypanosoma vivax, and other livestock hemopathogens. PLoS Pathog. 2025, 21, e1012570. [Google Scholar] [CrossRef] [PubMed]
- Maia, C. Sand fly-borne diseases in Europe: Epidemiological overview and potential triggers for their emergence and re-emergence. J. Comp. Pathol. 2024, 209, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Calchi, A.C.; Yogui, D.R.; Alves, M.H.; Desbiez, A.L.J.; Kluyber, D.; Vultão, J.G.; Arantes, P.V.C.; de Santi, M.; Werther, K.; Teixeira, M.M.G.; et al. Molecular detection of piroplasmids in mammals from the Superorder Xenarthra in Brazil. Parasitol. Res. 2023, 122, 3169–3180. [Google Scholar] [CrossRef] [PubMed]
- Sada, J.M.; Kluyber, D.; Lee, D.A.B.; Calchi, A.C.; Alves, M.H.; Machado, D.M.R.; Werther, K.; Machado, R.Z.; Desbiez, A.L.J.; André, M.R. Molecular detection and characterization of Anaplasmataceae agents, Bartonella spp. and hemoplasmas in armadillos and anteaters from Brazil. Acta Trop. 2024, 260, 107477. [Google Scholar] [CrossRef] [PubMed]
- QGIS Development Team. QGIS Geographic Information System; (Version 3.32.0); Open Source Geospatial Foundation Project: Beaverton, OR, USA, 2023; Available online: https://qgis.org (accessed on 27 March 2025).
- Birkenheuer, A.J.; Levy, M.G.; Breitschwerdt, E.B. Development and evaluation of a seminested PCR for detection and differentiation of Babesia gibsoni (Asian Genotype) and B. canis DNA in canine blood samples. J. Clin. Microbiol. 2003, 41, 4172–4177. [Google Scholar] [CrossRef] [PubMed]
- Zahler, M.; Rinder, H.; Schein, E.; Gothe, R. Detection of a new pathogenic Babesia microti-like species in dogs. Vet. Parasitol. 2000, 89, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Silveira, J.A.; Rabelo, E.M.; Ribeiro, M.F. Detection of Theileria and Babesia in brown brocket deer (Mazama gouazoubira) and marsh deer (Blastocerus dichotomus) in the State of Minas Gerais, Brazil. Vet. Parasitol. 2011, 177, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.F.; Girotto, A.; Brandão, P.E.; Da Silva, A.S.; França, R.T.; Lopes, S.T.; Labruna, M.B. Detection and molecular characterization of a canine piroplasm from Brazil. Vet. Parasitol. 2011, 180, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, M.; Tajima, T.; Torii, H.; Yabutani, M.; Ishii, J.; Harasawa, M.; Isogai, E.; Rikihisa, Y. Ehrlichia chaffeensis infection of sika deer, Japan. Emerg. Infect. Dis. 2009, 15, 1991–1993. [Google Scholar] [CrossRef] [PubMed]
- Massung, R.F.; Slater, K.; Owens, J.H.; Nicholson, W.L.; Mather, T.N.; Solberg, V.B.; Olson, J.G. Nested PCR assay for detection of granulocytic ehrlichiae. J. Clin. Microbiol. 1998, 36, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, J.; Ayoubi, P.; Blouin, E.F.; Almazán, C.; Naranjo, V.; Kocan, K.M. Gene expression profiling of human promyelocytic cells in response to infection with Anaplasma phagocytophilum. Cell Microbiol. 2005, 7, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Bown, K.J.; Lambin, X.; Ogden, N.H.; Petrovec, M.; Shaw, S.E.; Woldehiwet, Z.; Birtles, R.J. High-resolution genetic fingerprinting of European strains of Anaplasma phagocytophilum by use of multilocus variable-number tandem-repeat analysis. J. Clin. Microbiol. 2007, 45, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Criado-Fornelio, A.; Martinez-Marcos, A.; Buling-Saraña, A.; Barba-Carretero, J.C. Presence of Mycoplasma haemofelis, Mycoplasma haemominutum and piroplasmids in cats from southern Europe: A molecular study. Vet. Microbiol. 2003, 93, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Kewish, K.E.; Appleyard, G.D.; Myers, S.L.; Kidney, B.A.; Jackson, M.L. Mycoplasma haemofelis and Mycoplasma haemominutum detection by polymerase chain reaction in cats from Saskatchewan and Alberta. Can. Vet. J. 2004, 45, 749–752. [Google Scholar] [PubMed]
- Mongruel, A.C.B.; Spanhol, V.C.; Valente, J.D.M.; Porto, P.P.; Ogawa, L.; Otomura, F.H.; Marquez, E.S.; André, M.R.; Vieira, T.S.W.J.; Vieira, R.F.C. Survey of vector-borne and nematode parasites involved in the etiology of anemic syndrome in sheep from Southern Brazil. Rev. Bras. Parasitol. Vet. 2020, 29, e007320. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, J.; Ruiz-Fons, F.; Naranjo, V.; Torina, A.; Rodríguez, O.; Gortázar, C. Evidence of Anaplasma infections in European roe deer (Capreolus capreolus) from southern Spain. Res. Vet. Sci. 2008, 84, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Silveira, J.A.; Rabelo, E.M.; Ribeiro, M.F. Molecular detection of tick-borne pathogens of the family Anaplasmataceae in Brazilian brown brocket deer (Mazama gouazoubira) and marsh deer (Blastocerus dichotomus). Transbound. Emerg. Dis. 2012, 59, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Noyes, H.A.; Stevens, J.R.; Teixeira, M.; Phelan, J.; Holz, P. A nested PCR for the ssrRNA gene detects Trypanosoma binneyi in the platypus and Trypanosoma sp. in wombats and kangaroos in Australia. Int. J. Parasitol. 1999, 29, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Gilber, S.R.; Alban, S.M.; Gobor, L.; Bescrovaine, J.O.; Myiazaki, M.I.; Thomaz-Soccol, V. Comparison of conventional serology and PCR methods for the routine diagnosis of Trypanosoma cruzi infection. Rev. Soc. Bras. Med. Trop. 2013, 46, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Silveira, J.A.; Rabelo, E.M.; Lacerda, A.C.; Borges, P.A.; Tomás, W.M.; Pellegrin, A.O.; Tomich, R.G.; Ribeiro, M.F. Molecular detection and identification of hemoparasites in pampas deer (Ozotoceros bezoarticus Linnaeus, 1758) from the Pantanal, Brazil. Ticks Tick Borne Dis. 2013, 4, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Cortez, A.P.; Rodrigues, A.C.; Garcia, H.A.; Neves, L.; Batista, J.S.; Bengaly, Z.; Paiva, F.; Teixeira, M.M. Cathepsin L-like genes of Trypanosoma vivax from Africa and South America—Characterization, relationships and diagnostic implications. Mol. Cell. Probes 2009, 23, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, G.A.; Guhl, F.; Chiari, E.; Macedo, A.M. Species specific detection of Trypanosoma cruzi and Trypanosoma rangeli in vector and mammalian hosts by polymerase chain reaction amplification of kinetoplast minicircle DNA. Acta Trop. 1999, 72, 203–212. [Google Scholar] [CrossRef] [PubMed]
- El Tai, N.O.; Osman, O.F.; El Fari, M.; Presber, W.; Schönian, G. Genetic heterogeneity of ribosomal internal transcribed spacer in clinical samples of Leishmania donovani spotted on filter paper as revealed by single-strand conformation polymorphisms and sequencing. Trans. R. Soc. Trop. Med. Hyg. 2000, 94, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Moreira, S.M. Estudo Retrospectivo (1998–2001) da Erliquiose Canina em Belo Horizonte: Avaliação Clínica e Laboratorial de Infecções Experimentais. Master’s Thesis, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil, 2001. Available online: http://hdl.handle.net/1843/BUOS-8C8DX2 (accessed on 22 February 2025).
- Bastos, C.V.; Passos, L.M.; Facury-Filho, E.J.; Rabelo, E.M.; de la Fuente, J.; Ribeiro, M.F. Protection in the absence of exclusion between two Brazilian isolates of Anaplasma marginale in experimentally infected calves. Vet. J. 2010, 186, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.M.; Ribeiro, M.F.; Duarte, A.L.; Mangueira, J.M.; Pessoa, A.F.; Azevedo, S.S.; Barros, A.T.; Riet-Correa, F.; Labruna, M.B. Seroprevalence and risk factors for cattle anaplasmosis, babesiosis, and trypanosomiasis in a Brazilian semiarid region. Rev. Bras. Parasitol. Vet. 2013, 22, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Castillo, A.P.; Miranda, J.V.O.; Fonseca, P.L.C.; Silva, S.O.; Lopes, R.E.N.; Spanhol, V.C.; Moreira, R.G.; Nicolino, R.R.; Queiroz, D.C.; de Araújo e Santos, L.C.G.; et al. Evidence of SARS-CoV-2 infection and co-infections in stray cats in Brazil. Acta Trop. 2024, 249, 107056. [Google Scholar] [CrossRef] [PubMed]
- Estevam, L.G.T.M.; Fonseca, A.A., Jr.; Silvestre, B.T.; Hemetrio, N.S.; Almeida, L.R.; Oliveira, M.M.; Silva, S.M.; Ribeiro, M.F.B.; Silveira, J.A.G. Seven years of evaluation of ectoparasites and vector-borne pathogens among ring-tailed coatis in an urban park in southeastern Brazil. Vet. Parasitol. Reg. Stud. Rep. 2020, 21, 100442. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.D.; Simões, S.V.D.; Souza, F.A.L.; Silveira, J.A.G.; Ribeiro, M.F.B.; Cadioli, F.A.; Sampaio, P.H. Aspectos clínicos, epidemiológicos e diagnóstico da infecção por Trypanosoma vivax em rebanho bovino no estado do Maranhão. Pesqui. Vet. Bras. 2018, 38, 896–901. [Google Scholar] [CrossRef]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Neimark, H.; Johansson, K.E.; Rikihisa, Y.; Tully, J.G. Proposal to transfer some members of the genera Haemobartonella and Eperythrozoon to the genus Mycoplasma with descriptions of ‘Candidatus Mycoplasma haemofelis’, ‘Candidatus Mycoplasma haemomuris’, ‘Candidatus Mycoplasma haemosuis’ and ‘Candidatus Mycoplasma wenyonii’. Int. J. Syst. Evol. Microbiol. 2001, 51, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Deane, L.M. Tripanosomideos de mamíferos da Região Amazônica. I. Alguns flagelados encontrados no sangue de mamíferos silvestres do Estado do Pará. Rev. Inst. Med. Trop. São Paulo 1961, 3, 15–28. [Google Scholar]
- Lainson, R.; Shaw, J.J.; Fraiha, H.; Miles, M.A.; Draper, C.C. Chagas’s Disease in the Amazon Basin: I. Trypanosoma cruzi infections in silvatic mammals, triatomine bugs and man in the State of Pará, North Brazil. Trans. R. Soc. Trop. Med. Hyg. 1979, 73, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Miles, M.A.; Povoa, M.M.; de Souza, A.A.; Lainson, R.; Shaw, J.J.; Ketteridge, D.S. Chagas’s disease in the Amazon Basin: II. The distribution of Trypanosoma cruzi zymodemes 1 and 3 in Pará State, North Brazil. Trans. R. Soc. Trop. Med. Hyg. 1981, 75, 667–674. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, V.A.; Boité, M.C.; Cupolillo, E.; Jansen, A.M.; Roque, A.L. Mixed infection in the anteater Tamandua tetradactyla (Mammalia: Pilosa) from Pará State, Brazil: Trypanosoma cruzi, T. rangeli and Leishmania infantum. Parasitology 2013, 140, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Dias, F.B.; Quartier, M.; Romaña, C.A.; Diotaiuti, L.; Harry, M. Tamandua tetradactyla Linnaeus, 1758 (Myrmecophagidae) and Rhodnius robustus Larrousse, 1927 (Triatominae) infection focus by Trypanosoma rangeli Tejera, 1920 (Trypanosomatidae) in Attalea phalerata Mart. ex Spreng (Arecaceae) palm tree in the Brazilian Amazon. Infect. Genet. Evol. 2010, 10, 1278–1281. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.M.; Barreto, W.T.G.; de Macedo, G.C.; Barros, J.H.D.S.; Xavier, S.C.D.C.; Garcia, C.M.; Mourão, G.; de Oliveira, J.; Rimoldi, A.R.; Porfírio, G.E.O.; et al. The reservoir system for Trypanosoma (Kinetoplastida, Trypanosomatidae) species in large neotropical wetland. Acta Trop. 2019, 199, 105098. [Google Scholar] [CrossRef] [PubMed]
- Carcavallo, R.U. Climatic factors related to Chagas disease transmission. Mem. Inst. Oswaldo Cruz 1999, 94 (Suppl. 1), 367–369. [Google Scholar] [CrossRef] [PubMed]
- Ferro, E.; Silva, A.M.; Sobral-Souza, T.; Vancine, M.H.; Muylaert, R.L.; de Abreu, A.P.; Pelloso, S.M.; de Barros Carvalho, M.D.; de Andrade, L.; Ribeiro, M.C.; et al. Spatial prediction of risk areas for vector transmission of Trypanosoma cruzi in the State of Paraná, southern Brazil. PLoS Negl. Trop. Dis. 2018, 12, e0006907. [Google Scholar] [CrossRef]
- Dirección General de Epidemiología. Análisis de la Situación de Salud del Perú; Ministério de Salud del Perú: Lima, Peru, 2010. Available online: https://www.dge.gob.pe/publicaciones/pub_asis/asis25.pdf (accessed on 3 June 2025).
- Campigotto, G.; Volpato, A.; Galli, G.M.; Glombowsky, P.; Baldissera, M.D.; Miletti, L.C.; Jaguezeski, A.M.; Stefani, L.M.; Da Silva, A.S. Vertical transmission of Trypanosoma evansi in experimentally infected rats. Exp. Parasitol. 2017, 174, 42–44. [Google Scholar] [CrossRef] [PubMed]
- Herrera, H.M.; Dávila, A.M.; Norek, A.; Abreu, U.G.; Souza, S.S.; D’Andrea, P.S.; Jansen, A.M. Enzootiology of Trypanosoma evansi in Pantanal, Brazil. Vet. Parasitol. 2004, 125, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Hoare, C.A. The Trypanosomes of Mammals. A Zoological Monograph; Blackwell Scientific Publications: Oxford, UK, 1972. [Google Scholar]
- O’Donoghue, P. Haemoprotozoa: Making biological sense of molecular phylogenies. Int. J. Parasitol. Parasites Wildl. 2017, 6, 241–256. [Google Scholar] [CrossRef] [PubMed]
- OIE. Trypanosoma evansi Infection (Surra): Technical Disease Cards. OIE Terrestrial Manual. 2018, pp. 1–4. Available online: https://www.oie.int/fileadmin/Home/eng/Animal_Health_in_the_World/docs/pdf/Disease_cards/TRYPANO_EVANSI.pdf (accessed on 5 May 2025).
- Aregawi, W.G.; Agga, G.E.; Abdi, R.D.; Büscher, P. Systematic review and meta-analysis on the global distribution, host range, and prevalence of Trypanosoma evansi. Parasites Vectors 2019, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Luckins, A.G. Trypanosoma evansi in Asia. Parasitol. Today 1988, 4, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Olifiers, N.; Jansen, A.M.; Herrera, H.M.; Bianchi, R.C.; D’Andrea, P.S.; Mourão, G.M.; Gompper, M.E. Co-Infection and Wild Animal Health: Effects of Trypanosomatids and Gastrointestinal Parasites on Coatis of the Brazilian Pantanal. PLoS ONE 2015, 10, e0143997. [Google Scholar] [CrossRef] [PubMed]
- Maggi, R.G.; Compton, S.M.; Trull, C.L.; Mascarelli, P.E.; Mozayeni, B.R.; Breitschwerdt, E.B. Infection with hemotropic Mycoplasma species in patients with or without extensive arthropod or animal contact. J. Clin. Microbiol. 2013, 51, 3237–3241. [Google Scholar] [CrossRef] [PubMed]
- Maggi, R.G.; Mascarelli, P.E.; Havenga, L.N.; Naidoo, V.; Breitschwerdt, E.B. Co-infection with Anaplasma platys, Bartonella henselae and Candidatus Mycoplasma haematoparvum in a veterinarian. Parasites Vectors 2013, 6, 103. [Google Scholar] [CrossRef] [PubMed]
- Hornok, S.; Micsutka, A.; Meli, M.L.; Lutz, H.; Hofmann-Lehmann, R. Molecular investigation of transplacental and vector-borne transmission of bovine haemoplasmas. Vet. Microbiol. 2011, 152, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Tasker, S. Hemotropic Mycoplasma. Vet. Clin. N. Am. Small Anim. Pract. 2022, 52, 1319–1340. [Google Scholar] [CrossRef] [PubMed]
- Millán, J.; Martín-Maldonado, B.; Rodríguez-Pastor, R.; Martínez-Padilla, J.; Esperón, F. High diversity, novel genotypes, and vertical transmission of hemotropic Mycoplasma in micromammals. Comp. Immunol. Microbiol. Infect. Dis. 2024, 107, 102151. [Google Scholar] [CrossRef] [PubMed]
- Neimark, H.; Peters, W.; Robinson, B.L.; Stewart, L.B. Phylogenetic analysis and description of Eperythrozoon coccoides, proposal to transfer to the genus Mycoplasma as Mycoplasma coccoides comb. nov. and Request for an Opinion. Int. J. Syst. Evol. Microbiol. 2005, 55, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.S.; de Souza, R.S.; Carvalho de Araujo, A.; Silva, S.O.; Melo, M.N.; Melo, F.G.; Ribeiro, G.H.S.; de Sousa, F.G.; Bastos, C.V.; Moreira, T.F.; et al. Hemopathogens in naturally infected bovine fetuses in Brazil. Ticks Tick-Borne Dis. 2024, 15, 102351. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.; Shemesh, M.; Garrido, M.; Messika, I.; Einav, M.; Khokhlova, I.; Tasker, S.; Hawlena, H. Haemoplasmas in wild rodents: Routes of transmission and infection dynamics. Mol. Ecol. 2018, 27, 3714–3726. [Google Scholar] [CrossRef] [PubMed]
- Kreutz, K.; Fischer, F.; Linsenmair, K.E. Observations of intraspecific aggression in giant anteaters (Myrmecophaga tridactyla). Edentata 2009, 10, 6–7. [Google Scholar] [CrossRef]
- Kluyber, D.; Desbiez, A.L.J.; Attias, N.; Massocato, G.F.; Gennari, S.M.; Soares, H.S.; Bagagli, E.; Bosco, S.M.G.; Garcés, H.G.; Ferreira, J.D.S.; et al. Zoonotic parasites infecting free-living armadillos from Brazil. Transbound. Emerg. Dis. 2021, 68, 1639–1651. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, L.B.; Calchi, A.C.; Vultão, J.G.; Yogui, D.R.; Kluyber, D.; Alves, M.H.; Desbiez, A.L.J.; de Santi, M.; Soares, A.G.; Soares, J.F.; et al. Molecular investigation of haemotropic Mycoplasmas and Coxiella burnetii in free-living Xenarthra mammals from Brazil, with evidence of new haemoplasma species. Transbound. Emerg. Dis. 2022, 69, e1877–e1891. [Google Scholar] [CrossRef] [PubMed]
- Hokan, M.; Strube, C.; Radespiel, U.; Zimmermann, E. Sleeping site ecology, but not sex, affect ecto- and hemoparasite risk, in sympatric, arboreal primates (Avahi occidentalis and Lepilemur edwardsi). Front. Zool. 2017, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- Ezquiaga, M.C.; Gallo, J.A.; D’Agostino, R.L.; Udrizar Sauthier, D.E.; Abba, A.M.; Sanchez, J. Fleas and ticks in armadillos from Argentinean Patagonia: Diversity, abundance and distribution. Acta Trop. 2021, 219, 105911. [Google Scholar] [CrossRef] [PubMed]
- Miranda, F.R.; Teixeira, R.H.F.; Gazêta, G.S.; Serra-Freire, N.M.; Amorim, M. Presence of Amblyomma cajennense in wild giant armadillos (Priodontes maximus) of the Pantanal Matogrossense, Brazil. Edentata 2010, 11, 73–77. [Google Scholar] [CrossRef]
- Oliveira, G.M.B.; Martins, T.F.; Pereira, L.C.M.; Nicola, P.A.; Horta, M.C. Ocorrência de carrapatos em Tamandua tetradactyla (Linnaeus, 1758) na Caatinga, Nordeste do Brasil. Arq. Bras. Med. Vet. Zootec. 2017, 69, 865–869. [Google Scholar] [CrossRef]
- Soares, H.S.; Barbieri, A.R.; Martins, T.F.; Minervino, A.H.; de Lima, J.T.; Marcili, A.; Gennari, S.M.; Labruna, M.B. Ticks and rickettsial infection in the wildlife of two regions of the Brazilian Amazon. Exp. Appl. Acarol. 2015, 65, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Labruna, M.B.; Camargo, L.M.; Terrassini, F.A.; Schumaker, T.T.; Camargo, E.P. Notes on parasitism by Amblyomma humerale (Acari: Ixodidae) in the state of Rondônia, western Amazon, Brazil. J. Med. Entomol. 2002, 39, 814–817. [Google Scholar] [CrossRef] [PubMed]
| Sample Identification | Species | Age | Location: Country/State/County |
|---|---|---|---|
| CD002 | C. didactylus | Adult | Brazil/MA/São Luís |
| CD004 | C. didactylus | Adult | Brazil/PE/Igarassu |
| CD005 | C. didactylus | Adult | Brazil/PE/Timbaúba |
| CD006 | C. didactylus | Adult | Brazil/PE/Jaboatão dos Guararapes |
| CD008 | C. didactylus | Adult | Brazil/PA/Oriximiná |
| CD010 | C. didactylus | Adult | Brazil/RN/Goianinha |
| CD011 | C. rufus | Adult | Peru/Ucayali/Atalaya |
| CD012 | C. ida | Adult | Peru/Ucayali/Purús |
| CD015 | C. didactylus | Adult | Brazil/AM/Santa Isabel do Rio Negro |
| CD018 | C. ida | Adult | Peru/Loreto/Maynas |
| CD022 | C. didactylus | Adult | Brazil/MA/Rosário |
| CD026 | C. rufus | Adult | Brazil/RO/Espigão do Oeste |
| CD030 | C. thomasi | Adult | Brazil/AC/Porto Walter |
| CD032 | C. didactylus | Adult | Brazil/AM/Manaus |
| CD034 | C. dorsalis | Adult | Colombia/Santander/Girón |
| UFMG 6015 | C. rufus | Adult | Brazil/RO/Porto Velho |
| Agents | Primer | Sequences (5’-3’) | Target | Fragment (bp) | Reference | |
|---|---|---|---|---|---|---|
| Piroplasmida/ Hepatozoon spp. screening | 1st reaction | RIB-19 | CGGGATCCAACCTGGTTGATCCTGC | 18S rRNA | 1700 | [22] |
| RIB-20 | CCGAATTCCTTGTTACGACTTCTC | |||||
| 2nd reaction | BAB-Rum F | ACCTCACCAGGTCCAGACAG | 18S rRNA | 430 | [23] | |
| BAB-Rum R | GTACAAAGGGCAGGGACGTA | |||||
| Piroplasmida screening | HSP70F2 | GGATCAACAAYGGMAAGAAC | hsp70 | 720 | [24] | |
| HSP70R2 | GBAGGTTGTTGTCCTTVGTCAT | |||||
| Ehrlichia spp. screening | 1st reaction | N516SCH 1 F | ACGGACAATTGCTTATAGCCTT | 16S rRNA | 1195 | [25] |
| N516SCH 1 R | ACAACTTTTATGGATTAGCTAAAT | |||||
| 2nd reaction | N516SCH 2 F | GGGCACGTAGGTGGACTAG | 16S rRNA | 443 | [25] | |
| N516SCH 2 R | CCTGTTAGGAGGGATACGAC | |||||
| Anaplasma spp. screening | 1st reaction | GE3a | CACATGCAAGTCGAACGGATTATTC | 16S rRNA | 932 | [26] |
| GE10R | TTCCGTTAAGAAGGATCTAATCTCC | |||||
| 2nd reaction | GE9F | AACGGATTATTCTTTATAGCTTGCT | 16SrRNA | 546 | [26] | |
| GE2 | GGCAGTATTAAAAGCAGCTCCAGG | |||||
| Anaplasma phagocytophilum characterization | 1st reaction | MSP4AP5 | ATGAATTACAGAGAATTGCTTGTAGG | msp4 | 849 | [27] |
| MSP4AP3 | TTAATTGAAAGCAAATCTTGCTCCTATG | |||||
| 2nd reaction | msp4F | CTATTGGYGGNGCYAGAGT | msp4 | 450 | [28] | |
| msp4R | GTTCATCGAAAATTCCGTGGTA | |||||
| Hemotropic Mycoplasma screening/characterization | HBT F | ATACGGCCCATATCCCTACG | 16S rRNA | 600 | [29] | |
| HBT R | TCGCTCCACCACTTGTTCA | |||||
| Hemotropic Mycoplasma screening | HemF | ACGAAAGTCTGATGGAGCAATA | 16S rRNA | 170–193 | [30] | |
| HemR | ACGCCCAATAAATCCGRATAAT | |||||
| Hemotropic Mycoplasma characterization | 23S_HAEMO_F | TGA GGG AAA GAG CCC AGA C | 23S rRNA | 800 | [31] | |
| 23S_HAEMO_R | GGA CAG AAT TTA CCT GAC AAG G | |||||
| Anaplasma marginale screening/characterization | 1st reaction | MSP45 | GGGAGCTCCTATGAATTACAGAGAATTGTTTAC | msp4 | 872 | [32] |
| MSP43 | CCGGATCCTTAGCTGAACAGGAATCTTGC | |||||
| 2nd reaction | AnapF | CGCCAGCAAACTTTTCCAAA | msp4 | 294 | [33] | |
| AnapR | ATATGGGGACACAGGCAAAT | |||||
| Kinetoplastida screening | 1st reaction | SSU450F | TGGGATAACAAAGGAGCA | 18S rRNA | 928–1984 | [34] |
| SSU450R | CTGAGACTGTAACCTCAAAGC | |||||
| 2nd reaction | TRY927F | CAGAAACGAAACACGGGAG | 18S rRNA | 927 | [34] | |
| TRY927R | CCTACTGGGCAGCTTGGA | |||||
| Kinetoplastida screening/characterization | TCZ1 | CGAGCTCTTGCCCACACGGGTGCT | kDNA | 180 | [35] | |
| TCZ2 | CCTCCAAGCAGC GGATAGTTCAGG | |||||
| Trypanosoma evansi screening/characterization | 1st reaction | Te1F | GCACAGTATGCAACCAAAAA | ITS | 280 | [36] |
| Te1R | GTGGTCAACAGGGAGAAAAT | |||||
| 2nd reaction | Te2F | CATGTATGTGTTTCTATATG | ITS | 219 | [36] | |
| Te1R | GTGGTCAACAGGGAGAAAAT | |||||
| Trypanosoma vivax screening/characterization | DTO154 | ACAGAATTCCAGGGCCAATGCGGCTCGTGCTGG | Catepsin L | 500 | [37] | |
| DTO155 | TTAAAGCTTCCACGAGTTCTTGATGATCCAGTA | |||||
| Trypanosoma cruzi/ Trypanosoma rangeli screening/characterization | S35 | AAATAATGTACGGGKGAGATGCATGA | kDNA | 330 | [38] | |
| S36 | GGTTCGATTGGGGTTGGTGTAATATA | |||||
| Leishmania spp. screening/characterization | LITSR | CTGGATCATTTT CCGATG | ITS1 | 300–350 | [39] | |
| L5.8S | TGATACCACTTA TCGCACTT | |||||
| gapdh | GAPDH F | CCTTCATTGACCTCAACTACAT | gapdh | 400 | [21] | |
| GAPDH R | CCAAAGTTGTCATGGATGACC | |||||
| Sample Identification | Country | Agent | |||||||
|---|---|---|---|---|---|---|---|---|---|
| T. evansi | T. cruzi | T. vivax | Leishmania spp. | Mycoplasma spp. | Piroplasmida/Hepatozoon spp. | Ehrlichia spp. | Anaplasma spp. | ||
| CD002 C. didactylus | Brazil | Positive | - | - | - | - | - | - | - |
| CD004 C. didactylus | Brazil | Positive | - | - | - | - | - | - | - |
| CD005 C. didactylus | Brazil | - | - | - | - | Positive | - | - | - |
| CD006 C. didactylus | Brazil | - | - | - | - | Positive | - | - | - |
| CD008 C. didactylus | Brazil | Positive | - | - | - | Positive | - | - | - |
| CD010 C. didactylus | Brazil | - | - | - | - | Positive | - | - | - |
| CD011 C. rufus | Peru | - | Positive | - | - | Positive | - | - | - |
| CD012 C. ida | Peru | Positive | - | - | - | Positive | - | - | - |
| CD015 C. didactylus | Brazil | - | - | - | - | - | - | - | - |
| CD018 C. ida | Peru | Positive | - | - | - | Positive | - | - | - |
| CD022 C. didactylus | Brazil | - | - | - | - | Positive | - | - | - |
| CD026 C. rufus | Brazil | Positive | - | - | - | - | - | - | - |
| CD030 C. thomasi | Brazil | Positive | - | - | - | Positive | - | - | - |
| CD032 C. didactylus | Brazil | Positive | - | - | - | - | - | - | - |
| CD034 C. dorsalis | Colombia | - | - | - | - | - | - | - | - |
| UFMG 6015 C. rufus | Brazil | - | - | - | - | Positive | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, P.H.C.; Alves, J.P.S.; Miranda, F.R.; Rojano, C.; Silveira, J.A.G. Vector-Borne Agents in Species of Silky Anteater (Cyclopes Gray, 1821) from South America. Pathogens 2025, 14, 718. https://doi.org/10.3390/pathogens14070718
Rodrigues PHC, Alves JPS, Miranda FR, Rojano C, Silveira JAG. Vector-Borne Agents in Species of Silky Anteater (Cyclopes Gray, 1821) from South America. Pathogens. 2025; 14(7):718. https://doi.org/10.3390/pathogens14070718
Chicago/Turabian StyleRodrigues, Pedro Henrique Cotrin, João Paulo Soares Alves, Flávia Regina Miranda, Cesar Rojano, and Júlia Angélica Gonçalves Silveira. 2025. "Vector-Borne Agents in Species of Silky Anteater (Cyclopes Gray, 1821) from South America" Pathogens 14, no. 7: 718. https://doi.org/10.3390/pathogens14070718
APA StyleRodrigues, P. H. C., Alves, J. P. S., Miranda, F. R., Rojano, C., & Silveira, J. A. G. (2025). Vector-Borne Agents in Species of Silky Anteater (Cyclopes Gray, 1821) from South America. Pathogens, 14(7), 718. https://doi.org/10.3390/pathogens14070718






